IKK-β-mediated myeloid cell activation exacerbates inflammation and inhibits recovery after spinal cord injury
Abstract
Traumatic spinal cord injury (SCI) is followed by massive infiltration and activation of myeloid cells such as neutrophils and macrophages, but the functions of these cells are controversial. In this study, our objective was to elucidate the in vivo role of a signaling pathway involved in activation of these innate immune cells in SCI using myeloid cell-specific IκB kinase (IKK)-β conditional knockout (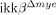 ) mice. In these mice, the ikkβ gene has been specifically deleted from myeloid cells, compromising their in vivo IKK/NF-κB-dependent activation. We found that
) mice. In these mice, the ikkβ gene has been specifically deleted from myeloid cells, compromising their in vivo IKK/NF-κB-dependent activation. We found that 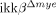 mice had significantly reduced neutrophil and macrophage infiltrations after SCI compared to ikkβ+/+ controls. SCI-induced proinflammatory gene expression was also reduced in
mice had significantly reduced neutrophil and macrophage infiltrations after SCI compared to ikkβ+/+ controls. SCI-induced proinflammatory gene expression was also reduced in 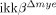 mice. Reduced neuroinflammation in
mice. Reduced neuroinflammation in 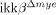 mice was accompanied by attenuated neuronal loss and behavioral deficits in motor activity. In addition, the SCI-induced expression of CXC ligand 1 was reduced in
mice was accompanied by attenuated neuronal loss and behavioral deficits in motor activity. In addition, the SCI-induced expression of CXC ligand 1 was reduced in 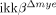 mice, which may be responsible for the reduced neutrophil infiltration in these mice. Our data demonstrate that IKK-β-dependent myeloid cell activation potentiates neuroinflammation and neuronal damage after SCI.
mice, which may be responsible for the reduced neutrophil infiltration in these mice. Our data demonstrate that IKK-β-dependent myeloid cell activation potentiates neuroinflammation and neuronal damage after SCI.
Introduction
Innate immune cells are actively involved in pathogenesis and recovery after spinal cord injury (SCI). In a rat SCI model, neutrophils infiltrate the primary lesion site within several hours, peak at 12–24 h and disappear after several days 1. Similarly, blood-derived monocytes/macrophages are detected in and around the lesion 2–3 days after injury and may persist for weeks 2. Studies on the roles of these immune cells indicate that myeloid cells in the spinal cord produce putative neurotoxic mediators, such as reactive oxygen species (ROS), cytokines and proteases, and thereby contribute to secondary damage after SCI 3. The neurotoxic effects of neutrophils were originally suggested by studies that blocked cell adhesion molecules with antibodies 1, 4. Administering anti-P-selectin or anti-ICAM-1 antibodies to SCI animal models reduced neutrophil infiltration into the spinal cord and attenuated tissue damage. Similarly, depleting blood monocytes by administering clodronate liposomes improved recovery of functional activity after SCI, indicating that blood-derived macrophages contribute to tissue damage 5.
Activated neutrophils and macrophages, on the other hand, not only express tissue-damaging molecules but also produce neurotrophic and anti-oxidant molecules 6, 7. In addition, macrophages contribute to neuroregeneration by removing myelin debris, which inhibits neurite outgrowth 8. Based on these findings, intralesional neutrophil/macrophage infiltration was argued to protect the spinal cord from secondary damage and facilitate recovery after SCI. In support of this model, depletion of blood neutrophils by i.v. administration of anti-Ly6G antibody was found to amplify neurological deficit after SCI 9. Activated macrophages can also be neuroprotective, as evidenced by a study in which implanting activated macrophages post-injury improved locomotive recovery 10. Such conflicting reports indicate that the in vivo roles of neutrophil/macrophage activation and infiltration in SCI are controversial and far from understood.
In this study, we revisited this question using IκB kinase (IKK)-β conditional knockout mice (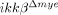 ), in which the ikkβ gene has been specifically deleted from myeloid cells, including the majority of neutrophil and macrophage populations 11. IKK-β is a protein kinase responsible for NF-κB activation via various inflammatory stimuli 12; NF-κB is a key transcription factor responsible for the expressions of inflammatory genes and adhesion molecules 13. We reasoned that the ikkβ deficiency would interfere with neutrophil/macrophage activation after SCI, allowing for the investigation of the in vivo role of IKK-β/NF-κB-mediated neutrophil and macrophage activation in SCI.
), in which the ikkβ gene has been specifically deleted from myeloid cells, including the majority of neutrophil and macrophage populations 11. IKK-β is a protein kinase responsible for NF-κB activation via various inflammatory stimuli 12; NF-κB is a key transcription factor responsible for the expressions of inflammatory genes and adhesion molecules 13. We reasoned that the ikkβ deficiency would interfere with neutrophil/macrophage activation after SCI, allowing for the investigation of the in vivo role of IKK-β/NF-κB-mediated neutrophil and macrophage activation in SCI.
