Integrated T-cell receptor and costimulatory signals determine TGF-β-dependent differentiation and maintenance of Foxp3+ regulatory T cells
Abstract
Foxp3-expressing Tregs play a non-redundant role in protecting against immune pathologies. Foxp3+ Tregs can arise intra- and extra-thymically, however, the signals directing their differentiation and maintenance in the periphery are not well understood. We show that stimulation of mouse naïve CD4+ T cells in vitro with optimal doses of anti-CD3/anti-CD28 resulted in high frequencies of Foxp3+ T cells via a TGF-β-dependent mechanism. Addition of TGF-β and retinoic acid overcame the inhibition of Foxp3 expression observed during high-strength anti-CD3/anti-CD28 stimulation. Reducing the strength of TCR or costimulatory signals with inhibitors of mammalian target of rapamycin (mTOR) or MEK/ERK signalling also enhanced expression of Foxp3 in a TGF-β-dependent manner. Addition of TGF-β was further required to maintain Foxp3 expression in ex vivo derived Foxp3+ Tregs upon prolonged anti-CD3/anti-CD28 signalling. Thus, induction/maintenance of Foxp3 expression by TGF-β is modulated by the integrated strength of TCR/costimulatory signals.
Introduction
Tregs expressing the transcription factor Foxp3 control the immune response to limit inflammatory pathologies 1. Foxp3-expressing Tregs can develop both in the thymus and in the periphery 2. High-affinity TCR signals 3, 4 together with costimulation through CD28 5, 6 are required for the development of Foxp3+ Tregs in the thymus. TGF-β promotes Foxp3+ Treg survival by antagonising negative selection 7 and is necessary for the maintenance of thymically derived Foxp3+ Tregs in the periphery 8. Low-level TCR signals promote differentiation of Tregs from naïve T cells in the periphery 9, 10, which is further enhanced by the addition of TGF-β or an absence of IL-2 10. It has been suggested that a high dose of antigen inhibits the differentiation of Foxp3+ Tregs from naïve T cells correlating indirectly with activation of the PI3K-Akt-mammalian target of rapamycin (mTOR) pathway 11, shown to be antagonistic for Foxp3 expression 12, 13 by a process suggested to be independent of TGF-β 11, 14. A requirement for costimulation through CD28 for extra-thymic Treg generation is unclear 15-17, although necessary for the maintenance of Foxp3+ Tregs in the periphery 18. IL-2 is also needed for peripheral Treg maintenance 19 and provision of IL-2, or co-transfer of conventional T cells, confers stability of Foxp3 expression in Tregs upon adoptive transfer into lymphopenic hosts, preventing their conversion to effector T cells 20-22. All-trans retinoic acid (RA) produced by specialised DCs in the gut 23-26 can enhance the differentiation of Tregs induced by TGF-β 10, 27-29 and is suggested to function in part by antagonising the negative regulation by CD4+CD44hi T cells in the context of CD28 signalling 30, 31. Here we provide data to clarify some of the conflicting reports on the role of costimulation on Foxp3 expression in Tregs. We show that induction and maintenance of TGF-β-dependent Foxp3 expression is modulated by the integrated strength of TCR/costimulator signals.
