Asymptomatic CMV infections in long-term renal transplant recipients are associated with the loss of FcRγ from LIR-1+ NK cells
Abstract
While it is established that cytomegalovirus (CMV) disease affects NK-cell profiles, the functional consequences of asymptomatic CMV replication are unclear. Here, we characterize NK cells in clinically stable renal transplant recipients (RTRs; n = 48) >2 years after transplantation. RTRs and age-matched controls (n = 32) were stratified by their CMV serostatus and the presence of measurable CMV DNA. CMV antibody or CMV DNA influenced expression of NKG2C, LIR-1, NKp30, NKp46, and FcRγ, a signaling adaptor molecule, on CD56dim NK cells. Phenotypic changes ascribed to CMV were clearer in RTRs than in control subjects and affected NK-cell function as assessed by TNF-α and CD107a expression. The most active NK cells were FcRγ–LIR-1+NKG2C– and displayed high antibody-dependent cell cytotoxicity responses in the presence of immobilized CMV glycoprotein B reactive antibody. However, perforin levels in supernatants from RTRs with active CMV replication were low. Overall we demonstrate that CMV can be reactivated in symptom-free renal transplant recipients, affecting the phenotypic, and functional profiles of NK cells. Continuous exposure to CMV may maintain and expand NK cells that lack FcRγ but express LIR-1.
Introduction
NK cells are large granular lymphocytes involved in control of virus-infected and tumor cells. Their function is regulated by diverse families of surface receptors with no gene rearrangement as the cells differentiate. NK cells are divided into two groups based on cell surface density of CD56; cytokine-producing CD56bright and cytotoxic CD56dim cells. CD56dim NK cells are more mature, less proliferative, and express inhibitory and activating receptors such as killer immunoglobulin-like receptors (KIR), C-type lectin-like receptors (e.g. CD94/NKG2C or NKG2A), leukocyte immunoglobulin receptors (e.g. LIR-1), and natural cytotoxicity receptors such as NKp30 and NKp46 1, 2. These receptors monitor changes in the expression of class I molecules on stressed cells to induce target cell lysis. Target cell lysis can also be initiated by CD16, a low-affinity Fc receptor on NK cells that initiates antibody-dependent cellular cytotoxicity (ADCC) after it is cross-linked by an IgG antibody 2-4.
Cytomegalovirus (CMV) is a herpes virus that persists without symptoms in an immunocompetent host, but can cause serious complications in immunosuppressed individuals such as renal transplant recipients (RTRs) 5. NK cells can control CMV infection even in the absence of an effective T-cell response 6. Moreover, CMV has the unique ability to imprint on the NK-cell receptor repertoire by altering expression of both inhibitory and activating receptors 7, 8. CMV also encodes proteins able to subvert NK-cell recognition of target cells. For example, LIR-1 binds with much higher affinity to CMV UL18 than to its natural ligand HLA-G 9. CMV-infected cells expressing UL18 inhibited LIR-1+ NK cells, whereas LIR-1– NK cells were activated in the presence of CMV UL18 10. Increased expression of LIR-1 on NK cells has been observed in CMV-seropositive individuals 7, 11, but the function of these cells is unclear. CMV also encodes UL40 that upregulates cell surface expression of HLA-E. NKG2C can recognize HLA-E carrying an appropriate peptide, but HLA-E binds with much higher affinity to NKG2A (an inhibitory receptor) to inhibit NK cell-mediated lysis 9.
CMV promotes the accumulation of NKG2C+ NK cells in healthy individuals 7, 12, 13. In recipients of solid organ and haematopoietic stem cell transplants, NKG2C+ NK cells expand following CMV reactivation and remain stable over time while there is no detectable CMV viraemia 12, 14. Most CMV-induced NKG2C+ NK cells express CD57, a marker of mature and functionally differentiated NK cells. NKG2ChiCD57hi NK cells have increased effector function and can lyse CMV-infected macrophages in the presence of CMV-specific antibodies via an ADCC mechanism 12, 15. In the presence of CMV-reactive antibodies, FcRγ-deficient (FcRγ–) NK cells from CMV-seropositive healthy donors display higher ADCC responses against CMV-infected targets than conventional NK cells. These NK cells lack the FcRγ signaling adaptor molecule, one of two adaptor chains (the other being CD3ζ) known to associate with the transmembrane domain of CD16 16. Thus, CMV alters NK-cell surface receptors and intracellular signalling adaptor molecules.
In the present study, we define the effects of CMV on NK-cell surface receptors, FcRγ and NK-cell function in RTRs stable for >2 years after transplantation with no symptoms of active CMV infection. Using a sensitive PCR, we detect subclinical CMV reactivations in RTRs and assess their effects on the NK-cell receptor repertoires and function.
Results
CMV antibody levels are higher in RTRs than in healthy controls
RTR with a median (range) age of 54 (27–71) years (15 females, 33 males) were recruited 8 (2–18) years after transplantation. All patients were free from clinical CMV disease or reactivation within 6 months of blood collection and were receiving no current anti-viral treatment. All patients were stable on maintenance immunosuppressive therapy [Tacrolimus (n = 28), Sirolimus (n = 12), Cyclosporin (n = 8)]. The drug regimen was not associated with CMV status (χ2, p = 0.26). Age-matched healthy controls (13 females, 19 males) were studied in parallel.