Results
Attenuated myeloid cell infiltration in 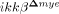 mice after SCI
mice after SCI
To study the in vivo role of IKK-β-mediated myeloid cell activation in SCI, we used a well-characterized SCI model, spinal cord hemisection at the T9 level 14 using wild-type (ikkβ+/+) and myeloid cell-specific IKK-β deficient (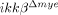 ) mice. Neutrophils infiltrated into the injured spinal cord tissue were counted using flow cytometry. Gr-1+ neutrophils were detected in ikkβ+/+ mouse spinal cord tissue 6 h post-injury, with numbers further increasing after 24 h (3.0% of total counted cells at 6 h and 5.6% at 24 h) (Fig. 1A and B). In comparison, the neutrophil number in
) mice. Neutrophils infiltrated into the injured spinal cord tissue were counted using flow cytometry. Gr-1+ neutrophils were detected in ikkβ+/+ mouse spinal cord tissue 6 h post-injury, with numbers further increasing after 24 h (3.0% of total counted cells at 6 h and 5.6% at 24 h) (Fig. 1A and B). In comparison, the neutrophil number in 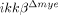 mouse spinal cord tissue was significantly less, 52 and 59% lower than those in ikkβ+/+ mice at 6 and 24 h respectively (Fig. 1A and B). No Gr-1+ cells were detected in spinal cords of sham-operated mice of either genotype (data not shown). Neutrophil infiltration in
mouse spinal cord tissue was significantly less, 52 and 59% lower than those in ikkβ+/+ mice at 6 and 24 h respectively (Fig. 1A and B). No Gr-1+ cells were detected in spinal cords of sham-operated mice of either genotype (data not shown). Neutrophil infiltration in 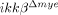 mice was similarly reduced in another SCI model, spinal cord crush injury (Supporting Information Fig. 1A). Macrophages infiltrated the lesion site at a considerably later time point following SCI. By flow cytometry, CD11b+/CD45high macrophages 15 in the injured spinal cord were found to increase in number to 2.6% at 5 days post-injury (dpi) in ikkβ+/+ mice (Fig. 2C and D), and only increased to 1.2% in
mice was similarly reduced in another SCI model, spinal cord crush injury (Supporting Information Fig. 1A). Macrophages infiltrated the lesion site at a considerably later time point following SCI. By flow cytometry, CD11b+/CD45high macrophages 15 in the injured spinal cord were found to increase in number to 2.6% at 5 days post-injury (dpi) in ikkβ+/+ mice (Fig. 2C and D), and only increased to 1.2% in 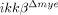 mice. The proportion of CD11b+/CD45low cells, which represent resident microglia 15, did not change significantly after SCI in either ikkβ+/+ or
mice. The proportion of CD11b+/CD45low cells, which represent resident microglia 15, did not change significantly after SCI in either ikkβ+/+ or 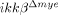 mice. It has been reported that macrophages exert different effects on spinal cord injuries depending on their activation type (M1 versus M2) 16. To characterize the type of tissue-infiltrating macrophages, we immunostained injured spinal cord tissues for iNOS and arginase 1, M1 and M2 macrophage markers, respectively. CD68+ macrophages in both ikkβ+/+ and
mice. It has been reported that macrophages exert different effects on spinal cord injuries depending on their activation type (M1 versus M2) 16. To characterize the type of tissue-infiltrating macrophages, we immunostained injured spinal cord tissues for iNOS and arginase 1, M1 and M2 macrophage markers, respectively. CD68+ macrophages in both ikkβ+/+ and 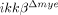 mice expressed iNOS but not Arginase 1 (Fig. 1E) at 5 dpi, indicating that these macrophages were mostly M1 macrophages. Taken together, these data show that deleting ikkβ from myeloid cells reduced neutrophil/macrophage infiltration into the spinal cord after SCI.
mice expressed iNOS but not Arginase 1 (Fig. 1E) at 5 dpi, indicating that these macrophages were mostly M1 macrophages. Taken together, these data show that deleting ikkβ from myeloid cells reduced neutrophil/macrophage infiltration into the spinal cord after SCI.
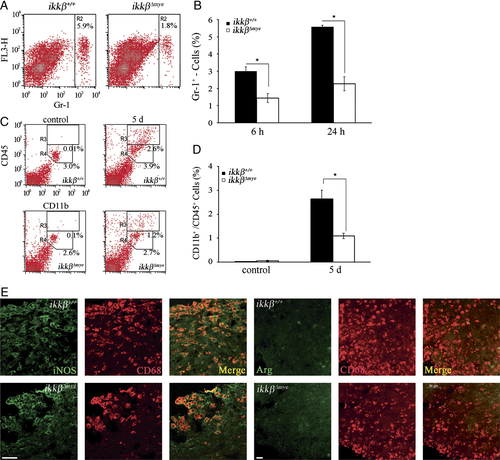
Neutrophil and macrophage infiltrations are reduced in 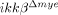 mice. (A) To quantify neutrophils, spinal cord cells were analyzed using flow cytometry with Gr-1 antibody 24 h post-injury. Representative data from three independent experiments with similar results are shown. (B) The graph shows the percentages of Gr-1+ neutrophils 6 and 24 h post-injury. The data are mean±SEM of three independent experiments (*p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (C and D) CD11b+/CD45high macrophage population (R3 gate) and CD11b+/CD45low microglia populations (R4 gate) were analyzed in the injured spinal cords of ikkβ+/+ mice and
mice. (A) To quantify neutrophils, spinal cord cells were analyzed using flow cytometry with Gr-1 antibody 24 h post-injury. Representative data from three independent experiments with similar results are shown. (B) The graph shows the percentages of Gr-1+ neutrophils 6 and 24 h post-injury. The data are mean±SEM of three independent experiments (*p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (C and D) CD11b+/CD45high macrophage population (R3 gate) and CD11b+/CD45low microglia populations (R4 gate) were analyzed in the injured spinal cords of ikkβ+/+ mice and 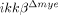 mice at 5 dpi using flow cytometry. Representative data from three independent experiments are shown (C), and the mean±SEM of three independent experiments are shown in a graph (D) (*p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (E) CD68+ (red) macrophages in the injured spinal cord sections of ikkβ+/+ mice and
mice at 5 dpi using flow cytometry. Representative data from three independent experiments are shown (C), and the mean±SEM of three independent experiments are shown in a graph (D) (*p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (E) CD68+ (red) macrophages in the injured spinal cord sections of ikkβ+/+ mice and 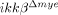 mice (5 dpi) were co-stained for iNOS (green in left panels) or Arginase 1 (Arg, green in right panels). Representative pictures from three independent experiments are shown. Scale bar, 20 μm. Original magnification, 400×.
