Spatiotemporal expression of endogenous TLR4 ligands leads to inflammation and bone erosion in mouse collagen-induced arthritis
Abstract
Increased expression of endogenous Toll-like receptor 4 (TLR4) ligands (e.g., Tenascin-C, S100A8/A9, citrullinated fibrinogen (cFb) immune complexes) has been observed in patients with rheumatoid arthritis (RA). However, their roles in RA pathogenesis are not well understood. Here, we investigated the expression kinetics and role of endogenous TLR4 ligands in the murine model of collagen-induced arthritis (CIA). Tenascin-C was upregulated in blood early in CIA, and correlated positively with the clinical score at day 56. Levels of S100A8/A9 increased starting from day 28, peaking at day 42, and correlated positively with joint inflammation. Levels of anti-cFb antibodies increased during the late phase of CIA and correlated positively with both joint inflammation and cartilage damage. Blockade of TLR4 activation at the time of the first TLR4 ligand upregulation prevented clinical and histological signs of arthritis. A TLR4-dependent role was also observed for Tenascin-C and cFb immune complexes in osteoclast differentiation in vitro. Taken together, our data suggests that the pathogenic contribution of TLR4 in promoting joint inflammation and bone erosion during CIA occurs via various TLR4 ligands arising at different stages of disease. The data also suggests that Blockade of TLR4 with monoclonal antibodies is a promising strategy in RA treatment.
Introduction
Toll-like receptor 4 (TLR4) is a pattern recognition receptor involved in the initiation of inflammatory responses to control pathogen infections 1. Excessive activation of TLR4 signaling pathways causes heightened inflammation and results in tissue damage 2. In addition to activation by pathogen-derived molecules, TLR4 has also been identified as a ‘danger-sensing’ receptor that recognizes host-derived damage associated molecular patterns (DAMPs; also known as danger-associated molecular pattern molecules) 3. Consistent with its role as a general ‘danger-sensing’ receptor, TLR4 is activated by many types of host-derived molecules including released intracellular proteins (e.g., S100A8/A9, high-mobility group protein B1 (HMGB1)), extracellular matrix components (e.g. extra domain A of fibronectin, Tenascin-C), modified molecules under disease situations (e.g. mmLDL, minimally modified low-density lipoprotein), and other soluble mediators (e.g., fibrinogen) 4-9.
A role of TLR4 in rheumatoid arthritis (RA) has been suggested due to its prominent role in inflammation. Two inflammatory cytokines downstream of TLR4 signaling, tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), have been validated as therapeutic targets in RA 10. Interestingly, deficiency in TLR4 signaling is protective in several animal models of arthritis 11-13. Furthermore, several endogenous ligands of TLR4, such as Tenascin-C, HMGB1 and S100A8/A9, are highly expressed in sera and synovial fluids of RA patients, with a role in arthritis being suggested in mouse models 14-18. The presence of anti-citrullinated protein antibodies (ACPA) is indicative of RA and, thus, used as a criteria for diagnosis 19. Immune complexes containing ACPA and citrullinated proteins, such as citrullinated fibrinogen (cFb) or citrullinated histone 2b (cH2b), have been reported as TLR4 activators 20, 21.
It is not yet known to what extent endogenous TLR4 ligands play a pathogenic role in RA. Thus, in this study, we investigated the spatiotemporal expression of several endogenous TLR4 ligands associated with RA using the mouse model of collagen-induced arthritis (CIA). An association of these ligands with signs of disease was observed and the role of TLR4 confirmed in vitro and in vivo using a blocking anti-mouse TLR4 monoclonal antibody (mAb). These findings provide a rationale to target TLR4 in RA, especially in those patients with high circulating levels of endogenous TLR4 ligands.