To understand the effect of CMV on NK-cell receptor repertoires and function, patients, and controls were stratified by the presence of CMV antibody and CMV DNA in plasma. Antibodies were detected using CMV lysate, CMV gB, and CMV IE-1 preparations (Fig. 1). Eight RTRs (17%) were classified as CMV seronegative (CMV Ab–). The 40 CMV seropositive RTRs were further subdivided into 33 RTRs (82%) without measurable CMV DNA (CMV Ab+) and 7 RTRs (18%) with measurable CMV DNA (CMV Ab+DNA+; 22–2717 CMV copies/ml). Of the 32 controls, 12 were CMV Ab– (38%). A representative sample of control plasmas was screened for CMV DNA and all were negative, as expected. CMV Ab+ RTRs had higher levels of IgG reactive with all three CMV antigens compared to CMV Ab+ controls (p < 0.05 to <0.0001). CMV Ab+DNA+ RTRs had slightly higher levels of antibody recognizing gB antigen than those without detectable viraemia (p = 0.03), but the presence of CMV DNA did not alter humoral responses to CMV lysate or IE-1 antigen (Fig. 1A–C).

CMV infection alters the phenotype of CD3¯CD56 dim NK cells in RTRs
CMV may alter NK-cell receptor expression 7, 17. We compared the effect of persistent (Ab+) and active (Ab+DNA+) CMV infection in RTRs and healthy controls. The proportions of CD3¯CD56bright NK cells were similar in all groups (data not shown; p = 0.08–0.75), but proportions of CD3¯CD56dim NK cells were lower in CMV Ab+DNA+ RTRs (p = 0.01) and CMV Ab+ RTRs (p = 0.004) than CMV Ab¯ RTRs. Frequencies were similar in CMV Ab¯ and CMV Ab+ controls (Fig. 2A). Accordingly, proportions of CD3¯CD56dim NK cells were inversely related to levels of antibody reactive with CMV gB or lysate in RTRs (r = −0.35, −0.36; p = 0.01, 0.01, resp.). The relationship was marginal in controls (r = −0.23, −0.30; p = 0.20, 0.10, resp.).

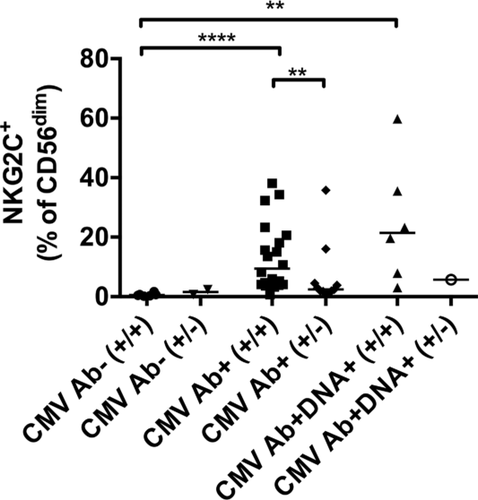
As proportions of CD56dim NK cells were affected by CMV in RTRs, we restricted our analysis to this subset. Consistent with earlier studies 7, 8, 14, persistent and/or active CMV in RTRs affected expression of NKG2C, LIR-1, NKp30, and NKp46 (Fig. 2B–E) on CD56dim NK cells (for gating strategy, please refer to Supporting Information Fig. 1). Expression of NKG2C was higher in CMV Ab+ RTRs (p < 0.0001) and CMV Ab+DNA+ RTRs (p = 0.002) than CMV Ab¯ RTRs. The median (range) frequency was marginally greater (p = 0.09) in CMV Ab+DNA+ RTRs [20 (3–60) %] than CMV Ab+ RTRs [5 (0.72–38) %]. Moreover, NKG2C expression was higher in CMV Ab+ controls (p = 0.0004) than CMV Ab¯ controls (Fig. 2B). To investigate variability in NKG2C expression observed in CMV-positive RTRs, all participants were genotyped for a deletion known to abrogate expression of NKG2C 18-20. As expected, heterozygous carriers (+/−) displayed lower expression of NKG2C. Induction of NKG2C expression by CMV was clearest in CMV-seropositive patients without the deletion (+/+) (Fig. 3).
When LIR-1 expression was assessed as median fluorescence intensity, levels were higher in CMV Ab+DNA+ RTRs than CMV Ab+ RTRs (p = 0.007) or CMV Ab¯ RTRs (p = 0.005), showing an effect of active CMV. CMV Ab+ RTRs had higher LIR-1 expression than CMV Ab+ controls (p = 0.001), but CMV Ab+ and Ab¯ controls were similar (Fig. 2C).
Expression of NKp46 and NKp30 was lower in CMV Ab+DNA+ RTRs than CMV Ab¯ RTRs (p < 0.05). CMV Ab+DNA+ RTRs also had lower expression of NKp46 compared to CMV Ab+ RTRs (p = 0.03), showing an effect of active CMV. Moreover, compared to CMV Ab+ controls, CMV Ab+ RTRs had decreased expression of NKp46 (p = 0.0007). Expression of NKp30 was lower in CMV Ab+ controls (p = 0.04) than CMV Ab¯ controls, but NKp46 expression was not affected by CMV in controls (Fig. 2D–E).
