CD38 identifies a hypo-proliferative IL-13-secreting CD4+ T-cell subset that does not fit into existing naive and memory phenotype paradigms
Abstract
CD38 is commonly regarded as an activation marker for human T cells. Herein, we show that CD38 expression identifies a hypo-proliferative CD4+ T-cell subset that, following TCR stimulation, retains expression of naive cell surface markers including CD45RA, CD62L and CCR7. Hypo-proliferation was mediated by reduced CD25 up-regulation upon TCR stimulation compared to CD4+CD38− cells and lack of responsiveness to exogenous IL-2. Instead, CD4+CD38+ T cells expressed CD127, and hypo-proliferation was reversed by addition of IL-7, further associated with increased STAT5 phosphorylation. This phenotype was exacerbated by addition of an agonistic CD38-binding antibody, suggesting that signaling through CD38 promotes this cell profile. Activated CD4+CD38+ cells had a bias towards IL-13 secretion, but not other Th2 cytokines such as IL-4 or IL-5. In comparison, the CD4+CD38− cells had a clear bias towards secretion of Th1-associated cytokines IFN-γ and TNF. The existence of such CD4+CD38+ T cells may play an important role in pathologies such as asthma, which are associated with IL-13, but not IL-4 and IL-5. Coupled with responsiveness to IL-7 but not IL-2, and the involvement of CD38 ligation, our results highlight a unique T-cell subpopulation that does not fit into existing naive and memory cell paradigms.
Introduction
The transition of CD4+ T cells from a naïve to an effector/memory state is a tightly regulated process requiring multiple stimuli. When naïve T cells first encounter their specific antigen presented by APCs, they are activated and carry out a range of effector functions before some cells are retained as long-term memory T cells. Surrounding cytokines play a crucial role not only in directing the immediate effector functions, but are also required for the cells development into functional memory cells. The common γ chain (γc)-dependent group of cytokines plays a particularly important role in regulating the homeostasis and transition of T-cell subsets and include IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21. IL-2 and IL-7 are two γc cytokines that have been extensively investigated for their role in maintaining naive, effector and memory cell survival, as expression of their high affinity receptors CD25 and CD127 has been demonstrated to play a key role in the controlling T-cell homeostasis, proliferation and differentiation 1.
IL-2 is produced rapidly by T cells following stimulation and is the major T-cell growth factor. The IL-2 receptor comprises α (CD25), β (CD122) and γ (CD132) chains, and the functional receptor is induced following TCR engagement 1. Signaling through the IL-2 receptor controls T-cell expansion and differentiation, and also supports memory cell division 2. The binding of IL-7 to its high-affinity receptor (α chain CD127 and γc CD132) is crucial for priming naive T-cell proliferation as it promotes up-regulation of anti-apoptotic molecule Bcl-2 and enhancement of TCR-mediated signaling 3, 4. In most T cells, CD127 is down-regulated following activation 5; however, recent studies investigating CD8+ T cells during viral infection showed that a small number of T cells retain or re-express CD127, predisposing them with a greater ability to become long-term memory cells 6, 7. Engagement of CD127 by IL-7 is not only crucial for T-cell effector progression into memory cells, it is also crucial for memory T-cell survival and proliferation 8. These stages of T-cell development are further tracked through changes in various surface markers. Typically for human PBMCs, naïve T cells express high levels of CD45RA and once activated down-regulate this isoform and express CD45RO 9, 10. The co-expression of other markers such as the secondary lymphoid organ (SLO) homing molecules CD62L and CCR7 helps to distinguish the migratory capacity of naïve and memory cells, as naïve and central memory cells express high levels of CD62L and CCR7 11. Additionally, there are numerous other T-cell markers that are used to distinguish the status of T-cell activation, including CD38. CD38 is type II trans-membrane glycoprotein with various functions including the enzymatic catalysis and hydrolysis of cyclic adenosine diphosphate ribose (cADPR) 12, a role as an endothelial adhesion molecule via binding to its ligand CD31 (PECAM-1) 13, 14, and a role in the transduction of activation and proliferation signals in both T and B cells 15. While CD38 is often used as an activation marker for human T cells, it is also known to be expressed on ‘naive’ CD4+CD45RA+ human T cells 16; therefore, our aim was to investigate the activation status and functionality of CD4+CD38+ T cells.
Here, we describe a subset of CD4+CD38+ T cells that curiously retain CD45RA, CD62L and CCR7 expression following in vitro stimulation and fail to up-regulate the classical activation marker CD45RO. These CD38+ cells are hypo-proliferative and display a CD25low/CD127high phenotype compared to CD38− cells, a phenotype that is exacerbated by CD38 ligation with agonistic monoclonal antibody IB4. Moreover, CD4+CD38+ T cells were responsive only to recombinant (r)IL-7, but not rIL-2, resulting in STAT5 phosphorylation and a subsequent increase in their proliferative capacity. Despite their limited proliferation and a reduced capacity to produce Th1 cytokines such as IFN-γ and TNF, CD4+CD38+ T cells were found to be the main IL-13 producing cell type following in vitro stimulation. Interestingly, this IL-13 secretion bias was not coupled with the secretion of other Th2 cytokines such as IL-4 and IL-5. Together these data highlight the existence of a distinctive subpopulation of CD4+ T cells that does not fit in conventional naïve and memory subset classifications, but display a unique activity and phenotypical profile upon stimulation.