Results and discussion
CD28 enhances or inhibits TGF-β-dependent Foxp3 expression depending on TCR signal strength
To determine whether different doses of TCR and costimulatory signals could influence the differentiation of naïve CD4+ T cells into Foxp3+ Tregs, we stimulated a homogeneous population of FACS-purified, CD4+CD44loCD25− T cells from a TCR transgenic mouse (TCR7) on a Rag−/− background with different doses of anti-CD3 (10, 2.5 and 1 μg/mL) and anti-CD28 (10, 2 and 0 μg/mL) in the presence of IL-2. To ensure that the Foxp3 expression detected arose from de novo differentiation, TCR transgenic Rag−/− mice were used where the starting T-cell population was naïve and did not contain any Foxp3+ Tregs. Maximal frequencies of Foxp3-expressing CD4+ T cells were observed at low doses of anti-CD3 without the need for addition of exogenous TGF-β, provided that cells were costimulated with anti-CD28 (Fig. 1A and B). Increasing the level of anti-CD3 stimulation resulted in a progressive decrease in Foxp3 expression in the presence of anti-CD28 costimulation. Foxp3 expression was minimal at the highest dose of anti-CD3/anti-CD28 costimulation, and was increased in the absence of anti-CD28 stimulation. This was not restricted to a single TCR as similar data were obtained using CD4+CD44loCD25−Foxp3GFP− T cells from C57BL/6 Foxp3GFP reporter mice with different TCR specificities (data not shown). These results are consistent with previous data showing that low antigen dose promotes Foxp3 expression 10, 11. However, we further demonstrate here that CD28 costimulation may enhance or inhibit Foxp3 induction depending on the strength of the TCR signal. Thus, optimal induction of Foxp3 expression in naïve CD4+ T cells results from integrated TCR/costimulator signals. This cumulative requirement for low thresholds of TCR together with high costimulatory signals may explain the mixed reports regarding the role of CD28 in Foxp3+ Treg generation.
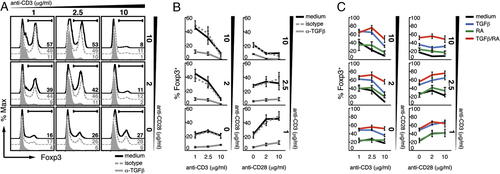
CD28 enhances or inhibits endogenous TGF-β-dependent Foxp3 expression depending on TCR signal strength; exogenous TGF-β and RA are required to induce Foxp3 expression at high-strength TCR/costimulatory signals. Naïve CD4+CD44loCD25− T cells from TCR7 Rag1−/− mice were cultured in the presence of increasing doses of plate-bound anti-CD3 and soluble anti-CD28 in complete RPMI medium (cRPMI) plus IL-2, alone or with anti-TGF-β or isotype control antibodies (A and B), or with TGF-β and/or RA (C). Numbers represent percentages of Foxp3-expressing cells gated on live CD4+ T cells depicted in histogram (A) or graphical form (B and C). Representative data are shown of three experiments performed (A); error bars in (B and C) refer to the SEM of three independent cultures.
The differentiation of Foxp3+ Tregs from naïve CD4+ T cells has previously been demonstrated to require the addition of TGF-β 10, 27-29. However, Turner et al. state that the induction of Foxp3 expression induced with low dose of antigen is independent of TGF-β as none was detected in culture supernatants 11. Thus, we sought to investigate whether our observed induction of Foxp3 expression at the optimal TCR/costimulator thresholds was dependent on signalling by endogenous TGF-β. Addition of neutralising antibodies directed against TGF-β to cultures of naïve CD4+ T cells stimulated as above resulted in almost complete inhibition of Foxp3 expression at all anti-CD3/anti-CD28 concentrations (Fig. 1A and B) as compared with isotype controls (Fig. 1A and B, dashed grey line). These results demonstrate that optimal TCR/costimulator thresholds promote Foxp3 expression in a TGF-β-dependent manner, although the exact signalling pathway was not examined.
Exogenous TGF-β and RA induce Foxp3 expression at high TCR/costimulatory signal strength
RA has recently been suggested to be required to overcome the negative effects of CD4+CD44hi effector T cells on TGF-β induced differentiation of Foxp3+ Tregs during high-level anti-CD3/anti-CD28 costimulation 30. This could account for earlier reports of highly efficient Foxp3+ Treg differentiation from naïve CD4+ TCR transgenic T cells on a Rag−/− background where TGF-β alone gave rise to high percentages of Foxp3-expressing CD4+ T cells 29. In addition to conflicting roles described for CD28 costimulation in the development and maintenance of extra-thymically derived Foxp3+ Tregs, there are mixed reports on its role in TGF-β and RA-induced differentiation of Foxp3+ Tregs 29-31. Here we investigated the effects of TGF-β and RA on Foxp3 expression across the different anti-CD3/anti-CD28 concentrations. Addition of TGF-β (Fig. 1C), and to a lesser extent RA (Fig. 1C), induced Foxp3 expression in naïve CD4+ T cells most effectively at high anti-CD3/anti-CD28 conditions. A combination of TGF-β and RA (Fig. 1C) was necessary to attain the highest frequencies of Foxp3-expressing CD4+ T cells and overcame the inhibition of Foxp3 expression observed at high-strength TCR/costimulator signalling. Thus, the addition of TGF-β plus RA appears to be most effective at high TCR/costimulator signal strength, although showing an effect throughout. Of note, while the viable cell numbers follow a typical dose response curve, the number of non-viable cells remains relatively constant across all groups demonstrating that differential cell death does not account for the differences in the proportions of viable Foxp3+ T cells (Supporting Information Fig. S1A and B). Furthermore, as both Foxp3+ and Foxp3− T cells have divided with similar kinetics, the percentages of Foxp3+ versus Foxp3− T cells in the cultures do not appear to be biased by their differential proliferation (Supporting Information Fig. S1C).