mice (5 dpi) were co-stained for iNOS (green in left panels) or Arginase 1 (Arg, green in right panels). Representative pictures from three independent experiments are shown. Scale bar, 20 μm. Original magnification, 400×.
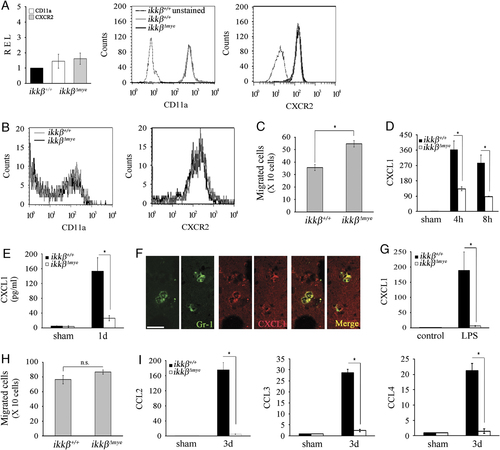
SCI-induced neutrophil-attracting chemokine expression is reduced in 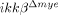 mice. (A) Total RNA was prepared from neutrophils isolated from the blood of ikkβ+/+ and
mice. (A) Total RNA was prepared from neutrophils isolated from the blood of ikkβ+/+ and 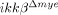 mice blood. CD11a and CXCR2 mRNA levels were measured by real-time RT-PCR (left graph). The relative gene expression levels (REL) in
mice blood. CD11a and CXCR2 mRNA levels were measured by real-time RT-PCR (left graph). The relative gene expression levels (REL) in 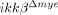 neutrophils compared to ikkβ+/+ neutrophils are presented (n=3). The data are mean±SEM of three independent experiments. CD11a and CXCR2 levels in ikkβ+/+ and
neutrophils compared to ikkβ+/+ neutrophils are presented (n=3). The data are mean±SEM of three independent experiments. CD11a and CXCR2 levels in ikkβ+/+ and 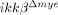 neutrophils were measured by flow cytometry (right histograms). Representative data from three independent experiments with similar results are shown. (B) CD11a and CXCR2 expressions in Gr-1+ neutrophils of injured-spinal cord tissue were analyzed by flow cytometry. Representative data from three independent experiments are shown. (C) Neutrophils isolated from ikkβ+/+ and
neutrophils were measured by flow cytometry (right histograms). Representative data from three independent experiments with similar results are shown. (B) CD11a and CXCR2 expressions in Gr-1+ neutrophils of injured-spinal cord tissue were analyzed by flow cytometry. Representative data from three independent experiments are shown. (C) Neutrophils isolated from ikkβ+/+ and 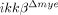 mice blood were used for migration assays. Neutrophils that migrated into the lower Transwell chamber were counted by light microscopy. (*p<0.05, one-way ANOVA followed by independent Tukey's post-hoc test). (D) CXCL1 mRNA level in injured spinal cord of ikkβ+/+ and
mice blood were used for migration assays. Neutrophils that migrated into the lower Transwell chamber were counted by light microscopy. (*p<0.05, one-way ANOVA followed by independent Tukey's post-hoc test). (D) CXCL1 mRNA level in injured spinal cord of ikkβ+/+ and 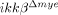 mice at 4 and 8 h was assayed using real-time RT-PCR. The data are mean±SEM of three independent experiments (*p<0.05, one-way ANOVA followed by independent Tukey's post-hoc test). (E) CXCL1 expression levels in injured spinal cord tissues at 1 dpi were measured by ELISA. The data are mean±SEM of three independent experiments (*p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (F) To test CXCL1 expression from tissue-infiltrating neutrophils, spinal cord tissue sections of ikkβ+/+ mice at 1 dpi were immunostained with anti-Gr-1 antibody (green) and anti-CXCL1 antibody (red). Representative figures from three independent experiments are shown. Scale bar, 20 μm. Original magnification, 630×; scan zoom, 2×. (G) Isolated neutrophils from blood were stimulated with LPS (50 ng/mL) for 3 h, and CXCL1 expression was measured by real-time RT-PCR. Means±SEM of three independent experiments are shown (*p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (H) Monocytes isolated from ikkβ+/+ and
mice at 4 and 8 h was assayed using real-time RT-PCR. The data are mean±SEM of three independent experiments (*p<0.05, one-way ANOVA followed by independent Tukey's post-hoc test). (E) CXCL1 expression levels in injured spinal cord tissues at 1 dpi were measured by ELISA. The data are mean±SEM of three independent experiments (*p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (F) To test CXCL1 expression from tissue-infiltrating neutrophils, spinal cord tissue sections of ikkβ+/+ mice at 1 dpi were immunostained with anti-Gr-1 antibody (green) and anti-CXCL1 antibody (red). Representative figures from three independent experiments are shown. Scale bar, 20 μm. Original magnification, 630×; scan zoom, 2×. (G) Isolated neutrophils from blood were stimulated with LPS (50 ng/mL) for 3 h, and CXCL1 expression was measured by real-time RT-PCR. Means±SEM of three independent experiments are shown (*p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (H) Monocytes isolated from ikkβ+/+ and 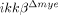 mice were used for migration assays. n.s., not significant. (I) CCL2, 3 and 4 expression in the injured spinal cords of ikkβ+/+ and