Expression of NKG2A, perforin, CD57, KLRG1, CD16, NKG2D, and CD62L was also assessed. CMV Ab+ RTRs had slightly less NKG2A+ cells compared to CMV Ab¯ RTRs (p = 0.05) (Fig. 2F). Perforin expression was higher in CMV Ab¯ RTRs than CMV Ab¯ controls (Fig. 2G), while expression of KLRG1 (Fig. 2I) was slightly lower, suggesting an effect of transplantation or immunosuppressive drugs. On the other hand, CD57, CD16, NKG2D, and CD62L did not exhibit any differences between groups (Fig. 2H, J, K and L).
We also assessed expression of KIR2DL1, KIR3DL1, KIR2DL2/DL3/DS2, and KIR2DS4 on CD56dim NK cells from individuals shown to carry the relevant genes after PCR amplification of extracted DNA (PCR data not shown). Expression of KIR2DL1, KIR3DL1, and KIR2DL2/2DL3/2DS2 were similar in RTRs and controls, with and without CMV (Fig. 2M, N and O). Most (32/48) RTRs did not express KIR2DS4, as they carried the KIR2DS4*003 variant in which a 22bp deletion abrogated cell surface expression 21 or lacked the KIR2DS4 gene.
Perforin secretion after stimulation is diminished in RTRs with active CMV infection
Natural cytotoxicity depends upon the secretion of perforin and granzymes following contact with a potential target. Lower perforin secretion has been reported in NK cells from older adults following stimulation with K562 cells 22, but the authors did not assess CMV serostatus or reactivation. This is addressed here following stimulation of PBMC with anti-CD16 or K562 cells.
Before in vitro stimulation, cells from CMV Ab+ RTRs had lower expression of perforin than CMV Ab¯ RTRs (p = 0.01) (Fig. 2G). After stimulation, perforin levels were similar in cultures from CMV Ab¯ RTRs and CMV Ab+ RTRs or CMV Ab¯ controls; therefore, transplantation itself did not impair perforin secretion. However, perforin levels in the supernatant of NK cells from CMV Ab+DNA+ RTRs was lower compared to CMV Ab+ RTRs (anti-CD16 p = 0.004; K562 p = 0.027) and CMV Ab¯ RTRs (anti-CD16 p = 0.05), and thus, active CMV replication may suppress or exhaust perforin release. Intermittent CMV reactivation in CMV Ab+ RTRs may activate NK cells as perforin secretion was higher in cultures from CMV Ab+ RTRs than CMV Ab+ controls (K562 p = 0.0009) (Fig. 4A and B).

CMV enhances the induction of CD107a on NK cells stimulated with anti-CD16
We assessed changes in NK-cell function (expression of CD107a and TNF-α) following in vitro stimulation with cytokines (IL-12 + IL-15 + IL-18), K562 cells or anti-CD16 (Fig. 4C–H). Expression of CD107a and TNF-α was measured on NK cells expressing NKG2C, LIR-1, NKp30, and NKp46 as CMV affected expression of these markers (Fig. 2). We included CD57+ CD56dim NK cells, as CD57 is a marker of mature and functional NK cells. Expression of TNF-α or CD107a on NKG2C+CD56dim NK cells was not quantitated in CMV Ab¯ subjects, as there were too few events (Fig. 4E).
Following cytokine stimulation, there were few differences between the five groups. However, CD107a expression by CD57+ and LIR-1+CD56dim NK cells was lower in CMV Ab+DNA+ RTRs than CMV Ab¯ RTRs (p = 0.02) (Fig. 4D and F), and CD107a expression by NKG2C+ CD56dim NK cells was higher in CMV Ab+ RTRs than CMV Ab+ controls (p = 0.01) (Fig. 4E).
Following K562 stimulation, CMV seropositivity or DNA in RTRs did not alter NK-cell function, but NKG2C+CD56dim NK cells had higher expression of CD107a in CMV Ab+ RTRs than CMV Ab+ controls (p = 0.03; Fig. 4E). Compared to CMV Ab¯ controls, CMV Ab+ controls had higher expression of CD107a on CD56dim (p = 0.03; Fig. 4C), CD57+CD56dim (p = 0.03; Fig. 4D) and NKp46+CD56dim NK cells (p = 0.03; Fig. 4H). No significant differences were observed with TNF-α responses (data not shown; p = 0.07–0.99).
After anti-CD16 stimulation, expression of CD107a on CD56dim, CD57+, LIR-1+, and NKp46+ NK cells from CMV Ab+ RTRs (p < 0.05) and CMV Ab+DNA+ RTRs (p < 0.05) was higher compared to CMV Ab¯ RTRs (Fig. 4C–H). NKG2C+CD56dim NK cells had higher expression of CD107a in CMV Ab+ RTRs than CMV Ab+ controls (p = 0.004; Fig. 4E). TNF-α production was higher on CD57+, LIR-1+, and NKp46+ NK cells from CMV Ab+ RTRs (p ≤ 0.05) than CMV Ab¯ RTRs (data not shown). These differences were not evident among controls. Thus, persistent CMV in RTRs enhances NK-mediated ADCC responses.