Results
CD4+CD38+ T cells have a hypo-proliferative profile when stimulated in vitro
CD4+CD38+ T cells are an abundant cell population making up 22.6% of peripheral CD4+ T cells and 12.1% of total peripheral T cells (CD4+: 22.63±1.68, CD3+: 12.1±1.35; n=11 control healthy donors). While CD38 is classically regarded as a T-cell activation marker, several studies have shown that expression of CD38 often coincides with expression of T-cell naive marker CD45RA 16 and our flow cytometry analysis confirmed these findings. Analysis of PBMC from 11 healthy donors showed that CD4+CD38+ T cells contained a greater percentage of CD45RA+CD45RO− cells (38+: 69.54±4%; 38−: 28.9±4%) while the CD38− cells contained a greater percentage of CD45RA−CD45RO+ cells (38−: 45.2±5%; 38+: 13.4%±2; Fig. 1A) suggesting CD38 is not simply a marker of CD4+ T-cell activation. To investigate the properties and function of these cells in more detail, CD4+ T cells were isolated to 92–99% purity and separated into CD38+ and CD38− subsets (Fig. 1B). The sorted cell subsets were equally capable of proliferating in vitro when stimulated via non-TCR-dependent stimulation (PMA/ionomycin); however, when stimulated with anti-CD3/CD28 for 3 days, CD4+CD38+ T cells displayed a greatly decreased proliferative capacity compared with CD4+CD38− cells (n=9, p=0002) (Fig. 1C). This hypo-proliferative profile was not due to a reduced activation threshold of the CD4+CD38+ T cells, as titrations of anti-CD3 and anti-CD28 monoclonal antibodies all yielded similarly decreased levels of proliferation for CD38+ when compared with CD38− T cells (Supporting Information Figs. S1A and S2A).
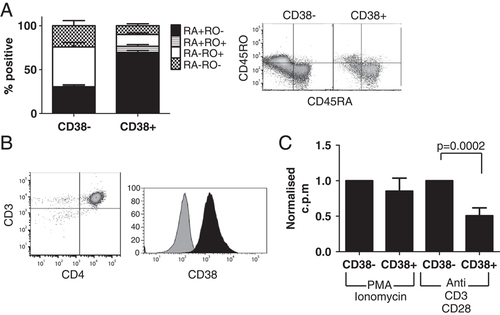
CD4+CD38+ T cells naturally express high levels of CD45RA and are hypoproliferative when stimulated in vitro via the TCR. (A) Phenotypic analysis was conducted on CD4+CD38+ and CD4+CD38− T cells from whole PBMC from 11 healthy donors. Percentages of CD45RA+CD45RO− (RA+RO−), CD45RA+CD45RO+ (RA+RO+), CD45RA−CD45RO+ (RA−RO+) and CD45RA−CD45RO− (RA−RO-) cells were determined. (n=11 donors; Mean+SEM) (B) CD3+CD4+ T cells were isolated to 92–99% purity, and then further separated into CD38+ (black) and CD38− (grey) cells. (C) Isolated cells were stimulated with PMA/Ionomycin or anti CD3/CD28 antibodies for 3 days, and their capacity to proliferate was determined. (CD38+ c.p.m normalized against CD38− c.p.m for each donor; Mean+SEM; n=3 PMA/Ionomycin, n=9 anti-CD3/CD28, p=0.0002 paired t-test).
CD4+CD38+ cells retain a ‘naive’ phenotype upon in vitro stimulation
Since freshly isolated CD4+CD38+ T cells have a natural bias for CD45RA co-expression, we investigated whether this phenotype was retained following in vitro stimulation. The CD4+CD38+ T cells retained high levels of CD38 together with CD45RA and did not acquire as high a level of CD45RO expression as compared with the CD38− cells (Fig. 2A). When whole CD4+ T cells were stimulated in vitro for 3 days with anti-CD3/CD28, these cells decreased expression of CD45RA while increasing expression of CD38 and CD45RO (data not shown). The retention of CD45RA and CD38 coupled with a reduced expression of CD45RO on isolated CD4+CD38+ cells indicate that pre-existing circulating CD38+ cells found in the periphery may act differently than CD38+ cells induced in vitro. Isolated CD4+CD38+ T cells also retained high expression of SLO homing receptors CD62L and CCR7 following TCR stimulation (Fig. 2A). CD62L (L-selectin) is known to be highly expressed on both naive 17 and central memory cells 11, as both cell types are required to migrate back to the lymph node in order to interact with APC. CD62L binds to peripheral node addressins on high endothelial venules allowing for T-cell attachment. The rolling lymphocytes use CCR7 to bind to CCL19 and CCL21, triggering firm binding and allowing trans-endothelial migration of the cell into the SLO 18.