mice were used for migration assays. n.s., not significant. (I) CCL2, 3 and 4 expression in the injured spinal cords of ikkβ+/+ and 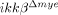 mice were measured by real-time RT-PCR. The data are mean±SEM of three independent experiments (*p<0.05, one-way ANOVA followed by independent Tukey's post-hoc test).
mice were measured by real-time RT-PCR. The data are mean±SEM of three independent experiments (*p<0.05, one-way ANOVA followed by independent Tukey's post-hoc test).
SCI-induced neutrophil chemoattractants are reduced in 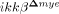 mice
mice
To test whether the reduced neutrophil infiltration in 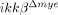 mice was due to innate characteristics of IKK-β-deficient neutrophils, we isolated neutrophils from both ikkβ+/+ and
mice was due to innate characteristics of IKK-β-deficient neutrophils, we isolated neutrophils from both ikkβ+/+ and 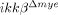 mice and assessed the expression of CD11a and CXCR2, an adhesion molecule and a chemokine receptor involved in neutrophil extravasation and migration 17. CD11a and CXCR2 expression in blood neutrophils from
mice and assessed the expression of CD11a and CXCR2, an adhesion molecule and a chemokine receptor involved in neutrophil extravasation and migration 17. CD11a and CXCR2 expression in blood neutrophils from 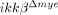 mice were comparable to ikkβ+/+ mice both at mRNA and protein levels (Fig. 2A). Similarly, CD11a and CXCR2 expression in tissue-infiltrating neutrophils after SCI were not altered in
mice were comparable to ikkβ+/+ mice both at mRNA and protein levels (Fig. 2A). Similarly, CD11a and CXCR2 expression in tissue-infiltrating neutrophils after SCI were not altered in 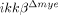 mice (Fig. 2B). We also tested the migratory activities of neutrophils from ikkβ+/+ and
mice (Fig. 2B). We also tested the migratory activities of neutrophils from ikkβ+/+ and 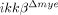 mice by incubating cells in media containing CXC ligand (CXCL) 1, a chemokine specific for neutrophil attraction using Transwell plates (Fig. 2C). Neutrophils from
mice by incubating cells in media containing CXC ligand (CXCL) 1, a chemokine specific for neutrophil attraction using Transwell plates (Fig. 2C). Neutrophils from 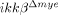 mice migrated more than cells from ikkβ+/+ mice (547±15 versus 357±13). These data suggest that the reduced neutrophil infiltration in
mice migrated more than cells from ikkβ+/+ mice (547±15 versus 357±13). These data suggest that the reduced neutrophil infiltration in 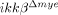 mice was not due to an altered migratory capacity of neutrophils in the blood after ikkβ deletion.
mice was not due to an altered migratory capacity of neutrophils in the blood after ikkβ deletion.
We tested whether 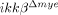 mice had altered expression of neutrophil-attracting chemokine in the injured spinal cord (Fig. 2D). At 4 h after SCI, CXCL1 mRNA levels increased by 364-fold in the injured tissue of ikkβ+/+ mice and declined slightly to 286-fold at 8 h. However, in
mice had altered expression of neutrophil-attracting chemokine in the injured spinal cord (Fig. 2D). At 4 h after SCI, CXCL1 mRNA levels increased by 364-fold in the injured tissue of ikkβ+/+ mice and declined slightly to 286-fold at 8 h. However, in 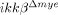 mice, CXCL1 mRNA only increased 134-fold at 4 h and 88-fold at 8 h. Similarly, CXCL1 protein expression was reduced in
mice, CXCL1 mRNA only increased 134-fold at 4 h and 88-fold at 8 h. Similarly, CXCL1 protein expression was reduced in 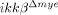 mice by more than 83% (153±82 versus 26±12) at 1 dpi (Fig. 2E). These data suggest that reduced expression of neutrophil-attracting chemokines in the injured spinal cord of
mice by more than 83% (153±82 versus 26±12) at 1 dpi (Fig. 2E). These data suggest that reduced expression of neutrophil-attracting chemokines in the injured spinal cord of  mice may, in part, account for low neutrophil infiltration in the lesion site.
mice may, in part, account for low neutrophil infiltration in the lesion site.