Loss of FcRγ is a feature of LIR-1+ NK cells from CMV-seropositive individuals
FcRγ-deficient cells are reported to respond robustly to anti-CD16 stimulation, and CMV seropositivity increases the percentage of FcRγ– NK cells in healthy controls 16. Accordingly, CMV Ab+ controls and CMV Ab+ RTRs had higher proportions of FcRγ– NK cells than CMV Ab– controls (p < 0.0001) and CMV Ab– RTRs (p < 0.0001), respectively. CMV Ab+DNA+ RTRs had slightly higher proportions of FcRγ– NK cells than CMV Ab+ RTRs (p = 0.08) and had significantly higher than CMV Ab– RTRs (p < 0.0001). Interestingly, CMV Ab+ RTRs had higher proportions of FcRγ– NK cells than CMV Ab+ controls (p = 0.04; Fig. 5A).
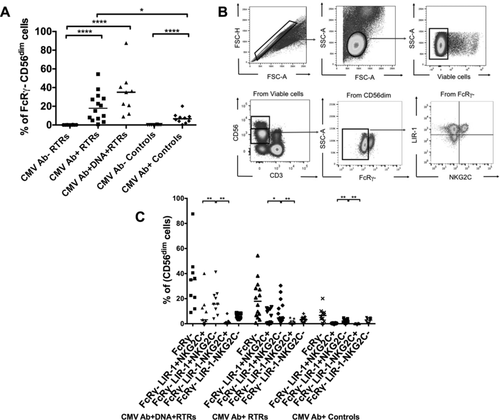
We then assessed expression of LIR-1 and NKG2C on FcRγ– NK cells (Fig. 5B). CMV Ab+ RTRs, CMV Ab+DNA+ RTRs, and CMV Ab+ controls had more FcRγ–LIR-1+NKG2C+ NK cells than CMV Ab– RTRs (p < 0.0001) or CMV Ab– controls (p = 0.0007) (data not shown). Interestingly, more CD56dim NK cells had the phenotype FcRγ–LIR-1+NKG2C– than FcRγ–LIR-1–NKG2C+ or FcRγ–LIR-1+NKG2C+. This was seen in CMV Ab+ RTRs (p < 0.05), CMV Ab+DNA+ RTRs (p < 0.01), and CMV Ab+ controls (p < 0.01) (Fig. 5C). Expansion of NKG2C–FcRγ– NK cells has been observed in NKG2C heterozygous carriers 23. Here expansion of FcRγ–LIR-1+NKG2C– NK cells was found irrespective of the NKG2C genotype. When proportions of FcRγ–LIR-1+NKG2C– NK cells were assessed according to NKG2C genotype, approximately 67% of CMV Ab+DNA+ RTRs (6/9), 64% of CMV Ab+ RTRs (9/14) and 61% of CMV Ab+ controls (11/18) were homozygous for the NKG2C gene, whereas 33% of CMV Ab+DNA+ RTRs (3/9), 36% of CMV Ab+ RTRs (5/14), and 39% of CMV Ab+ controls (7/19) were heterozygotes (NKG2C+/−). Proportions of FcRγ–LIR-1+NKG2C– NK cells were then compared between NKG2C homozygous and heterozygous carriers. Among CMV Ab+ RTRs, higher proportions of these cells (p = 0.01) were observed in NKG2C homozygous carriers [9.5 (0.7–38)] than NKG2C heterozygous carriers [2.4 (0.9–36)]. Among controls, the median (range) in NKG2C homozygous carriers was higher [3.8 (1.1–31)] than NKG2C heterozygous carriers [1.7 (0.2–28)], with no significant difference between the two.
In comparison to CMV-positive RTRs, higher expression of NKG2C on FcRγ–LIR-1+ NK cells was not observed in CMV Ab+ controls, suggesting that intermittent CMV reactivation may be required to upregulate and/or maintain NKG2C on FcRγ–LIR-1+ NK cells.
FcRγ–LIR-1+ NK cells from CMV+ RTRs display the highest ADCC responses
FcRγ– NK cells show increased production of IFN-γ and expression of CD107a following stimulation designed to elicit ADCC 16. Here, we induced ADCC (induction of CD107a and TNF-α) by cross-linking receptors on NK cell from RTRs and healthy controls with CMV gB-specific antibody bound to immobilized antigen. Purified anti-CD16 was used as a positive control. Plasma from CMV-seronegative controls did not activate NK cells in PBMC preparations or purified cultured NK cells (data not shown).
FcRγ–LIR-1+NKG2C– NK cells from CMV-positive RTRs and controls had higher ADCC responses than FcRγ–LIR-1–NKG2C+ (p < 0.05) NK cells when cross-linked with anti-CMV gB antibody (Fig. 6A) or with anti-CD16 (Fig. 6B). FcRγ–LIR-1+NKG2C– also had higher responses than FcRγ–LIR-1–NKG2C+ following K562 stimulation (data not shown; p = 0.003 to 0.008). Thus, FcRγ–LIR-1+NKG2C– NK cells exhibit higher ADCC and NK-cell cytotoxicity.