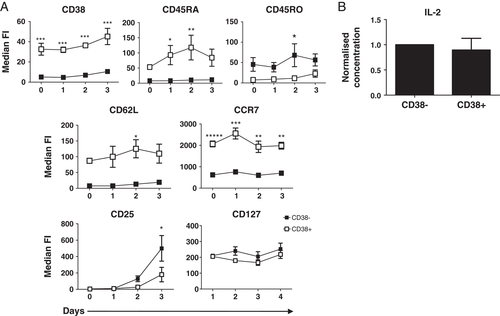
CD4+CD38+ T cells retain CD45RA, CD62L and CCR7 expression upon in vitro stimulation. (A) Isolated CD4+CD38+ (□) and CD4+CD38− (▪) T cells were stimulated with anti-CD3/CD28 antibodies for 0–3 days and expression of the markers indicated were analyzed by flow cytometry. (MFI±SEM; n=5 for CD38, CD45RA, CD45R0, CD25; n=4 for CD62L and CCR7; n=3 for CD127; *p<0.05, **p<0.01, ***p<0.001; two-way ANOVA). For the analysis of CD25 and CD127, both cell subsets were initially depleted of CD25 to avoid interference from recently activated cells and/or regulatory cells. (B) Sorted CD4+CD38+ and CD4+CD38− T cells were cultured for 3 days with anti-CD3/CD28 antibodies. Culture supernatants were tested for IL-2 by cytometric bead array. (Concentrations for CD38+ cells are normalized against CD38−; mean+SEM, n=14).
Proliferation of T cells is dependent on TCR stimulation together with co-stimulation through CD28, but also requires support from several cytokines that promote cell division, including IL-2 and IL-7. In order to determine whether the hypo-responsive nature of CD4+CD38+ compared with CD4+CD38− cells was due to their differential capacity to respond to these cytokines, we first examined expression of the receptors' α-chains, CD25 and CD127, over the 3-day time course. CD25+ cells were initially depleted from both populations to avoid any interference from recently activated cells and/or regulatory cells. While CD38+ cells did express some CD25 after 3 days in vitro stimulation, these levels were significantly lower compared with those of CD38− cells, suggesting a decreased ability to respond to IL-2 (Fig. 2A: p<0.05 two-way ANOVA; n=5). The expression of CD127, in contrast, was similar across the 3-day time course for both cell subsets. Following 3 days culture, both cells had similar levels of IL-2 detectable in the supernatant; therefore, the hypo-proliferative nature of the CD38+ cells was not due to a lack of IL-2 production (Fig. 2B IL-2; n=14). Levels of IL-7 were not detectable for either subset as expected, since T cells are known not to secrete IL-7 (data not shown, n=7).
Ligation of CD 38 with IB4 exacerbates the hypo-proliferative CD127high/CD25low phenotype
As CD38 is a multifunctional molecule, we investigated whether the unique phenotypic profile and hypo-proliferative capacity was due to the function of the molecule itself. CD38 belongs to the family of ectoenzymes, which are a diverse group of membrane-bound proteins that have their catalytically active sites in the extracellular environment 19. Ectoenzymes are often co-expressed therefore expression of CD38 with ectoenzymes CD39 and CD73 were investigated. While neither subset expressed CD73, the CD4+CD38+ T cells did co-express high levels of CD39 compared to a very small population within CD4+CD38− T cells (Supporting Information Fig. S2.).
Ectoenzymes are classified according to their enzymatic activites; CD38 is an ADP-ribosyl cyclase and an ADP-ribosyltransferase, and thus catalyzes the formation of several products from the substrate NADP (Nicotinamide adenine dinucleotide phosphate), including nicotinamide, cADPR, ADPR and nicotinic-acid-adenine dinucleotide 19, 20. Although cADPR makes up only 1–3% of the final product 19, it is the most commonly studied product of CD38 activity and once formed, induces intracellular calcium release 21. In order to determine whether cADPR produced by CD38 was involved in maintaining their ‘naïve’ phenotype after stimulation, we added the cADPR antagonist, 8-bromo-cADPR to CD4+CD38+ T cells for their 3 day in vitro culture with anti-CD3/CD28, and assessed expression of key surface markers. Supporting Information Fig. S3 shows that expression of CD45RA, CD25 and CD127 was not altered when cADPR activity was antagonized (1, 2 and 5 μM 8-bromo-cADPR tested and 2 μM used in Supporting Information Fig. S3).