In order to compare the functional activity of the Foxp3+ Tregs generated by the different in vitro regimens, a conventional in vitro suppression assay was performed. The capacity of the different in vitro-derived Foxp3-expressing T cells as compared with that of ex vivo-derived Foxp3+ Tregs to inhibit the proliferation of naïve CellTrace Violet-labelled responder T cells was examined. As shown in the Supporting Information Fig. S2A and B, Foxp3+ Tregs FACS-sorted after 7 days of stimulation with anti-CD3/anti-CD28 (2.5/10 μg/mL) in medium and IL-2 alone (iTreg (medium)), or with added TGF-β and RA (iTreg (TGF-β/RA)) showed significant regulatory activity comparable with that of ex vivo-derived Foxp3+ Tregs (nTreg).
Exogenous TGF-β prevents prolonged TCR signalling induced Foxp3 downregulation
Premature termination of TCR signalling has previously been shown to favour the differentiation of Foxp3+ Tregs from naïve CD4+ T cells in vitro 14. In view of our findings that high TCR/costimulator signals inhibit the induction of Foxp3 expression, we next sought to investigate the effect of prolonged TCR signalling on the stability of ex vivo-derived Foxp3+ Tregs. To this end, we stimulated FACS-purified CD4+Foxp3GFP+ T cells from Foxp3GFP C57BL/6 mice with different doses of anti-CD3 and anti-CD28 in medium containing IL-2 and either removed the cells or kept them on anti-CD3-coated plates after 72 h stimulation. Removal of cells from anti-CD3 stimulation resulted in greater percentages of Tregs retaining Foxp3 expression than continued TCR triggering (between 50–70% compared with 25–50%, respectively) (Fig. 2A and B, black line). This effect was dominant and occurred at all anti-CD3/anti-CD28 doses, although varied to some extent depending on the dose of anti-CD3/anti-CD28. In order to exclude the possibility that the accumulation of the Foxp3− T cells was due to differential proliferation rates of the populations that have retained Foxp3 expression versus those that have not, we have labelled the CD4+Foxp3GFP+ Tregs with CellTrace Violet and followed its dilution during the course of the culture period. As shown in the Supporting Information Fig. S3, most of the cells in the medium control group have divided by the end of the culture period, with Tregs retaining Foxp3 dividing at a similar rate to those that had lost Foxp3 expression under both short and prolonged conditions of TCR triggering. Thus, the percentages of Foxp3-retaining Tregs do not appear to be biased by the differential proliferation rate of T cells that have lost Foxp3 expression. Addition of TGF-β to the cultures sustained Foxp3 expression under both conditions (Fig. 2A and B; and Supporting Information Fig. S3), although its effect was more prominent when Tregs were exposed to prolonged anti-CD3 signaling. Thus, in addition to playing a role in the differentiation of Foxp3+ Tregs, TGF-β also ensures that Foxp3 expression is maintained during continued, high-level TCR signalling.