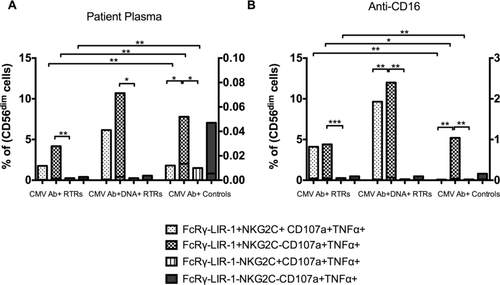
ADCC responses of cells defined by FcRγ–, LIR-1, and NKG2C were similar in CMV Ab+ RTRs and CMV Ab+DNA+ RTRs after cross-linking with patient plasma or anti-CD16 (Fig. 6A–B), but were much greater than the responses seen in CMV Ab+ controls (right hand axes).
Discussion
We have shown that active and latent CMV infections alter NK-cell profiles in RTRs who have been clinically stable for >2 years after transplantation. Specifically, CMV influenced expression of NKG2C, LIR-1, NKp30, NKp46, and FcRγ. NK-cell function was assessed by induction of TNF-α and CD107a. Most functional NK cells had the phenotype FcRγ–LIR-1+ NKG2C– and displayed high ADCC responses in the presence of anti-CMV gB antibody.
To understand the effect of CMV, RTRs and controls were stratified based on their CMV serostatus and/or the presence of CMV DNA. A higher frequency of CMV reactivation in RTRs may plausibly explain their higher levels of CMV-reactive antibody. Interestingly, CMV Ab+DNA+ RTRs had particularly higher levels of antibody reactive with gB antigen. CMV gB is present on the viral envelope or exists on the surface of an infected cell and is crucial for entry into the cell 24, 25. In sera from CMV-seropositive individuals, up to 70% of neutralizing antibodies can be gB-specific 26, 27. Thus, CMV Ab+DNA+ RTRs may have higher gB neutralization antibody titres to control CMV replication. However, gB had little role in viral neutralisation in vitro 28; therefore, anti-gB antibody in CMV Ab+DNA+ RTRs may be involved in activation of the complement pathway or cross-linking of FcγRIII (CD16) receptor on NK cells. Alternatively, high levels may simply reflect higher antigen burdens. Unlike gB antibody, IE-1 antibody levels were not higher in CMV Ab+DNA+ RTRs compared to other groups. This may arise because CMV-seronegative RTRs and controls had antibody that is able to bind bacterial contaminants of the IE-1 preparation.
The suggestion that CMV infection may imprint on the NK-cell receptor repertoire is not new 7, 29. Similar to other studies 12-14, we showed that CMV induced expression of NKG2C on CD56dim NK cells in RTRs and controls. However, no greater expansion was observed in CMV Ab+DNA+ RTRs than CMV Ab+ RTRs. This differs from an earlier study of solid organ transplant patients 12, but the time of sample collection relative to a burst of CMV replication may be critical. Moreover, few RTRs had detectable CMV DNA, limiting the number of patients in this group. However, the patient with the highest levels of CMV DNA had abundant NKG2C+ NK cells (data not shown).
CMV infection can also increase the expression of LIR-1 on NK cells from CMV-seropositive healthy controls 7. LIR-1 (CD85j/ILT2) is an inhibitory NK-cell receptor that binds to classical (HLA-A, B, and C) and nonclassical (HLA-G) MHC Class I molecules 30. We found that the expression of LIR-1 on CD56dim NK cells was high in CMV Ab+DNA+ and CMV Ab+ RTRs. In a cohort of lung transplant patients, CMV disease was associated with increased expression of LIR-1 that occurred even before viral DNA became detectable 31. Thus, high LIR-1 expression in our cohort may reflect intermittent CMV reactivation and mark patients at higher risk for progression to clinical disease. LIR-1 is known for its affinity for UL18, a CMV evasive protein 9, 32, so upregulation of UL18 during intermittent CMV reactivation may induce LIR-1 expression. CMV immune-evasion proteins can also suppress the expansion of cytotoxic CD56dim NK cells; thus, persistent and active CMV infections were associated with lower proportions of CD56dim NK cells in our cohort.
CMV infection increases degranulation and IFN-γ production by mature NK cells 15. NK-cell cytotoxicity is mediated through the release of perforin and granzymes 33. No previous studies have assessed how CMV affects perforin secretion after NK-cell activation. Here, perforin was assessed in culture supernatants following anti-CD16 and K562 stimulation. RTRs with CMV DNA generated lower levels of perforin in culture, suggesting active CMV replication may impair perforin release. This needs further investigation by assessing perforin levels at the immunological synapse and binding of perforin to the target cell membrane 22. As the CMV viral load increases in healthy individuals above 70 years of age 34, it is interesting that NK cells from older adults also displayed low perforin levels following K562 stimulation 22. While Hazeldine et al. 22 did not assess CMV DNA, CMV reactivation in elderly donors may impair polarization of lytic granules to the immunological synapse. Intermittent CMV reactivation and effective control of the virus in CMV Ab+ RTRs may also explain why their levels of intracellular perforin were higher than CMV Ab+ controls or CMV Ab+DNA+ RTRs.