CXCL1 is reportedly expressed in spinal cord-infiltrating neutrophils after SCI 18. We also detected CXCL1 expression in Gr1+ neutrophils in injured spinal cords (Fig. 2F). To test whether induction of these genes in neutrophils depends on IKK-β expression, we stimulated neutrophils with LPS, and compared CXCL1 expression in vitro. In neutrophils from ikkβ+/+ mice, LPS stimulation increased CXCL1 transcript levels by 192-fold, whereas the induction was almost completely abrogated in cells from 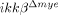 mice (Fig. 2G). Taken together, these data suggest the possibility that the first neutrophils to arrive at the SCI lesion are less activated in
mice (Fig. 2G). Taken together, these data suggest the possibility that the first neutrophils to arrive at the SCI lesion are less activated in 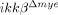 than in WT mice, resulting in reduced chemokine expression. This might further attenuate neutrophil recruitment to the lesion at later time points.
than in WT mice, resulting in reduced chemokine expression. This might further attenuate neutrophil recruitment to the lesion at later time points.
We also assessed monocyte migration in ikkβ+/+ and 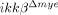 mice. Migration of
mice. Migration of 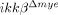 monocytes stimulated by CCL2 was not significantly different from ikkβ+/+ monocytes (Fig. 2H). Expression of CD11b or CD11c, adhesion molecules preferentially expressed on myeloid cells, was not significantly different in ikkβ+/+ or
monocytes stimulated by CCL2 was not significantly different from ikkβ+/+ monocytes (Fig. 2H). Expression of CD11b or CD11c, adhesion molecules preferentially expressed on myeloid cells, was not significantly different in ikkβ+/+ or 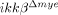 blood leukocytes (Supporting Information Fig. 2). However, CCL2, 3 and 4 expression in the injured spinal cord was almost completely blocked in
blood leukocytes (Supporting Information Fig. 2). However, CCL2, 3 and 4 expression in the injured spinal cord was almost completely blocked in 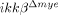 mice (Fig. 2I). These data once again suggest that reduced myeloid cell infiltration in
mice (Fig. 2I). These data once again suggest that reduced myeloid cell infiltration in 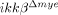 mice is not due to reduced migratory activity of the IKK-β-deficient cells but is more likely due to the reduced expression of chemoattractants in lesions of the
mice is not due to reduced migratory activity of the IKK-β-deficient cells but is more likely due to the reduced expression of chemoattractants in lesions of the 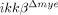 mice.
mice.
Compromise of SCI-induced neuronal cell death and behavioral deficit in 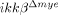 mice
mice
To address the effects of myeloid cell activation and infiltration on SCI, we measured lesion volume in the spinal cords of ikkβ+/+ and 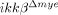 mice after hemisection injury (Fig. 3A). The lesion volume in
mice after hemisection injury (Fig. 3A). The lesion volume in 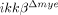 mice spinal cord was 30% less than the volume in ikkβ+/+ mice at 28 dpi. We then examined neurons in the coronal and longitudinal sections of injured spinal cords by immunohistochemistry using anti-NeuN antibody. Five days after SCI, there were 46% fewer NeuN+ neurons in the region ipsilateral to the lesion site in ikkβ+/+ mice than in sham-operated mice (Fig. 3C). However, in
mice spinal cord was 30% less than the volume in ikkβ+/+ mice at 28 dpi. We then examined neurons in the coronal and longitudinal sections of injured spinal cords by immunohistochemistry using anti-NeuN antibody. Five days after SCI, there were 46% fewer NeuN+ neurons in the region ipsilateral to the lesion site in ikkβ+/+ mice than in sham-operated mice (Fig. 3C). However, in 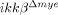 mice, more NeuN+ neurons were detected in the injured spinal cord (Fig. 3B). In
mice, more NeuN+ neurons were detected in the injured spinal cord (Fig. 3B). In 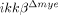 mice, there were 33% more NeuN+ neurons per unit area rostral (−500 μm) to the lesion site than in ikkβ+/+ mice (33 versus 44) (Fig. 3C). Similarly, we found an increase in NeuN+ cells in the longitudinal sections (±500 μm to the lesion site) of injured spinal cords in
mice, there were 33% more NeuN+ neurons per unit area rostral (−500 μm) to the lesion site than in ikkβ+/+ mice (33 versus 44) (Fig. 3C). Similarly, we found an increase in NeuN+ cells in the longitudinal sections (±500 μm to the lesion site) of injured spinal cords in 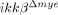 mice (Fig. 3D). Compared to
mice (Fig. 3D). Compared to  mice, the number of NeuN+ neurons in ikkβ+/+ mice was 54% (25 versus 48) at the rostral site and 49% (17 versus 37) at the caudal site (Fig. 3E). Neuronal cell death in
mice, the number of NeuN+ neurons in ikkβ+/+ mice was 54% (25 versus 48) at the rostral site and 49% (17 versus 37) at the caudal site (Fig. 3E). Neuronal cell death in  mice was also compromised after spinal cord crush injury (Supporting Information Fig. 1B). These data demonstrate that IKK-β-mediated myeloid cell activation and infiltration in injured spinal cords contribute to neuronal cell death after SCI.
mice was also compromised after spinal cord crush injury (Supporting Information Fig. 1B). These data demonstrate that IKK-β-mediated myeloid cell activation and infiltration in injured spinal cords contribute to neuronal cell death after SCI.