Results
Expression of endogenous TLR4 ligands during the development of CIA
Increased expression of several endogenous TLR4 ligands has been reported in patients with rheumatoid arthritis 14-17. To evaluate the potential role of these ligands, their levels were measured over time in the blood and in the joints of DBA/1 mice during the development of CIA. As expected, the mice manifested clinical signs of arthritis starting around 32 days after the first immunization with collagen type II (CII) and CFA (Supporting Information Fig. 1). Knee joints (one per mouse) were obtained and processed in order to quantify levels of TLR4 ligands from fluids of a ‘joint/patella washout’ (See ‘Materials and methods’). In addition, blood was collected from the mice.
A baseline level for Tenascin-C of 302 ± 60 ng/mL was observed in blood, increasing to 795 ± 440 ng/mL at day 7 after the first immunization (Fig. 1A, left panel). A further substantial increase of Tenascin-C levels in the blood (1382 ± 142 ng/mL) was observed at day 14. These levels were sustained to reach 1549 ± 185 ng/mL at day 56. The baseline level of Tenascin-C in the joint washout was not detectable, increasing to 12.8 ± 3.2 ng/mL at day 21 and 82.1 ± 36.7 ng/mL at day 28 (Fig. 1A, right panel). The levels were further increased to 163.4 ± 18.7 ng/mL at day 56. The levels of S100A8/A9 in blood were only increased at the late stage (day 56) of disease (Fig. 1B, left panel). In contrast, increased levels of S100A8/A9 were observed in the joint washout starting from day 28 and peaking at day 42 with a mean value of 2836 ± 591 ng/mL (Fig. 1B, right panel). Very low levels of baseline HMGB1 were detected in blood (20.3 ± 8.0 ng/mL) and the joint washout (58.3 ± 15.8 ng/mL) (Fig. 1C), which increased to 49.9 ± 12.9 ng/mL in blood and 536.8 ± 93.8 ng/mL in the joint washout at day 56 without reaching statistical significance.
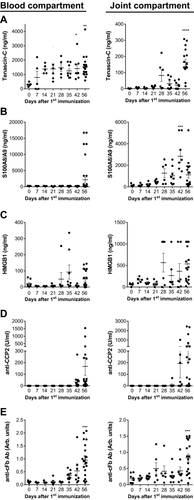
As the presence of ACPA is a hallmark of RA and immune complexes containing citrullinated fibrinogen (cFb-IC) have been reported as TLR4 ligands 19, 21, the levels of anti-cyclic citrullinated peptide 2 (anti-CCP2, as a measure of ACPA levels) and anti-cFb antibodies (Ab) were investigated. No anti-CCP2 Ab was detected in the blood or joint washout of non-immunized DBA/1 mice (Fig. 1D). Increased levels of anti-CCP2 Abs were detected from day 42 in both blood and joints of CIA mice. The highest levels were observed at day 56, with a mean value of 168 ± 77 U/mL in blood and 372 ± 153 U/mL in the joint washout (Fig. 1D). An increase of anti-cFb Ab was first detected in blood at day 35, with the highest levels attained in both blood and the joint washout at day 56 (0.95 ± 0.16 arbitrary (arb.) units in blood and 0.83 ± 0.12 arb. units in joint washout, Fig. 1E).
Correlation of disease severity with the levels of Tenascin-C and anti-CCP2 Ab in CIA
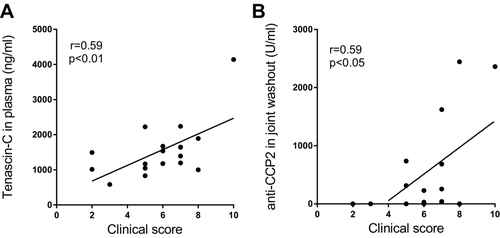
Association of the levels of TLR4 ligands with disease severity in CIA was next investigated. Tenascin-C levels in blood correlated positively with disease severity at day 56 (r = 0.59, 95% confidence interval (CI): 0.17 to 0.83, p < 0.01, Fig. 2A). The levels of anti-CCP2 Ab in the joint washout correlated with disease severity at day 56 (r = 0.59, 95% CI: 0.17–0.83, p < 0.05, Fig. 2B).