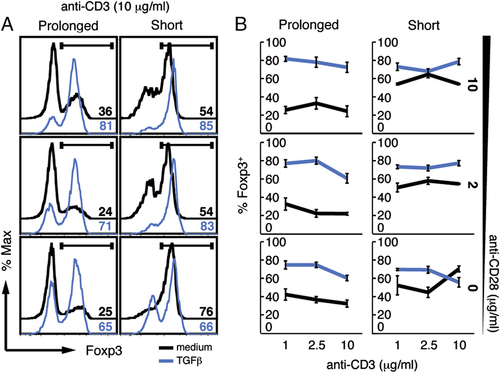
Exogenous TGF-β prevents prolonged TCR-signalling-induced Foxp3 downregulation. CD4+Foxp3GFP+ Treg cells were cultured in the presence of increasing doses of plate-bound anti-CD3 and soluble anti-CD28 in cRPMI plus IL-2, alone or with TGF-β. On day 3, cells were either removed from (short) or kept on (prolonged) the anti-CD3-coated plates until the end of culture period. Numbers represent percentages of Foxp3-expressing T cells assessed by nuclear stain with anti-Foxp3 gated on live cells depicted in histogram (A) or graphical form (B). Representative data are shown of three experiments performed (A); error bars in (B) refer to the SEM of three independent cultures.
Inhibition of MEK/ERK or mTOR induces Foxp3 expression in a TGF-β-dependent manner
TCR/costimulator ligation can trigger multiple downstream signalling pathways 32. The strength of TCR/costimulatory signals can be moderated by inhibiting their downstream signalling pathways using an inhibitor of MEK1/2, PD0325901, which blocks downstream ERK1/2 activation 33, or rapamycin, an inhibitor of mTOR. In keeping with our findings that the cumulative strength of TCR/costimulator signals determines the induction of Foxp3 expression in naïve CD4+ T cells, inhibition of mTOR by rapamycin resulted in significantly (p<0.001, in anti-CD28-treated cultures only) enhanced Foxp3 expression at high-dose anti-CD3 (10 μg/mL) and anti-CD28 (10, 2 and 0 μg/mL) stimulation as compared with vehicle control (Fig. 3A and B). Of note, as the number of non-viable cells remained relatively constant, differential cell death did not appear accountable for the differences in the proportions of viable Foxp3+ cells. However, the number of live cells recovered was decreased, indicative of a shift in the dose response curve resulting from the inhibition of signalling pathways downstream of TCR/costimulatory signals (Supporting Information Fig. S4). The frequencies of Foxp3 Treg in cultures containing rapamycin were only marginally increased upon further addition of TGF-β, although still reached statistical significance when compared to rapamycin alone (Fig 3A and B). Our findings are in keeping with previous reports that blocking the PI3K-Akt-mTOR pathway induces Foxp3-expressing Tregs 12-14. Importantly, however, we show that the induction of Foxp3 expression by rapamycin is partially dependent on endogenous TGF-β, since significantly reduced frequencies of Foxp3-expressing CD4+ T cells were detected in the presence of anti-TGF-β antibody as compared to rapamycin alone (Fig 3A and B). Our results differ from the previous study by Sauer et al. where TGF-β appeared dispensable for PI3K/mTOR inhibitor-driven Foxp3 induction, and these differences may be possibly due to variations in experimental protocols 14. While Sauer et al. used naïve CD4+CD62L+CD25− T cells from WT mice still containing CD44hi T cells, which could potentially affect Foxp3 induction 30, we used CD4+CD44loCD25− T cells from Rag−/− TCR transgenic mice ensuring their naïve state. Furthermore, Sauer et al. used one dose of TCR/costimulator (200 ng/mL anti-TCRβ and 2 μg/mL anti-CD28) stimuli with shorter kinetics in contrast to our range of doses (10 μg/mL anti-CD3 and 10, 2 and 0 μg/mL anti-CD28) and a more extended culture. As the two protocols differ considerably, it is difficult to draw any comparisons between the two studies, although each may have relevance to different in vivo scenarios. Despite mTOR being predominantly downstream of CD28, we also show that the effect of rapamycin was still evident in the absence of costimulation through CD28, implying activation of mTOR downstream of CD3 signalling. Inhibition of ERK activation upon PD0325901 addition also led to significantly (p<0.01, in anti-CD28-treated cultures only) increased frequencies of Foxp3-expressing CD4+ T cells via a TGF-β-dependent mechanism, although to a lower extent than observed upon addition of rapamycin (Fig 3A and B). This is in contrast to previous reports that suggested that TGF-β-driven Foxp3 induction may not be affected by ERK activation, and may again reflect differing experimental approaches 14. Namely, PD0325901 used here inhibits MEK1 with greater potency and specificity than PD98059 33 used in the Sauer et al. study 14, which may possibly explain why increased induction of Foxp3 was observed upon PD0325901 treatment. Addition of TGF-β further enhanced the increased Foxp3 expression seen in the presence of PD0325901 (Fig 3A and B), which reached statististical significance. Taken together, these results confirm that reducing CD3 and CD28 signals, here with inhibitors of downstream signalling pathways, results in high levels of Foxp3 expression in response to TGF-β.