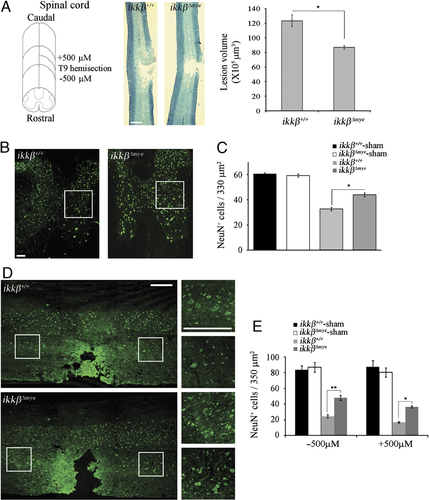
SCI-induced neuronal cell death is attenuated in  mice. (A) At 28 dpi, coronal and longitudinal sections were prepared from tissue within 500 μm of the lesion in SCI-induced or sham-operated mice. Spinal cord sections were stained with luxol fast blue. Scale bar, 500 μm. Total lesion volume was calculated from 68 sections per animal (n=3, *p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (B) Neurons in coronal sections of
mice. (A) At 28 dpi, coronal and longitudinal sections were prepared from tissue within 500 μm of the lesion in SCI-induced or sham-operated mice. Spinal cord sections were stained with luxol fast blue. Scale bar, 500 μm. Total lesion volume was calculated from 68 sections per animal (n=3, *p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (B) Neurons in coronal sections of 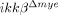 mice and ikkβ+/+ mice were detected by immunohistochemistry using anti-NeuN antibodies. Scale bar, 100 μm. Original magnification, 100×. A representative picture of a coronal section at −500 μm in the ipsilateral region is shown. (C) NeuN+ cells in the 330 μm2 white rectangle area (ipsi-region) −500 μm from the lesion site in the spinal cord of sham-operated ikkβ+/+ mice (ikkβ+/+-sham), sham-operated
mice and ikkβ+/+ mice were detected by immunohistochemistry using anti-NeuN antibodies. Scale bar, 100 μm. Original magnification, 100×. A representative picture of a coronal section at −500 μm in the ipsilateral region is shown. (C) NeuN+ cells in the 330 μm2 white rectangle area (ipsi-region) −500 μm from the lesion site in the spinal cord of sham-operated ikkβ+/+ mice (ikkβ+/+-sham), sham-operated 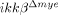 mice (
mice (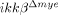 -sham), SCI-injured ikkβ+/+ mice (ikkβ+/+) and SCI-injured
-sham), SCI-injured ikkβ+/+ mice (ikkβ+/+) and SCI-injured 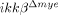 mice (
mice (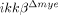 ) were quantified. Six slides from three animals were counted, and the means±SEM are presented (*p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (D) Neurons in the spinal cord longitudinal sections were detected by immunohistochemistry using anti-NeuN antibody. Scale bar, 100 μm. Original magnification, 100×. A representative image is shown. (E) NeuN+ cells in the 350 μm2 white rectangle area ±500 μm from the lesion site in the spinal cord of ikkβ+/+-sham,
) were quantified. Six slides from three animals were counted, and the means±SEM are presented (*p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (D) Neurons in the spinal cord longitudinal sections were detected by immunohistochemistry using anti-NeuN antibody. Scale bar, 100 μm. Original magnification, 100×. A representative image is shown. (E) NeuN+ cells in the 350 μm2 white rectangle area ±500 μm from the lesion site in the spinal cord of ikkβ+/+-sham, 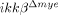 -sham, ikkβ+/+ and
-sham, ikkβ+/+ and 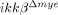 mice were quantified. Eight slides from four animals were counted, and the means±SEM are presented (*p<0.005, **p<0.05, one-way ANOVA followed by independent Tukey's post-hoc test).
mice were quantified. Eight slides from four animals were counted, and the means±SEM are presented (*p<0.005, **p<0.05, one-way ANOVA followed by independent Tukey's post-hoc test).