Kinetics of joint inflammation and destruction is correlated with the levels of TLR4 ligands in CIA
Cell infiltration in the joint, assessed by H&E staining of paraffin-embedded sections, was first detected at day 14 post immunization, with infiltration detected in majority of the mice at day 28 (Fig. 3A,). The infiltration index increased significantly over baseline at day 42 and continued to increase to the end of the experiment (day 56). Cartilage damage, assessed by the decrease of Safranin-O staining, was first observed at day 21 and a significant increase was observed at days 42 and 56 (Fig. 3B). The number of osteoclasts in the joint, the main cell type involved in bone erosion, was assessed by measuring the area of TRAP-positive staining in joint sections. A substantial increase of osteoclast number was first observed at day 35 and continued to increase with disease progression until the end of the experiment (day 56) (Fig. 3C).

Association between the histological signs and levels of TLR4 ligands was analyzed. S100A8/A9 demonstrated a significant positive correlation with cell infiltration in the joint (r = 0.84, 95% CI: 0.24–0.98, p < 0.05, Fig. 3D) at the peak of the protein's levels in the joint washout during CIA development (i.e., day 42). The levels of anti-cFb Ab in blood demonstrated a significant positive correlation with cell infiltration (r = 0.56, 95% CI: 0.19–0.79, p < 0.01, Fig. 3E) and cartilage damage (r = 0.55, 95% CI: 0.19–0.78, p < 0.01, Fig. 3F) as well as a trend toward correlation with osteoclast numbers in the joint (r = 0.38, 95% CI: 0–0.68, p = 0.08, Fig. 3G) late in the disease, i.e., at day 56.
Blockade of TLR4 activity ameliorates disease severity and histological parameters in CIA
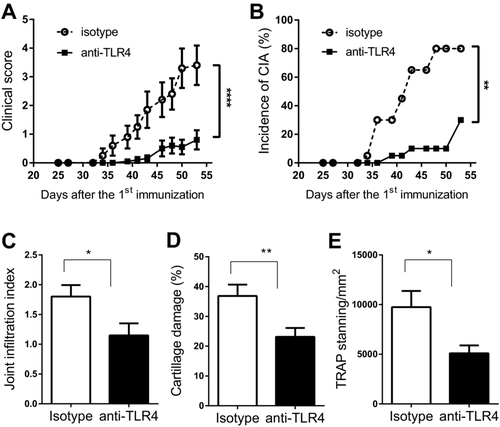
Blockade of TLR4 signaling was performed with the anti-mouse TLR4 mAb, 5E3, which binds to mouse TLR4 at a region involved in receptor dimerization, and thus blocks TLR4 signaling independent of ligand type 22, 23. As the earliest substantial increase of TLR4 ligands was observed at day 14 after the first immunization, treatment with 5E3 was initiated at this point to block any potential TLR4 activation mediated by upregulation of endogenous TLR4 ligands. Administration of the anti-TLR4 mAb delayed disease onset and decreased both incidence and severity of disease as compared to the isotype control antibody (Fig. 4A & 4B). The impact of TLR4 blockade on inflammatory cell infiltration, cartilage damage and osteoclast number was also investigated at the end of the experiment. Cell infiltration into the joint was reduced with anti-TLR4 treatment (Fig. 4C), with the mean infiltration index decreasing from 1.80 ± 0.19 in the isotype control group to 1.14 ± 0.20 in the anti-TLR4 mAb treated group. Cartilage damage, assessed by Safranin O staining, was also significantly reduced from a mean damage area of 36.8% ± 3.8% in the isotype control group to a mean damage area of 23.1% ± 3.0% in the anti-TLR4 treated group (Fig. 4D). The number of osteoclasts, assessed by the area of TRAP staining, was also significantly reduced by anti-TLR4 treatment (Fig. 4E). Taken together, the results demonstrate that blockade of TLR4 activity significantly ameliorated both clinical and histological parameters in CIA.