We also assessed expression of CD107a (also known as lysosome-associated membrane protein; LAMP-1) and TNF-α after stimulating NK cells with cytokines (IL-12 + IL-15 + IL-18), K562 target cells or anti-CD16. CD57 is a marker of T-cell replicative senescence, whereas on NK cells, it is regarded as a marker of maturation. CD57+ NK cells proliferate less and produce less IFN-γ in response to cytokines, but have higher cytotoxic capability and the ability to produce abundant cytokines when activated by a potential target cell 35. Unlike other studies 12, 36, we found no increase in CD57 expression with CMV. After cytokine stimulation, expression of CD107a on CD57+ CD56dim cells was lower in CMV Ab+DNA+ RTRs. This may reflect lower expression of IL-12 and IL-18 receptors on CD57+ NK cells 35, 37.
Expression of CD107a on NK cells stimulated with anti-CD16 was high in RTRs with active or persistent CMV. CD56dim, CD57+, LIR-1+, and NKp46+ NK cells exhibited higher CD107a expression in CMV Ab+DNA+ RTRs. This is a paradoxical finding in view of the low levels of perforin in supernatants. CD107a (LAMP-1) binds adaptor-protein 1 (AP-1) sorting complex, which is essential for perforin trafficking to lytic granules. Decreased levels of LAMP-1 or defects in AP-1 can impair perforin secretion 38. Increased expression of CD107a and decreased perforin in the supernatants from CMV Ab+DNA+ RTRs implies a defect in AP-1. This warrants further investigation.
Another plausible explanation for higher expression of CD107a after anti-CD16 stimulation can be a loss of FcRγ. CMV seropositivity correlated with loss of FcRγ, a signaling adaptor molecule that associates with the transmembrane domain of CD16. This loss increases NK cell-mediated ADCC 16. The decrease in FcRγ expression seen in RTRs could explain their higher expression of CD107a after anti-CD16 stimulation. In haematopoietic cell transplant recipients, CMV reactivation caused significant loss of FcRγ expression at 6 months and 1-year posttransplant 39. Accordingly, CMV Ab+DNA+ RTRs had slightly more FcRγ– NK cells (p = 0.08) than CMV Ab+ RTRs. An increase in FcRγ– NK cells in CMV Ab+ RTRs compared with CMV Ab+ healthy controls may simply reflect intermittent episodes of CMV reactivation driven by immunosuppressive drugs, inflammation or donor and recipient CMV serostatus at the time of transplantation.
In CMV-positive people, the FcRγ– phenotype aligns with expression of NKG2C and decreased expression of NKp30 36, which was confirmed here in both the RTRs and controls. As in other studies, only a subset of FcRγ– NK cells expressed NKG2C. We noted similar frequencies of LIR-1+ and FcRγ– NK cells in a patient with the highest levels of CMV DNA (data not shown) and observed increased expression of LIR-1 and NKG2C on FcRγ– NK cells. Interestingly, FcRγ–LIR-1+NKG2C– NK cells were observed in CMV-positive RTRs and controls. Expansion of FcRγ–NKG2C– NK cells is observed in NKG2C heterozygous people 23. However in our study, expansion of FcRγ–LIR-1+NKG2C– NK cells was independent of the NKG2C genotype, so it may reflect CMV reactivation. It is unclear why NK cells downregulate FcRγ in relation to CMV infection. Following CMV infection, NK cells undergo profound epigenetic changes resulting in the downregulation of several proteins including signaling adaptor proteins (e.g. FcRγ) and transcription factors (e.g. PLZF – promyelocytic leukaemia zinc finger) and in turn epigenetically resemble CD8+ T cells 39. The resultant cells are termed adaptive NK cells. The underlying mechanisms remain unclear. LIR-1 binds with high affinity to the CMV protein UL18 9. This interaction may trigger epigenetic changes; therefore, higher proportions of FcRγ–LIR-1+NKG2C– NK cells are observed in CMV-positive people. Further studies are required to understand the stages of differentiation.
FcRγ– NK cells display increased ADCC responses in the presence of CMV-specific antibodies 16, 39. Similarly, we observed higher CD107a and TNF-α expression in FcRγ–LIR-1+NKG2C– and in FcRγ–LIR-1+NKG2C+ NK cells (Fig. 6). Increased polyfunctional responses in FcRγ–LIR-1+NKG2C– NK cells were also induced by K562 target cells (data not shown). Thus, CMV-driven FcRγ–LIR-1+NKG2C– NK cells have enhanced ADCC and NK-cell cytotoxicity. However, whether FcRγ–LIR-1+NKG2C– NK cells can induce higher NK-cell cytotoxicity in the presence of CMV-infected cells requires investigation.
Adaptive NK cells have memory-like properties 16. Expansion of NKG2C+ NK cells was observed in CMV seropositive, but not seronegative bone marrow transplant recipients given NKG2C+ NK cells from a CMV-seropositive donor 40. Here some CMV Ab+DNA+ RTRs had high proportions of FcRγ–LIR-1+NKG2C+ NK cells and raised strong ADCC responses, but this was variable. Many patients with high ADCC responses may have active CMV replication, and thus, active CMV may be essential to expand the adaptive NK cells, which may in turn contribute to the control of CMV. Longitudinal studies should address whether these cells are a stable feature of some RTRs or are a response to protracted CMV replication.