CD38 also has a role as an adhesion molecule, with CD31 expressed on endothelial cells being a natural ligand of human CD38 14, 22. Several monoclonal antibodies mimic this natural binding and translate either an agonistic or antagonistic signal to the T cell. To investigate whether ligation of the CD38 molecule is involved in the CD4+CD38+ T cell novel characteristics, several anti-CD38 antibodies were added to the 3-day in vitro culture together with anti-CD3/CD28. While most antibodies had little effect on the CD4+CD38+ cell phenotype, ligation with the agonistic antibody clone IB4 significantly further decreased CD25 expression in 3/3 donors and significantly increased CD127 expression in 2/3 donors (Fig. 3A, n=3, p<0.05; two-way ANOVA). Expression levels of CD45RA did not change after addition of the anti-CD38 antibodies (Supporting Information Fig. S4.). Further titration of agonist IB4 demonstrated its ability to promote a CD25low/CD127high phenotype (Fig. 3B). Coinciding with the promotion of the cells' ‘naïve’ phenotype, IB4 also further exacerbated the CD4+CD38+ cells' hypo-proliferative profile as addition of IB4 at 30 μg/mL significantly decreased the CD4+CD38+ T cells' already low proliferative capacity (Fig. 3C; n=4 p<0.01; 30 μg/mL IB4 versus 30 μg/mL Isotype/Anti-CD3/CD28 alone; repeated measure one-way ANOVA).
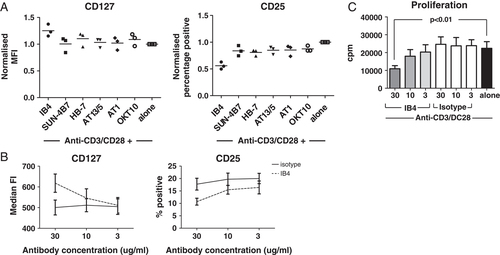
Ligation of CD38 with agonistic monoclonal antibody IB4 exacerbates CD4+CD38+ cells CD127hi CD25lo hypo-proliferative phenotype. (A) Sorted CD4+CD38+ T cells were cultured for 3 days with anti-CD3/CD28 antibodies plus 10 μg/mL of various anti-CD38 antibodies (IB4, SUN-4B7, HB-7, AT13/5, AT1 and OKT10) and their MFI expression of CD127 and percentage positive CD25 was determined using flow cytometry. (n=3, readout normalized against values for anti-CD3/CD28 alone for each donor. For CD127: IB4 versus alone p<0.05 for two out of three donors; for CD25: IB4 versus alone p<0.05 for three out of three donors; Two-way ANOVA). (B and C) The effect of agonistic monoclonal antibody IB4 on anti-CD3/CD28 stimulated CD4+CD38+ T cells was further investigated using a wider IB4 concentration range. (B) CD127 and CD25 expression was analyzed (Mean±SEM, solid line IgG2a isotype control, broken line IB4; n=5), as well as (C) the CD4+CD38+ T cells proliferative response (Mean+SEM; grey bars: IB4 30, 10 and 3 μg/mL; white bars: IgG2a isotype control, 30, 10 and 3 μg/mL; black bar: anti-CD3/CD28 alone; n=4; 30 μg/mL IB4 versus 30 μg/mL Isotype/Anti-CD3/CD28 alone p<0.01; repeated measure one-way ANOVA).
Addition of exogenous IL-7, but not IL-2, can increase the CD4+CD38+ cells' hypo-proliferative profile
The hypothesis that the hypo-proliferative phenotype of CD4+CD38+ T cells was linked to their inability to respond to IL-2, but potential responsiveness to IL-7, was confirmed by addition of exogenous recombinant cytokines to anti-CD3/CD28 stimulated cultures. Figure 4A again shows that the CD4+CD38+ T cells' proliferative capacity was significantly lower than that of CD4+CD38− T cells; however, proliferation of CD4+CD3+ T cellswas increased following addition of 1000 pg/mL rIL-7, but not 100 U rIL-2. Addition of either rIL-2 or rIL-7 to CD4+CD38− T cells had no enhancing effect, consistent with an already maximal proliferative response evident by the initial anti-CD3/CD28 stimulation. To further investigate the downstream pathways associated with IL-7 and IL-2 stimulation, STAT5 phosphorylation levels were determined following anti-CD3/CD28 stimulation with or without the addition of exogenous cytokines. Figure 4B and C show that upon anti-CD3/CD28 stimulation alone or with addition of IL-2, STAT5 phosphorylation was much higher for the CD4+CD38− cells compared with the CD4+CD38+ cells (CD38+ versus CD38− anti-CD3/CD28 (p<0.01) and with rIL-2 (p<0.001)). When rIL-7 was added, however, STAT5 phosphorylation levels for both subsets were very high and comparable, indicating that the CD4+CD38+ cells require IL-7 to induce STAT5-mediated signaling for TCR-induced proliferation.