TLR4 blockade reduces levels of Ab to cFb but not to collagen type II (anti-CII) in CIA
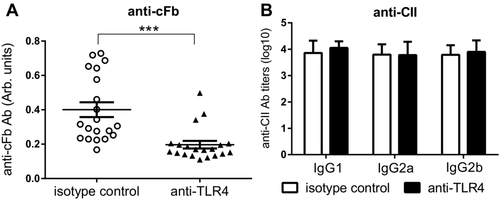
As the presence of Ab to cFb was shown to be associated with histological signs in CIA (Fig. 3E–G), we measured its levels at day 56. TLR4 blockade significantly reduced the levels of anti-cFb in the blood (Fig. 5A). In contrast, levels of anti-CII antibodies were not affected by anti-TLR4 treatment (Fig. 5B). Levels of Tenascin-C, HMGB1, S100A8/A9 and anti-CCP2 were also not altered by anti-TLR4 treatment (Supporting Information Fig. 2).
TLR4 blockade reduces levels of IL-6 and IL-1β but not that of TNF-α in CIA
In a separate experiment, the impact of neutralizing TLR4 on the inflammatory cytokine levels in blood of mice with CIA was assessed. Treatment with anti-TLR4 mAb significantly reduced the levels of IL-6 from 251.8 ± 42.39 pg/mL in the isotype control group to 100.0 ± 21.41 pg/mL in the anti-TLR4 treated group (Supporting Information Fig. 3A), and IL-1β significantly reduced from 13.63 ± 1.13 pg/mL to 8.730 ± 1.415 pg/mL (Supporting Information Fig. 3B). In contrast, the level of TNF-α was not affected (Supporting Information Fig. 3C).
Endogenous TLR4 ligands overexpressed in arthritic mice promote osteoclast differentiation in vitro
As anti-cFb Ab showed a trend of correlation with the number of osteoclasts in the arthritic joints and TLR4 was expressed on osteoclasts, the potential of TLR4 ligands to directly induce osteoclast differentiation was assessed. Mouse bone marrow cells were differentiated into osteoclasts in the presence of RANKL and M-CSF. TLR4 ligands were added to the culture on day 3 and the number of osteoclasts was quantified after 4 additional days. Osteoclasts in the cultures were identified as multinucleated and TRAP positive cells. The addition of native fibrinogen (nFb), in addition to RANKL and M-CSF, had no effect on the number of osteoclasts (Fig. 6A and Supporting Information Fig. 4). In contrast, the number of osteoclasts substantially increased from 20 (± 3)/well in the control group to 32 (± 0.7)/well when stimulated with cFb. An even higher number of osteoclasts (44 ± 2.4/well) was obtained when stimulated with cFb-IC. Treatment with anti-TLR4 mAb abrogated the increase of osteoclast numbers induced by cFb and cFb-IC as compared to the isotype control antibody (Fig. 6A and Supporting Information Fig. 4). Stimulation with Tenascin-C also significantly increased the number of osteoclasts, which was again inhibited by treatment with the anti-TLR4 mAb (Fig. 6B).

As TLR4 signaling induces the production of TNF-α and IL-6, which promote osteoclast differentiation 24, the involvement of TNF-α and IL-6 in TLR4-mediated osteoclast differentiation was investigated. Stimulation with LPS significantly increased osteoclast differentiation, and was inhibited by TLR4 blockade. LPS-induced osteoclast differentiation was also inhibited by anti-TNF-α and anti-IL-6R blocking mAbs to the same extent as the anti-TLR4 mAb, while the isotype control antibody had no impact (Fig. 6C). These results suggest that TLR4 activation promotes osteoclast differentiation by upregulation of TNF-α and IL-6.
Discussion
TLR4 is a mediator of the innate inflammatory response 1. The presence of endogenous TLR4 ligands in RA patients suggests that TLR4 activation may be a trigger of the inflammatory process in RA. To address the role of individual TLR4 ligands in arthritis pathogenesis, we investigated the spatiotemporal expression and role of multiple endogenous TLR4 ligands in CIA. The study demonstrated that multiple TLR4 ligands were temporally upregulated and associated with clinical and histological manifestation of CIA at different stages of disease development. The direct involvement of TLR4 activation in CIA pathogenesis and osteoclast differentiation was confirmed using a neutralizing mAb against TLR4.