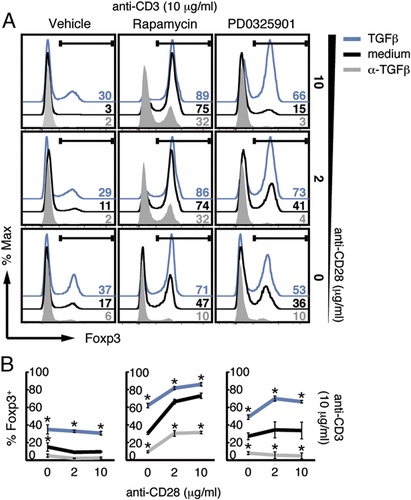
Inhibition of MEK/ERK or mTOR induces Foxp3 expression in a TGF-β-dependent manner. Naïve CD4+CD44loCD25− T cells from TCR7 Rag1−/− mice were cultured in the presence of plate-bound anti-CD3 at 10 μg/mL and soluble anti-CD28 (10, 2, 0 μg/ml) in cRPMI plus IL-2, alone or with anti-TGF-β antibody or TGF-β, in the absence (DMSO control) or presence of rapamycin or PD0325901. Data are presented as in Fig. 1, representative data are shown of three experiments performed (A) with SEM of three independent cultures (B). *p<0.05 as determined by two-way ANOVA.
Concluding remarks
In summary, we have shown that induction of Foxp3 expression in naïve CD4+ T cells by TGF-β is modulated by the combined strength of TCR/costimulatory signals and that prolonged TCR signalling can result in the loss of Foxp3 expression in ex vivo derived Foxp3+ Tregs. We have confirmed the role of PI3K-Akt-mTOR pathway in inhibiting Foxp3 expression, recently shown to act by inactivating Foxo proteins 34. However, we additionally show that this enhancement of Foxp3 expression observed during CD3/CD28 stimulation in the presence of rapamycin is still, at least partially, dependent on TGF-β signalling. We further identified the ERK MAPK pathway in the negative regulation of Foxp3 expression, albeit to a lesser extent than the effect of mTOR signalling. These findings demonstrate that the strength of integrated signals delivered through the TCR and costimulatory pathways impinge on the level and maintenance of Foxp3 expression induced by TGF-β.
Directing T-cell differentiation towards Foxp3+ Treg development by pharmacological means may have potential therapeutic implications for the treatment of immune-mediated diseases including autoimmunity or immune pathology where induction of Foxp3+ Tregs could aid in the prevention of unwanted immune responses. The conversion of naïve T cells into Foxp3+ Tregs has been demonstrated in vivo using delivery of antigen under subimmunognic conditions 10. However, as these are difficult to define owing to the range of different TCR affinities and/or overall avidities for an antigen, inclusion of rapamycin in the tolerization regimen would be of therapeutic value ensuring that no untoward inflammation occurs.