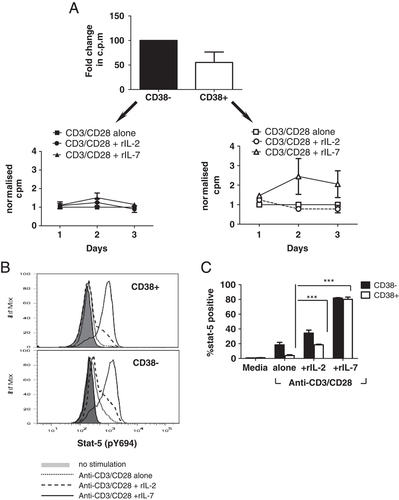
The hypo-proliferative profile of the CD4+CD38+ T cells can be reversed with addition of exogenous IL-7, but not IL-2. (A) Isolated CD4+CD38+ and CD4+CD38− T cells were stimulated with anti-CD3/CD28 antibodies for 3 days, alone or together with either 1000 pg/mL rIL7 or 100 U rIL2, and cell proliferation measured. (Mean+SEM, c.p.m for each donor for each day was normalized against c.p.m for anti-CD3/CD28 alone n=3). (B and C) Isolated CD4+CD38+ and CD4+CD38− T cells were either left unstimulated or stimulated with anti-CD3/CD28 antibodies alone, or together with 100 U rIL-2 or 1000 ρg/mL rIL-7, for 5 h and the level of STAT5 phosphorylation was determined using intracellular flow cytometry. (B) A representative plot of n=3 donors. (C) The graph shows the percentage of STAT5-positive CD4+CD38− (black bars) and CD4+CD38+ (white bars) T cells (Mean+SEM; n=3; ***p<0.001 two-way ANOVA.
IL-7 is known to induce proliferation of naïve T cells and also promotes cell survival by up-regulating expression of anti-apoptotic marker Bcl-2 23, 24. Figure 5A shows that following 3 day in vitro culture with anti-CD3/CD28, CD4+CD38+ T cells have a higher percentage of Bcl-2 compared with the CD4+CD38− T cells (n=3; p=0.0013 paired t-test). This high level of Bcl-2 expression was not further increased by addition of exogenous IL-2 or IL-7 at various concentrations (Fig. 5B). By contrast, as previously noted, proliferation was significantly increased after the addition of 1000 pg/mL rIL-7, suggesting that Bcl-2 expression may be already at a maximal level and cannot be further increased.
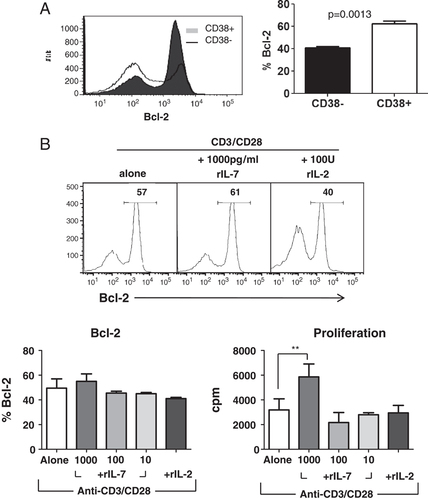
The anti-apoptotic potential of the CD4+CD38+ cells is significantly greater than that of CD4+CD38− cells. (A) Following 3 days of stimulation with anti-CD3/CD28 antibodies, intracellular expression of the anti-apoptotic marker Bcl-2 was determined for CD4+CD38+ and CD4+CD38− T cells. The histogram (left) is a representative of n=3 donors. The graph (right) shows percentage of Bcl-2-expressing cells for each cell type and is the mean+SEM of n=3 donors from independent experiments; p=0.0013 paired t-test). (B) Bcl-2 expression, as well as proliferative response, was further analyzed in CD4+CD38+ T cells stimulated with anti-CD3/CD28 antibodies alone, or either plus rIL-7 or rIL2, (Mean+SEM; n=2 Blc-2, n=5 proliferation; **p<0.01 repeated measures one-way ANOVA).
CD4+CD38+ T cells secrete significantly higher levels of IL-13
Despite their hypo-proliferative profile and their ‘naive’ T-cell phenotype, the CD4+CD38+ T cells do have some functionality as they display a unique cytokine profile following stimulation. They secrete significantly higher levels of IL-13 (p=0.0225; n=16) and significantly lower levels of IFN-γ (p<0.0001, n=16) and TNF (p=0.0204, n=14) compared with CD4+CD38− cells (Fig. 6A). This cytokine bias was highlighted when the ratio of IL-13 to IFN-γ was determined for each donor and found to be significantly higher for CD38+ cells compared with CD38− cells (Fig. 6B, n=18; p=0.0018 paired t-test). Moreover, the addition of 1000 ρg/mL IL-7 did not alter this ratio (n=6, p=0.0002 paired t-test). The CD4+CD38+ T cells' IL-13 secretion bias was not coupled with secretion of other Th2 cytokines such as IL-4 and IL-5, suggesting that these cells have a specific role related to IL-13 secretion that is independent of a generalized Th2 response.