Tenascin C is an extracellular matrix protein and has been reported as a TLR4 activator 5. Although Tenascin C is not detected in healthy adult tissues, it has been detected in the blood of healthy subjects with its source not well determined 16, 25. Circulating Tenascin-C levels are increased in many inflammatory indications, including RA 16. The administration of Tenascin-C intra-articularly into the mouse knee joint has been demonstrated to induce destructive joint inflammation 5. We observed that the blood levels of Tenascin-C were significantly upregulated in CIA and trended toward a positive correlation with the clinical score. Increased levels of Tenascin-C have also been observed in RA patients at the early stage of the disease, with levels correlated positively with bone erosion 16. In agreement with its in vivo association with bone erosion, we observed that stimulation of osteoclast precursors with Tenascin-C promoted osteoclast differentiation in vitro. The results suggest that Tenascin-C is involved in bone erosion during the early stage of arthritis by enhancing osteoclast differentiation.
S100A8/A9, a putative endogenous TLR4 ligand, is broadly upregulated in inflammatory indications 26. Although S100A8/A9 stimulates cellular signaling through various receptors, its inflammatory effects on myeloid cells were mainly mediated by TLR4 activation 27. Increased levels of S100A9 in the afflicted joint and its correlation with joint inflammation after disease onset have been reported in CIA as well as antigen-induced arthritis (AIA) 28, 29. Unfortunately, no time course analysis of S100A8/A9 expression was performed in these studies. Our detailed time course data thus expands the observations of these studies by illustrating that upregulation of S100A8/A9 expression occurs in relevant joints during the onset of CIA. Furthermore, the increased levels of S100A8/A9 were maintained during disease progression and peaked at day 42. Consistent with reported data in RA patients 30, 31, a positive correlation of S100A8/A9 levels with joint inflammation was observed in our study. Due to the small sample size, further investigation is required to confirm this observation.
The presence of ACPA is a characteristic feature of RA 32. The discovery of immune complexes containing cFb and cH2b as TLR4 activators suggests that a subgroup of ACPA from RA patients may form TLR4-activating immune complexes to enhance joint damage and drive disease progression 20, 21. In CIA, a significant increase of ACPA levels (measured by anti-CCP2 assay) was only observed after disease onset. This is different from patients with RA, where ACPA positivity was observed long before disease onset 33. Nonetheless, consistent with a role of TLR4 activation by ACPA in RA, we demonstrated that the levels of anti-cFb Ab were positively associated with joint inflammation and osteoclast number at day 56, when the levels of anti-cFb peaked in CIA. Only a single subtype of ACPA (anti-cFb Ab) has been assessed in this study, thus it would be relevant to investigate whether ACPA with other specificities (for example, anti-cH2b Ab) are upregulated and play a role in CIA progression. Anti-cFb Ab were detected earlier than anti-CCP2 Ab in CIA. This may be due to the presence of citrullinated epitopes in cFb that are distinct from those mimicked by the CCP2 peptides but are recognized by ACPA from CIA mice. Alternatively, this discrepancy could be explained by higher sensitivity of the anti-cFb Ab assay. The level of HMGB1 was also elevated late in CIA development (day 56). However, no correlation with any disease parameter was observed, suggesting that immune complexes containing ACPA may dominate over HMGB1 in mediating TLR4 activation late in disease progression.