To characterize the nature of cell death after SCI, we measured apoptotic cells in the lesion area by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (Fig. 4A). One day after SCI, TUNEL-positive apoptotic cells were detected close to the lesion site in ikkβ+/+ mice, co-localizing with NeuN+ neurons (Fig. 4A, white arrows), shown magnified in Fig. 4B. In addition, active caspase-3 expression co-localized to NeuN+ neurons at 24 h post-injury (Fig. 4D, white arrows). Thus, neurons in the lesion underwent apoptosis 1 day after spinal hemisection injury in ikkβ+/+ mice. In 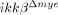 mice, however, few TUNEL+ or caspase-3+ neurons were detected at the lesion (Fig. 4A, lower panels, and D, right panel, and 4C). Since we did not detect any obvious macrophage infiltration into the lesion site within 24 h of injury, IKK-β-mediated neutrophil activation was likely involved in neuronal apoptosis at 1 dpi. This might eventually have resulted in the reduced number of neurons in ikkβ+/+ mice compared to
mice, however, few TUNEL+ or caspase-3+ neurons were detected at the lesion (Fig. 4A, lower panels, and D, right panel, and 4C). Since we did not detect any obvious macrophage infiltration into the lesion site within 24 h of injury, IKK-β-mediated neutrophil activation was likely involved in neuronal apoptosis at 1 dpi. This might eventually have resulted in the reduced number of neurons in ikkβ+/+ mice compared to  mice at the later time point.
mice at the later time point.
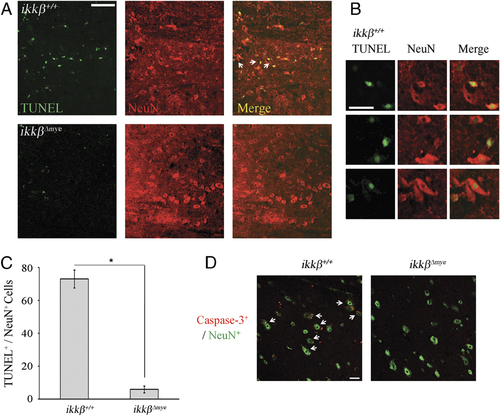
Apoptotic neuronal cell death is reduced in  mice. (A) Spinal cord sections prepared 1 dpi were used for TUNEL (green) staining, and the same sections were used for anti-NeuN (red) immunostaining. DNA fragmentation in neurons was detected at the lesion boundary of ikkβ+/+ mice. Scale bar, 50 μm. Arrows indicate areas that are magnified in (B). Original magnification, 200×. A representative figure from three independent experiments with similar results is shown. (B) Magnified images indicated by arrows in (A) Scale bar, 20 μm. (C) All TUNEL+ and NeuN+ cells in each section were counted and shown in a graph. Means±SEM of three independent experiments are shown (*p<0.005, one-way ANOVA followed by independent Tukey's post-hoc test). (D) Spinal cord sections prepared 1 dpi were immunostained for active caspase-3 (red) and NeuN+ (green). Immunoreactivity of active caspase-3 was detected in NeuN+ cells in the sections of ikkβ+/+ mice (arrows). Representative figure from three independent experiments with similar results is shown. Scale bar, 20 μm. Original magnification, 400×.
mice. (A) Spinal cord sections prepared 1 dpi were used for TUNEL (green) staining, and the same sections were used for anti-NeuN (red) immunostaining. DNA fragmentation in neurons was detected at the lesion boundary of ikkβ+/+ mice. Scale bar, 50 μm. Arrows indicate areas that are magnified in (B). Original magnification, 200×. A representative figure from three independent experiments with similar results is shown. (B) Magnified images indicated by arrows in (A) Scale bar, 20 μm. (C) All TUNEL+ and NeuN+ cells in each section were counted and shown in a graph. Means±SEM of three independent experiments are shown (*p<0.005, one-way ANOVA followed by independent Tukey's post-hoc test). (D) Spinal cord sections prepared 1 dpi were immunostained for active caspase-3 (red) and NeuN+ (green). Immunoreactivity of active caspase-3 was detected in NeuN+ cells in the sections of ikkβ+/+ mice (arrows). Representative figure from three independent experiments with similar results is shown. Scale bar, 20 μm. Original magnification, 400×.
To test whether the reduced cell death in  mice affects functional recovery from SCI, we determined the Basso Mouse Scale (BMS) scores 19 of injured mice (Fig. 5). Up to 3 days after injury, no difference in motor function was observed between ikkβ+/+ and
mice affects functional recovery from SCI, we determined the Basso Mouse Scale (BMS) scores 19 of injured mice (Fig. 5). Up to 3 days after injury, no difference in motor function was observed between ikkβ+/+ and 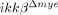 mice. After 7 days, the average BMS score of
mice. After 7 days, the average BMS score of 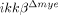 mice was higher than ikkβ+/+ mice, showing that lack of myeloid IKK-β improved recovery after SCI.
mice was higher than ikkβ+/+ mice, showing that lack of myeloid IKK-β improved recovery after SCI.
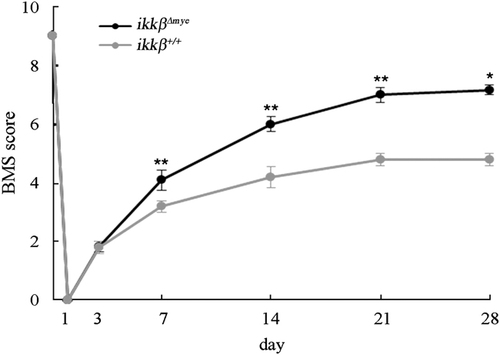
Improved functional recovery after SCI in 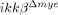 mice. BMS tests were carried out in an open field over 28 days after SCI in ikkβ+/+ (n=5) and
mice. BMS tests were carried out in an open field over 28 days after SCI in ikkβ+/+ (n=5) and 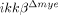 mice (n=6) (*p<0.05, **p<0.5, two-way ANOVA followed by Tukey's post-hoc test).
mice (n=6) (*p<0.05, **p<0.5, two-way ANOVA followed by Tukey's post-hoc test).