Immunosuppressive drugs or other clinical variables such as age at transplant and the CMV serostatus of the donor and the recipient before transplantation may have altered the NK-cell receptors in RTRs; however, due to small sample size and lack of clinical data, the effect of these variables on NK-cell receptor expression could not be determined. In spite of these limitations, our findings align with the other studies 16, 39 that demonstrate that the loss of FcRγ and increase in the expression of LIR-1 or NKG2C is associated with CMV seropositivity. Expansion of FcRγ–LIR-1+NKG2C+ and FcRγ–LIR-1+NKG2C– NK cells should be confirmed in future in a larger cohort of CMV Ab+DNA+ RTRs.
The changes to NK-cell phenotypes associated here with CMV seropositivity (which carries a likelihood of intermittent reactivation) are likely to be clinically important because CMV may promote vascular disease directly via infection of the endothelium 41 or indirectly by stimulating immune responses and inflammation 42. In response to CMV infection, the recruitment of NK or T cells for its control may induce endothelial injury and promote atherogenesis. CMV-stimulated production of IFN-γ and TNF-α by CD4 T cells can induce the chemokine fractalkine, which can activate NK cells to cause endothelial damage 43. Moreover, NK cells can destroy endothelial cells following activation by xenoantigen-specific antibodies 44. Adaptive NK cells induced by CMV may play a role in the pathogenesis of vascular disease, as these cells are resistant to apoptosis during inflammatory conditions, have high proliferation rates upon engagement of an activating receptor and are highly responsive after cross-linking with CMV-specific antibodies 39. Therefore, in an attempt to control CMV infection, enhanced activation may drive NK cells to damage endothelial cells and cause vascular disease. High CMV antibody levels in healthy individuals are linked with ischemic heart disease, and expansion of NKG2C+ NK cells is associated with carotid atherosclerotic plaques in CMV seropositive patients 45, 46. Thus, expression of LIR-1 and/or loss of FcRγ could be used as a biomarker to assess the risk of developing vascular disease. In summary, we demonstrate that CMV infection affects NK-cell receptor expression and expands FcRγ–LIR-1+ NK cells by reactivating intermittently in clinically stable RTRs.
Materials and methods
Patients and controls
Renal transplant recipients were recruited from Royal Perth Hospital (RPH), Western Australia. All patients were clinically stable, free from clinical CMV disease or reactivation within 6 months of blood collection and receiving no current anti-viral treatment. All patients were stable on maintenance immunosuppressive therapy. Age-matched healthy controls were recruited from laboratory staff and colleagues. All participants provided written informed consent, and the project was approved by the Human Research Ethics Committees of RPH, the University of Western Australia and Curtin University. PBMC were isolated from heparin-treated blood using Ficoll–Paque (GE Healthcare, Little Chalfont, UK) density gradient centrifugation and cryopreserved in liquid nitrogen. Plasma samples were aliquoted and stored at −80°C.
Detection of CMV DNA and antibody
Plasma from RTRs was screened for CMV DNA in the Department of Microbiology (RPH) using commercial kits (Abbott Diagnostics, IL) able to quantitate >20 copies/mL. This value was used as a cut-off.
CMV-reactive IgG was quantitated by ELISA using a lysate of human foreskin fibroblasts (HFF) infected with AD169. Cells were harvested after 7 days, sonicated and stored at −80°C. Uninfected HFF were prepared as a negative control. Parallel microtitre plates were coated with CMV glycoprotein prepared in hamster ovary cells (Chiron, CA) and CMV immediate-early 1 (IE-1) prepared in E. coli (Miltenyi Biotec, Germany) at 0.5 μg/mL. Plates were coated overnight (4°C), washed with PBS/0.05% Tween, blocked with 5%BSA/PBS and washed. Plasma samples prediluted in 2%BSA/PBS were run alongside control plasma from a CMV-seropositive healthy individual assigned a value of 100 arbitrary units (AU). Plates were washed after 2 hours, and horse-radish peroxide conjugated anti-human IgG (Sigma-Aldrich, MO) diluted 1:4000 in 2%BSA/PBS was added. Tetramethylbenzidine substrate (Sigma-Aldrich) was added, color development was stopped with 1M H2SO4 and plates were read at 450 nm. CMV seropositivity was defined as >2 standard deviations above the mean antibody levels derived for a set of 11 samples that had been deemed seronegative by the ARCHITECT CMV IgG assay (Abbott Diagnostics, IL).
Immunophenotyping
Cryopreserved PBMC were thawed and washed, and aliquots of 106 cells were stained for 15 min in the dark at room temperature (RT) with antibodies recognizing cell surface antigens: V500-anti-CD3 (UCHT1), V450-anti-CD56 (B159), APC-H7-anti-CD16 (3G8), APC-anti-CD57 (NK-1), PE-anti-NKp46 (9E2/NKp46), PE-anti-NKp30 (p30-15), PE-Cy7-anti-NKG2D (1D11; BD Biosciences, NJ), PerCPCy5.5-anti-CD85j (HP-F1; eBioscience, CA), Alexa Fluor 488 anti-NKG2C (134591), PE-anti-KIR2DL1 (143211; R&D systems, MN), PE-anti-KIR2DL2/L3/DS2 (GL183; Beckman Coulter, CA), PerCPCy5.5-anti-CD62L (DREG-56), PE-KIR3DL1 (DX9; Biolegend, CA), PerCPCy5.5-anti-KIR2DL1/S1 (HP-MA4; eBioscience). For intracellular markers, cells were incubated for 20 min at 4°C with Cytofix/Cytoperm solution after surface staining and washed twice with cold 1X perm wash buffer (BD Cytofix/Cytoperm kit). Cells were then stained with 5 μL PerCPCy5.5-anti-perforin (DG9; Biolegend) for 30 min in the dark at RT. After incubation, cells were washed twice with cold 1X perm wash buffer. At least 100 000 events were acquired in the lymphocyte gate on a FACSCanto II instrument (BD Biosciences). Data were analyzed with FlowJo v10 software (Tree Star, OR).