Mice genetically deficient in TLR4 demonstrate reduced incidence and severity of CIA 13. It is not clear whether this protection is due to developmental defects or a deficiency in TLR4 signaling during CIA pathogenesis. Using a LPS mimetic, short-term disease amelioration has been confirmed in CIA 34. However, the mice were only treated for 4–6 days and long-term effects of TLR4 blockade by the LPS mimetic in the CIA model were not investigated. Furthermore, TLR4 activation stimulated by certain ligands, such as Tenascin-C and extra domain A (EDA) of fibronectin, is not blocked by an LPS mimetic, which blocks TLR4 activation by competing with LPS for TLR4/MD-2 binding 5, 35. The results obtained with an LPS mimetic may therefore not reflect the full potential of TLR4 blockade in CIA. In the current study, we have used the previously described anti-TLR4 mAb, 5E3, which has the potential to block TLR4 activation stimulated by all TLR4 ligands 22, 23. Using this antibody and a prolonged treatment schedule (i.e., 6 weeks), we have demonstrated long-term amelioration of disease severity in CIA. To avoid interfering with adaptive immune responses elicited by CII immunization, and to align the timing of TLR4 blockade with the initial increase of TLR4 ligands, administration of the 5E3 Ab was initiated at day 14 after the first immunization with CII. The results demonstrated that TLR4 blockade inhibited joint inflammation and damage without altering the immune response to CII immunization.
TLR4 deficiency has been reported not to inhibit the T cell immune response in mice with CIA 13. This is consistent with our observations that anti-TLR4 mAb treatment does not alter the immune response to CII and does not alter TNF-α levels. Interestingly, the levels of anti-cFb Ab were significantly reduced by neutralizing TLR4. As neutralizing TLR4 is not expected to interfere with the T cell response, the change in anti-cFb Ab levels might be due to a reduced production of cFb in response to reduced inflammation in the joint. Further investigation is required to confirm this speculation.
Surprisingly, the levels of Tenascin-C, HMGB1 and S100A8/A9 were not affected by treatment with anti-TLR4 mAb. As TLR4 deficiency does not inhibit T cell immune response in mice with CIA 13, the production of the above TLR4 ligands could be associated with the activation of the adaptive immune response linked to immunization with CII and thus was not affected by TLR4 blockade. Nonetheless, the ligands were not able to promote disease progression when TLR4 activation was blocked. The levels of IL-6 and IL-1β were reduced when TLR4 signaling was blocked, suggesting that TLR4 promotes arthritis by acting upstream of proinflammatory cytokine secretion post TLR4 ligand activation of macrophages.
Using an in vitro system, two TLR4 ligands upregulated in the CIA model and RA patients, Tenascin-C and cFb-IC, have been demonstrated in this study to promote osteoclast differentiation through TLR4 activation. To investigate the mechanisms underlying TLR4-mediated osteoclast differentiation, LPS has been used as a model ligand as it is the best characterized TLR4 ligand. Our results demonstrated that TNF and IL-6 are responsible for osteoclast differentiation induced by TLR4 activation. As osteoclast differentiation stimulated by Tenascin C and cFb-IC is also mediated by TLR4, we expect that TNF-α and IL-6 are also involved in these stimulations. Consistent with this prediction, Tenascin C has been reported to stimulate IL-6 and TNF-α production in macrophages 5. As both IL-6 and TNF-α are clinically validated therapeutic targets in RA and multiple TLR4 ligands are upregulated in RA, TLR4 blockade offers a novel targeted strategy for RA patients that are unresponsive to these validated therapies.
Materials and methods
Mice
Studies were conducted with 8-week-old male DBA/1J mice (Janvier Laboratories, Le Genest-St-Isle, France). Mice were housed in groups of 10. Animal experiments were conducted after obtaining the permission of the Swiss veterinary office for animal experimentation.
Induction of collagen-induced arthritis (CIA) and treatment with anti-TLR4 antibody
The induction of CIA has been described previously 36. Briefly, male DBA/1J mice were immunized intradermally with 100 μL emulsion of bovine collagen type II (CII, MD Biosciences, Zurich, Switzerland) emulsified in an equal volume of Complete Freund's adjuvant (CFA) (2 mg/mL Mycobacterium tuberculosis; Difco, San Jose, CA, USA) at the base of the tail. After 21 days, mice received a secondary intradermal immunization with 100 mg bovine CII emulsified in Incomplete Freund's Adjuvant (IFA). Clinical scores were assessed every 2 days. CIA severity was graded by overall assessment of inflammation on individual paws, applying a scale ranging from 0 to 4. Each paw was graded according to the following system: 0, no inflammation; 1, swelling of at least one digit; 2, swelling of all the digits and inflammation of the paw; 3, severe inflammation of whole paw and digits or ankylosis; 4, necrosis. The sum of all four paws corresponds to the total clinical score, i.e. the maximal clinical score for an individual mouse is 16.