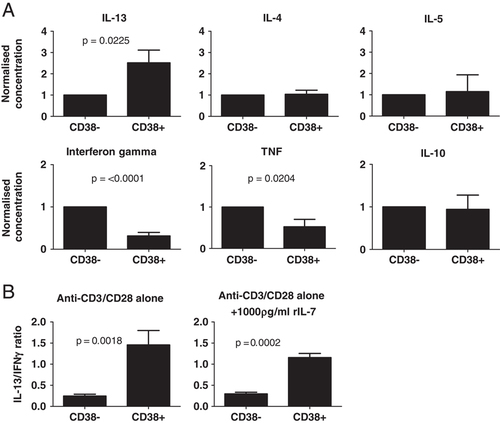
CD4+CD38+ T cells secrete significantly higher levels of IL-13 than CD4+CD38− T cells, and significantly lower levels of IFN-γ. (A) Sorted CD4+CD38+ and CD4+CD38− T cells were cultured for 3 days with anti-CD3/CD28 antibodies and the concentration of various secreted cytokines, within the culture supernatants, was determined (CD38+ concentrations were normalized against CD38− concentrations for each cytokine, for each donor; Mean+SEM; IL-13: p=0.0225, n=16; IFN-γ: p<0.0001, n=16; TNF: p=0.0204, n=14; IL-4: n=13; IL-5: n=9; IL-10: n=14; paired t-test). (B) Using the raw concentrations, the ratio of secreted IL-13 to IFN-γ was determined when cells were stimulated with anti-CD3/CD28 antibodies alone (left) (Mean+SEM; n=18; p=0.0018; paired t-test), or together with 1000 pg/mL rIL-7 (right) (Mean+SEM; n=6; p=0.0002 paired t-test). There were no significant correlations for IL-13/TNF or IL-4/IFN-γ between CD38+ and CD38− cells.
Discussion
A subset of T cells that express CD38 and display unique activation properties is described herein. Following in vitro stimulation via TCR, the CD4+CD38+ T cells retained high levels of CD45RA and Bcl-2, low levels of CD25 and CD45RO and were hypo-proliferative. Ligation of the CD38 molecule with agonistic monoclonal antibody IB4 exacerbated this low proliferation profile and the CD25low/CD127high profile. CD4+CD38+ T-cell proliferation and STAT5 phosphorylation could be increased following addition of rIL-7, but not rIL-2. The CD4+CD38+ T cell were not totally inactive, however, as they secreted high levels of IL-13, compared with the Th1-biased CD4+CD38− T cells, while not secreting other Th2 cytokines.
CD38 was first observed on T lymphocytes in 1980 25 and has since been found to be expressed on a variety of immune cells 20. While CD38 is commonly used to identify stimulated T cells in both mice 26 and humans 27, an association between CD38 and CD45RA expression on CD4+ T cells has been previously reported in both peripheral blood 16 and cord blood 28. In our study, circulating CD4+CD38+ T cells from healthy donors contained a greater percentage of CD45RA+CD45RO− cells while the CD38− cells contained a greater percentage of CD45RA−CD45RO+ cells. Upon isolation and stimulation with anti-CD3/CD28 for 3 days, CD4+CD38+ T cells displayed a significant hypo-proliferative profile compared with the CD4+CD38− cells, even though T-cell stimulation via a non-TCR pathway (PMA/Ionomycin) showed that the cells were equally capable of proliferating. Hypo-proliferative CD4+CD45RA+ T cells have been previously reported 16; however, hypo-proliferative CD4+CD38+ T cells have not. As we found that ligation of CD38 with an agonistic monoclonal antibody IB4 enhanced this hypo-proliferative phenotype, this strongly suggests that the CD38 molecule may be directly associated with signaling via TCR. Previous studies in both T cells and T-cell lines have shown that CD38 is closely associated with the TCR/CD3 complex 29 as signaling through either molecule induced phosphorylation of an intracellular protein involved in cell activation, phospholipase C-γ 30. Based on our findings, we hypothesize that while lack of responsiveness to IL-2 contributes to the CD4+CD38+ T cells' hypo-proliferative phenotype, engagement of the CD38 molecule itself may be additionally involved in regulating intracellular pathways following TCR stimulation. By contrast, our findings suggest that the enzymatic activity of CD38, leading to changed levels of cADPR, is not involved in enhancing the cells' ‘naive’ phenotype. Nevertheless, it is still possible that other less direct enzymatic pathways, perhaps linked to the co-expression of other ectoenzymes may be involved. In this context it is interesting to note that we found CD39 expression preferentially associated with CD4+CD38+ T cells rather than CD4+CD38− cells. CD39 is a nucleoside triphosphate diphosphohydrolase that converts ATP/ADP to AMP, which is then degraded into nucleosides such as adenosine by ectoenzyme CD73 (reviewed in 19). Since CD4+CD38+ cells did not further co-express CD73, it is possible that AMP may accumulate in culture and future studies could determine its range of modulatory effects on TCR transduction pathways.
The observation that CD4+CD38+ T cells' limited proliferative capacity could be reversed by the addition of exogenous IL-7, but not IL-2, further demonstrates the cells' unique activation profile. Naïve cells initially rely on IL-7 for their survival and proliferation 31 and once activated, switch to IL-2 dependency as indicated by down-regulation of CD127 and up-regulation of CD25. Following this effector phase, IL-7 once again becomes important as it supports memory CD4+ T-cell expansion, survival and homeostasis (reviewed in 32). As T cells do not to produce IL-7, the CD38+ cells cannot regulate their own proliferative capacity, unlike conventional T cells that produce and consume IL-2. Therefore, CD4+CD38+ T cells require other cells, such as stromal cells, to supply IL-7 in vivo in order to undergo proliferation and cell expansion. Interestingly, IL-7 has been shown to sustain the expression of CD38 ligand, CD31, on naive adult T cells and also promotes their proliferation, without decreasing their CD31 expression levels 33. In this context, it is interesting to note that ligation of CD38 with the IB4 further increased CD127 and decreased CD25 expression. CD31+ naive T cells are thought to be recent thymic emigrants 34 and CD31 expression is lost on T cells following TCR stimulation 35. It is thus tempting to speculate that part of IL-7 regulation of CD4+CD38+ T cells is to promote other T cells to express its ligand CD31, which in turn can feed back to enhance its responsiveness to IL-7. The CD4+CD38+ T cells also seemed to have a high threshold for IL-7 responsiveness as 1000 pg/mL (1 ng) was required to induce proliferation, whereas 100 pg/mL and 10 ρg/mL doses had no effect, suggesting that expansion of the CD4+CD38+ T cells may only occur in vivo when levels of IL-7 are particularly high.