Perforin ELISA
PBMCs were stimulated with anti-CD16 or K562 cells for 6 h, and supernatants were collected after 6 h and stored at −80°C. Perforin levels were measured using a commercial human Perforin ELISA kit (Abcam, Cambridge, UK) able to detect < 40 pg/mL.
Functional assays
Cryopreserved PBMC were thawed, washed and stimulated (0.5 × 106 cells/100 μL) with cytokines [IL-12 (10 ng/mL; R & D systems) + IL-15 (10 ng/mL; R & D systems) + IL-18 (10 ng/mL; MBL, Japan)] for ∼ 16 h at 37°C and 5% CO2. Remaining PBMC were rested overnight, and next day these cells (5 × 105/100 μL) were stimulated for 6 h at 37°C with RPMI 1640/10% FBS, 10 μg anti-CD16 (BD Biosciences) bound to flat-bottomed 96-well plates or K562 cells at an effector-to-target ratio of 2:1. BV786-anti-CD107a (H4A3; BD Biosciences) was added to all wells. After 1 h, brefeldin A and monensin (BD Biosciences) were added and cells were incubated for a further 5 h at 37°C. Cells were then stained with a live/dead dye, FVS700 (BD Biosciences) for 30 min, followed by BUV395-anti-CD3 (UCHT1), BV510-anti-NKp46 (9E2/NKp46), PE-CF594-anti-CD57 (NK-1), BV421-anti-NKp30 (p30-15; BD Biosciences), PE.Cy7-anti-CD56 (HCD56; Biolegend), APC-anti-NKG2C (134591; R&D systems), and PE-anti-LIR-1 (HP-F1; eBioscience) for 15 min in the dark at RT. Cells were then fixed for 20 min at 4°C, permeabilized (BD Cytofix/Cytoperm™; BD Biosciences), stained for intracellular antigens using BV650-anti-TNF-α (MAb11; Biolegend) and FITC-anti-FceRIγ (Merck Millipore, Germany) for 30 min and was washed twice with cold 1X perm wash buffer. At least 100,000 events were acquired in the lymphocyte gate on a BD LSR II Fortessa Instrument (BD Biosciences). Data were analyzed with FlowJo v10 software (Tree Star, OR).
ADCC assay
Ninety-six-well plates were coated with CMV gB antigen at 0.5 μg/mL and incubated overnight at 4°C. Wells were washed and blocked for 60 min at RT with 10% FBS/RPMI. Hundred microliters aliquots of heat-inactivated (56°C, 1 h) autologous plasma were added to the coated wells, incubated overnight at 4°C. The optimal dilution (1:300) was selected in preliminary experiments that displayed highest expression of CD107a and production of TNF-α. Wells were washed and 5 × 105 PBMCs were added with BV786-anti-CD107a (H4A3; BD Biosciences) for 1 h followed by brefeldin A and monensin (BD Biosciences) for a further 5 h at 37°C. Cells were stained as described under the functional assay.
Genotyping
A deletion mutation abrogating expression of NKG2C was assessed by a nested PCR-based method with two primer pair sequences 18 to distinguish homozygosity and heterozygosity.
Statistical analyses
Statistical analyses were performed using Prism 5 (GraphPad Software, CA). Nonparametric tests were conducted to determine statistical significances. Mann–Whitney tests were used to compare results between groups. Wilcoxon matched pairs tests were used to compare results within a patient or control group. Correlation coefficients were calculated with Spearman's tests. All p values ≤0.05 were considered to be statistically significant.
Acknowledgments
The authors thank Anne Warger and Bronwyn Reid for assistance with patient recruitment. We also thank the patients and controls who donated blood. We thank Prof Hanspeter Pircher (Department of Virology, University of Freiburg, Germany) for his kind donation of anti-human AF488-KLRG1. We thank Dr Dianne De Santis (Department of Clinical Immunology, Fiona Stanley Hospital, Australia) for the KIR PCR primers and reagents.
The study was funded by the Medical Research Foundation of Royal Perth Hospital and the National Health and Medical Research Council of Australia (Grant 1068652). Nandini Makwana was supported by an India SIRF scholarship. Bree Foley and Sonia Fernandez were supported by priming grants from the Raine Medical Research Foundation.
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
Abbreviations
-
- ADCC
-
- antibody-dependent cell cytotoxicity
-
- CMV
-
- cytomegalovirus
-
- HFF
-
- human foreskin fibroblast
-
- KIR
-
- killer immunoglobulin-like receptor
-
- RPH
-
- Royal Perth Hospital
-
- RT
-
- room temperature
-
- RTR
-
- renal transplant recipient