The anti-mouse TLR4 monoclonal antibody 5E3 has been described previously 23. The mice were treated intraperitoneally (i.p.) with 100 mg/kg 5E3 or mouse IgG2a isotype control starting from day 14 after the first immunization (treatment schedule: day 14, 16, 18, 21, 28, 35, 42, and 49).
Sampling of the blood plasma and washout of knee joint capsules
Plasma samples were prepared from cardiac puncture blood samples. To prepare the washout of the joint capsule, the knee joint capsules with patella from the rear legs of the CIA mice were dissected under a dissection microscope at the indicated time points. The patella with surrounding tissue was collected in an Eppendorf tube. The needle of a syringe containing 100 μl of PBS was pushed through the articular space from the patellar side to make a hole at the opposite side. The 100 μl of PBS was then injected into the articular space from the patellar side and the flow-through coming out from the hole at the opposite side was collected into the Eppendorf tube containing the patella from the same joint. The retrieved solution was incubated with the patella overnight at 4°C. The supernatant was then collected from the Eppendorf tube and considered as the joint washout sample .Blood plasma and joint washout samples were stored at –20°C until the measurement of ACPA and TLR4 endogenous ligands.
Measurement of anti-CII antibodies, ACPA, and TLR4 ligands
Levels of anti-CII IgG1, IgG2a, and IgG2b were determined by ELISA. Briefly, serially diluted CIA plasma samples were added to each well of bovine CII-precoated immunoplates (eBioscience, San Diego, CA, USA). After incubation (2 h, 37°C), plates were washed with PBS/0.05% Tween 20 and bound IgGs were measured using peroxidase-conjugated goat Ab to mouse IgG1, IgG2a and IgG2b (Jackson ImmunoResearch Europe, Suffolk, UK). The titer was designated as log10 of the maximum fold of dilution that gave a positive signal for each sample. ACPA levels were determined using the anti-CCP2 kit (second generation peptides, Axis-Shield Diagnostics Ltd, Dundee, UK) following manufacturer's instructions. The levels of antibodies specific for cFb were determined by ELISA. 96-well plates (eBioscience, San Diego, CA, USA) were coated (overnight, 4°C) with 1 μg/mL cFb (Modiquest, Oss, The Netherlands) or native fibrinogen (nFb) as a control. Plates were washed with PBS–0.05% Tween-20, blocked with 1% bovine serum albumin (BSA), and incubated (2 h, 37°C) with mouse CIA plasma diluted 1/100 in PBS. Bound IgGs were measured using peroxidase-conjugated Ab to mouse and expressed in arbitrary units based on optical density (od) at 450 nm. Plasma and joint washout samples were characterized for levels of HMGB1, S100A8/A9 and Tenascin-C (TNC) using commercial ELISA kits (IBL, Minneapolis, MN, USA and Immundiagnostik, Bensheim, Germany) according to the manufacturers’ protocols.
Histological assessment of arthritis
For each indicated time point, at least five animals per group were euthanized, and joints were collected for histological assessment of synovial inflammation, cartilage damage and osteoclast number. The joints were fixed (10 days, room temperature) in 10% neutral buffered formalin (Sigma-Aldrich, St. Louis, MO, USA) and decalcified (3 weeks, room temperature) in an EDTA-based decalcifying solution (Merck, Darmstadt, Germany). The samples were rinsed in PBS, dehydrated, and embedded in paraffin blocks using an automated tissue processor (Microm, STP 120). Serial midsagittal sections (8 μm thick) of the whole knee joint were stained with H&E to assess synovial inflammation. Synovial inflammation was scored using an established scoring system: 0 = synovium with no hyperplasia to 3 = severe synovial hyperplasia and cellular infiltration. Safranin-O/fast green staining was performed on serial sections (one in every 5 was analyzed) to visualize proteoglycan for cartilage damage assessment. Safranin-O positive area (red-color staining) was quantified using the ImageJ software. Cartilage damage was calculated using the following formula: cartilage damage% = (1- Safranin-O positive area in the sample/ Safranin-O positive area in normal mice) x 100%. Osteoclast number was measured with Tartrate Resistant Acid Phosphatase (TRAP)-staining according to the manufacturer's protocol (acid phosphatase leucocyte kit, Sigma, St Louis, MO, USA) and quantified using Image J.