Materials and methods
Mice
TCR transgenic C57BL/6 (TCR7) mice specific for hen egg lysozyme 35 crossed to Rag1−/− (TCR7 Rag1−/−), or to Foxp3GFP C57BL/6 mice 36 (Foxp3GFP TCR7 Rag1−/−) and WT C57BL/6 mice were bred and maintained under specific pathogen-free conditions at NIMR according to the Home Office UK Animals (Scientific Procedures) Act 1986 and used at 8–12 wk of age.
T-cell sorting and in vitro assays
Naïve T cells were FACS-sorted on a MoFlo cytometer (Beckman Coulter) for CD4+CD44loCD25− or CD4+Foxp3GFP+ (>98% purity) from CD4-enriched spleens using CD4 (RM4-5), CD44 (IM7) and CD25 (PC61.5) antibodies (eBioscience). FACS-purified T cells, where indicated labelled with 2.5 μM CellTrace Violet or CFSE (Invitrogen), were plated at 5×104 per well in flat-bottom 96-well plates previously coated with anti-CD3 (145-2C11) at 1, 2.5 and 10 μg/mL in cRPMI medium 35 containing 5 ng/mL recombinant mIL-2 (Insight Biotechnology), with or without soluble anti-CD28 (37.51) at 2 or 10 μg/mL for 7 days. On day 3, cells were customarily removed from anti-CD3-coated wells. Where indicated, 10 μg/mL of anti-TGF-β (1D11.16) or isotype control (TC32.27F11) antibodies, 0.05 ng/mL (Figs. 1 and 3) or 5 ng/mL (Fig. 2) recombinant hTGF-β1 (Insight Biotechnology), 10 nM all trans-RA (Sigma-Aldrich), 50 nM rapamycin (Invitrogen) or 0.1 μM PD0325901 or similar volumes of DMSO vehicle were added to T-cell cultures. Alternatively, in Fig. 2, cells were removed or not removed from anti-CD3, supplemented with IL-2 only and cultured for additional 4 days. For in vitro suppression assays, induced CD4+Foxp3GFP+CD25hi T cells from TCR7 Foxp3GFP Rag1−/− mice stimulated with 2.5 μg/mL of anti-CD3 and 10 μg/mL of anti-CD28 for 7 days in the presence of IL-2 or additionally of TGF-β and RA, or CD4+Foxp3GFP+ isolated directly ex vivo from Foxp3GFP C57BL/6 mice as a control, were FACS-sorted and co-cultured with naïve CD4+CD44loCD25− T cells from TCR7 Rag1−/− mice labelled with CellTrace Violet (Invitrogen). Cells were plated at 5×104 per well in round-bottom 96-well plates and stimulated with 0.5 μg/mL soluble anti-CD3 along with 1×105 CD3-depleted, irradiated (3000 Rad) splenocytes from C57BL/6 mice as APCs. After 3 days, cells were harvested, stained with propidium iodide (Sigma-Aldrich) and CD4 to exclude dead cells and analysed on LSR II (BD) flow cytometer.
Flow cytometry
Expression of Foxp3 after culture was evaluated by staining dead cells with LIVE/DEAD® Fixable Red Dead Cell Staining Kit (Invitrogen), surface staining for CD4 BD HorizonTM V500 (BD) and intracellular staining for FoxP3 eFluor® 450 with Foxp3 Fixation/Permeabilization buffers (eBioscence) on LSR II (BD) flow cytometer and collected data analysed with FlowJo (Tree Star) software.
Statistical analysis
Results are presented as mean±SEM. Statistical analyses were performed where stated using GraphPad Prism (Graphpad Software) software. The statistical significance of differences between data groups was determined by one-way or two-way ANOVA at the 95% confidence level.
Acknowledgements
At NIMR, we thank A. Rae, G. Preece and N. Biboum for assistance in flow cytometry cell sorting; Biological Services unit for animal husbandry and breeding; G. Kassiotis, B. Seddon, V. Tybulewicz and M. Wilson for discussions and feedback on the manuscript and Ashleigh Howes for critical reading of the manuscript. We thank P. Cohen, University of Dundee, UK, for his generosity in providing the inhibitor PD0325901. This work was supported by the Medical Research Council, UK (U117565642).
Conflict of interest: The authors declare no financial or commercial conflict of interest.