The proliferation profile and bias to respond to IL-7 raises questions as to where the CD4+CD38+ T cells fit in to classical naive/effector/memory cell subsets. Their retention of high levels of CD45RA and lower levels of CD45RO following in vitro stimulation also challenges the classical T-cell activation dogma that states that the high molecular mass isoform containing the A exon (CD45RA) is commonly expressed on naive T lymphocytes and following activation, the low molecular mass 180-kDa isoform is expressed (CD45RO) and maintained on the majority of primed cells 9, 10. While this is the classical phenotype switch that occurs after activation, it is known that the CD45RA/CD45RO switch in CD8+ T cells does not always follow this pattern, as CD8+CD45RA+ cells stimulated with PHA were found to retain CD45RA expression while co-expressing memory T-cell marker CD11a 36. Also, CD4+CD45RA+ cells with functional properties have been identified and include IL-13-secreting CD4+CD45RA+ cells 37 as well as memory T cells that respond to recall antigens 38. Taken together, our data demonstrate that within the CD4+ T-cell population, CD38+ T cells that have been stimulated via the TCR do not acquire full/classical effector capacity. Their high level of Bcl-2 expression also ties in with their unique activation state as they appear to be remaining in a ‘static’ state, not progressing to classical activation and subsequent activation-induced cell death. Their potential to migrate to the SLO, through expression of CD62L and CCR7, may be tied in with their responsiveness and/or function as migration would allow for contact with stromal cell derived IL-7 for their expansion, and/or migrate to areas such as the gut epithelia or lung, where they could mediate localized IL-13 secretion. It will be important to directly assess the migratory capacity of CD4+CD38+ cells in vivo in future studies to further understand the cells role in vivo.
One clear function of CD4+CD38+ T cells that was observed was their bias for IL-13 secretion over IFN-γ and TNF, which was not coupled with secretion of other Th2 cytokines IL-4 and IL-5. Collectively, Th2 cytokines are involved in promoting humoral immunity including induction of IgE synthesis, promoting MHC class II expression, inhibition of inflammatory cytokine production and mediating the growth and survival of eosinophils 39-41. While early studies suggested functional redundancy between Th2 cytokines IL-13 and IL-4, 42, animal models have helped to clarify distinct roles for IL-13, including initiating and progressing allergic asthma and airway hyper-responsiveness in mice 43 and as a crucial factor in clearing several parasitic infections (reviewed in 39). Interestingly, primed CD4+CD45RA+ T cells that secrete IL-13 and fail to up-regulate CD45RO have been reported previously 37. The biological significance of IL-13-secreting CD4+ T cells that respond to IL-7 and can migrate to SLO may lay in their capacity to potentially interact with B cells. B-cell exposure to IL-13 is known to induce IgG4 and IgE synthesis and direct IgE isotype switching in human B cells 44. Interestingly, a Th2 bias coupled with high serum IgE levels has been reported in patients with HIV 45, and high levels of CD38 expression on T cells is widely known to correlate directly with poor HIV prognosis 46, 47. This link suggests that there may be a direct correlation between CD38+ T cells and secretion of IgE. As IgE is also known to play a role in allergic conditions such as asthma, future studies could investigate the potential role of CD4+CD38+ (CD45RA+CD62L+CCR7+) cells and their responsiveness to IL-7 in these disease models.
While there are conventional models for T-cell activation, it is important to acknowledge that alternative pathways may exist and certain cells may be geared to utilize certain pathways. We show that ‘non-classical’ CD4+ T cells do exist and these CD38+ cells display characteristics that fall between classical resting/naive and effector/memory cells.
Materials and methods
Study subjects
Buffy coats were obtained from routine blood bank donations from anonymous healthy adult donors acquired by the Australian Red Cross Blood Service (Approval number 05-04VIC-08/09-02VIC-01). PBMCs were separated through Ficoll–Paque (Amersham Pharmacia Biotech, Sweden) density gradients. Local Ethical Committee approval was received for these studies.