In vitro differentiation and stimulation of osteoclasts
To obtain murine osteoclasts, bone marrow cells collected from tibiae and femora of naïve DBA/1J mice were cultured in MEM medium containing 5% FBS and gentamycin (complete medium). Osteoclast differentiation was induced for 7 days with 30 ng/mL of recombinant murine M-CSF and 20 ng/mL RANKL (R&D systems, Minneapolis, MN, USA). To analyze the in vitro effect of TLR4 ligands, nonadherent cells were remove after 3 days and adherent cells were cultured for four additional days in complete medium in the presence of cFb-immune complexes (cFb-IC) or Tenascin-C (5 μg/mL). The TLR4 ligand LPS (100 ng/mL) was used as a control for TLR4-dependent induction of osteoclastogenesis. For preparation of cFb-IC, flat-bottomed 96-well cell culture plates were coated (overnight, 4°C) with 20 μg/mL of cFb or nFb as a control, washed in PBS containing 0.05% Tween-20 and incubated (2 h, 4°C) with or without 10 ng/mL of anti-cFb mAb (clone 3D1, Modiquest, Oss, The Netherlands). The endotoxin level was lower than 0.08 EU/mL in the final assay mix according to the Limulus amebocyte lysate (LAL) test. This level of endotoxin was not able to stimulate any TLR4 activation on mouse myeloid cells according to a LPS dose response study (data not shown). For inhibition experiments, the cells were preincubated with 100 μg/mL anti-TLR4 (5E3) or the isotype control for 30 min before stimulation with cFb-IC, Tenascin-C or LPS. In some experiments anti-IL-6R (clone 2B10) or anti-TNFα (clone V1q) mAbs were also included. TRAP-positive multinucleated cells with three or more nuclei were defined as differentiated osteoclasts.
Statistics
The data were displayed as mean ± SEM. Statistical analysis was performed using GraphPad Prism version 6.04 for Windows (GraphPad Software, San Diego, CA, USA). Statistically significant differences between groups were indicated as: *p < 0.05; **p < 0.01; ***p < 0.001, and ****p < 0.0001.
Acknowledgements
We thank Laurence Chatel and Laura Cons for technical support and Professor Jean-Claude Martinou from University of Geneva for scientific discussion. This research was supported by the Seventh Framework Program and Marie Curie Actions (European Commission, FP7-PEOPLE-2011-ITN-289150 “Osteoimmune”). GWK is supported by a Marie Curie Innovative Training Networks (ITN) doctoral fellowship. G.W.K., E.H., W.F., M.K.V., and L.S. were involved in designing the study plan. G.W.K., E.H., S.H., and I.D. were involved in data collection and interpretation of data. E.H., W.F., M.K.V., W.R., P.L.vL., and L.S. were involved in interpretation of data and revising the manuscript.
Conflict of interest
G.W.K., E.H., S.H., M.K.V., W.F., and L.S. are employees of Novimmune SA, whose anti-TLR4 antibody was used in this study. The other authors declare no conflict of interests.
References
Abbreviations
-
- Ab
-
- antibodies
-
- ACPA
-
- Anti-citrullinated protein antibodies
-
- CIA
-
- collagen-induced arthritis
-
- cFb
-
- citrullinated fibrinogen
-
- mAb
-
- monoclonal antibodies
-
- RA
-
- rheumatoid arthritis
-
- TLR4
-
- Toll-like receptor 4