Cell isolation/purification
CD4+ T cells were purified from PBMC using human CD4 negative isolation kits (Miltenyi Biotech, Germany) according to manufacturer's guidelines. Flow cytometry analysis established that the purified fraction consistently contained 92–99% CD3+CD4+ T cells. Purified CD4+ T cells were first depleted of CD25+ cells using anti-CD25 PE antibody (BD Pharmingen, USA) and anti-PE beads (Miltenyi Biotech) on an LD column to gain the CD25− fraction, to deplete recently activated cells and/or regulatory cells. The CD38+ and CD38− sub-fractions were subsequently sorted using either anti-CD38 FITC or APC antibody (BD Pharmingen) and anti-FITC beads or anti-APC beads (Miltenyi Biotech) and run on an MS column (Miltenyi Biotech) to isolate CD38+ cells, followed by an LD column (Miltenyi Biotech) to sort the CD38− cells.
Stimulation assays
The proliferative capacity of sorted populations (1×105 cells/50 μL) was tested by stimulation with 50 ng/mL PMA and 1 μg/mL Ionomycin for 3 days. TCR stimulation was tested by adding sorted cells into 96-well U-bottom plates pre-coated with anti-CD3 (2.5 μg/mL clone OKT3, Biolegend) together with soluble 1.25 μg/mL anti-CD28 (clone CD28.2; BD Pharmingen), in the presence or absence of recombinant human IL-7 (1000, 100, 10 pg/mL; R&D systems) or recombinant human IL-2 (100 U; R&D systems) (The efficacy of these cytokines were initially tested on CD4+ T cells stimulated for 18 h with anti-CD3/CD28 plus either rIL-2 or rIL-7 and both cytokines enhanced the T-cell expression of CD25; Supporting Information data S5.). Anti-CD38 antibodies were kindly donated by Professor Fabio Malavasi, Universita Di Torino: IB4, SUN-4B7, HB-7, AT13/5, AT1 and OKT10. All antibodies were used at a final concentration of 10 μg/mL and were purified and sterile, with the exception of OKT10 (ascites+NaN3), which was used at 1/500. Further analyses of IB4 were compared to isotype control mIgG2a (10 μg/mL). On day 3 of culture, 20 μL of supernatant was collected from triplicates for cytokine analysis. Tritiated thymidine (TRK 120 Amersham Life Sciences, UK) was then added to achieve a final concentration of 1 μCi/mL, and plates incubated overnight. AIM-V medium (Invitrogen) supplemented with 5% normal human AB serum (NHS, Sigma) was used for all assays.
Flow cytometry
In total, 105–106 cells were blocked with PBS/5% NHS for 30 min on ice, then incubated with appropriate dilutions of fluorochrome-labelled antibodies (BD Pharmingen) diluted in PBS/2% FCS. Intracellular staining for Bcl-2 was performed using the eBioscience fixation/permeabilization buffer kit. Cells were washed and resuspended in 1% paraformaldehyde solution and acquired on a FACSARIA or an LSRII (Becton Dickinson, USA), collecting a minimum of 50 000 events per sample. Data were analyzed using the FlowJo software (Treestar).
STAT5 tyrosine phosphorylation analysis
Sorted cells were incubated for 5 h either with no stimulus, anti-CD3/CD28 alone or together with either 1000 pg/mL rIL-7 or 100 U rIL-2, washed with cold PBS/2% NHS and blocked on ice with PBS/2% NHS for 10 min. The cells were washed, then resuspended in 50 μL warm PBS/2% NHS and left for 5 min at 37°C. Aliquots of 50 μL of Cytofix (BD Pharmingen) were added for a further 10 min at 37°C, then cells were washed and 100 μL Perm Buffer III (BD Pharmingen) was added for 30 min on ice. Cells were washed and incubated with anti-phospho-STAT5 (pY694) antibody coupled to Alexa Fluor 488 at room temperature for 30 min. Samples were acquired on LSRII (Becton Dickinson) collecting a minimum of 50 000 events per sample. Data was analyzed using the FlowJo software (Treestar).
Cytokine analysis
Cytometric bead array Flex sets for IL-4, IL-5, IL-10, IL-13, IFN-γ, TNF, IL-2 and IL-7 (Pharmingen, BD Biosciences) were used to determine the concentration of secreted cytokines in cell culture supernatants. Standards and samples were prepared according to the manufacturer's recommendations. Samples were run on the FACSCanto or LSRII (Becton Dickinson), collecting 3000–5000 events per flex set and data were analyzed using BD Cellquest software.
Statistical analysis
Statistical significance was determined using a two-way ANOVA with a Bonferoni post-test for time-course studies and repeated measures one-way ANOVA with Bonferoni post-test for multiple group analysis. For CD38+ versus CD38− comparisons, a paired parametric two-tailed Student's t-test was used for normalized data. Phenotype data are displayed as average MFI or percentage positive for 4–11 donors±SEM. All p values are cited in the text except those of p>0.05.
Acknowledgements
We would like to thank Professor Jennifer Rolland and Dr. Anja Scholzen for their critical feedback and advice regarding this manuscript and Chindu Govindaraj for her technical assistance. This work was funded by grants from the National Health and Medical Research Council of Australia (NHMRC). M.P. is an NHMRC Senior Fellow.
Conflict of interest: The authors declare no financial or commercial conflict of interest.