Reduced ROS and reactive nitrogen species production after SCI in 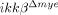 mice
mice
To identify the mechanisms underlying differences in the rates of neuronal apoptosis, we measured neurotoxic gene expression in injured spinal cord tissue. Previous studies suggest that neuronal cell death is induced by proinflammatory cytokines and ROS generated by neutrophils 20, 21. Therefore, we measured the mRNA expression of IL-6, IL-1β and TNF-α in injured spinal cords of ikkβ+/+ and 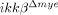 mice. Four hours after injury, when neutrophils begin to infiltrate the injured tissue, IL-6, IL-1β and TNF-α mRNAs increased by 85-, 160- and 21-fold, respectively, in ikkβ+/+ mice (Fig. 6A). However, in
mice. Four hours after injury, when neutrophils begin to infiltrate the injured tissue, IL-6, IL-1β and TNF-α mRNAs increased by 85-, 160- and 21-fold, respectively, in ikkβ+/+ mice (Fig. 6A). However, in 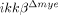 mice, the mRNA levels increased by only 18-, 43- and 3-fold respectively. Similarly, induction of iNOS, which is involved in reactive nitrogen species production, and COX-2, which is involved in ROS generation, were attenuated in
mice, the mRNA levels increased by only 18-, 43- and 3-fold respectively. Similarly, induction of iNOS, which is involved in reactive nitrogen species production, and COX-2, which is involved in ROS generation, were attenuated in  mice compared to ikkβ+/+ (two-fold versus five-fold for iNOS expression at 8 h; four-fold versus 13-fold for COX-2 expression at 4 h) (Fig. 6B and C). Nitric oxide (NO) and superoxides generated by iNOS and COX-2 may cause neuronal cell death via nitrotyrosylation and DNA damage 22, 23. To examine whether protein nitrotyrosylation and DNA damage were involved in SCI-induced neuronal death, we measured the levels of nitrotyrosylated proteins and 8-hydroxy-guanine (8-OHG) incorporation into DNA in the spinal cord by immunostaining. After injury, nitrotyrosine and 8-OHG-immunoreactive cells were detected in the lesion areas of ikkβ+/+ mice (Fig. 6D and E). The number of immunoreactive cells was much lower in
mice compared to ikkβ+/+ (two-fold versus five-fold for iNOS expression at 8 h; four-fold versus 13-fold for COX-2 expression at 4 h) (Fig. 6B and C). Nitric oxide (NO) and superoxides generated by iNOS and COX-2 may cause neuronal cell death via nitrotyrosylation and DNA damage 22, 23. To examine whether protein nitrotyrosylation and DNA damage were involved in SCI-induced neuronal death, we measured the levels of nitrotyrosylated proteins and 8-hydroxy-guanine (8-OHG) incorporation into DNA in the spinal cord by immunostaining. After injury, nitrotyrosine and 8-OHG-immunoreactive cells were detected in the lesion areas of ikkβ+/+ mice (Fig. 6D and E). The number of immunoreactive cells was much lower in 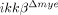 mice (Fig. 6D and E). NO activates neuronal matrix metalloproteinase-9 (MMP-9) through nitrotyrosylation, leading to neuronal apoptosis 24. To test if SCI-induced NO production and subsequent tyrosine nitrosylation leads to neuronal MMP-9 activation, we measured the MMP-9 activity in the injured spinal cord by in situ zymography (Fig. 7A). In ikkβ+/+ mice, gelatinolytic activity was detected in many cells near the lesion site, which mostly co-localized with NeuN+ neurons, but not with Gr-1+ neutrophils (Fig. 7A and B). However, the injured spinal cord of
mice (Fig. 6D and E). NO activates neuronal matrix metalloproteinase-9 (MMP-9) through nitrotyrosylation, leading to neuronal apoptosis 24. To test if SCI-induced NO production and subsequent tyrosine nitrosylation leads to neuronal MMP-9 activation, we measured the MMP-9 activity in the injured spinal cord by in situ zymography (Fig. 7A). In ikkβ+/+ mice, gelatinolytic activity was detected in many cells near the lesion site, which mostly co-localized with NeuN+ neurons, but not with Gr-1+ neutrophils (Fig. 7A and B). However, the injured spinal cord of 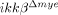 mice showed markedly reduced gelatinase-active neuronal cells (Fig. 7A, lower panels). We also measured MMP-9 mRNA in the spinal cord. MMP-9 transcripts were upregulated by ten-fold at 8 h post-injury in ikkβ+/+ spinal cords, with little or no induction in
mice showed markedly reduced gelatinase-active neuronal cells (Fig. 7A, lower panels). We also measured MMP-9 mRNA in the spinal cord. MMP-9 transcripts were upregulated by ten-fold at 8 h post-injury in ikkβ+/+ spinal cords, with little or no induction in 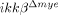 mice (Fig. 7C). These data show that increased MMP-9 expression in injured ikkβ+/+ mice might contribute to the enhanced MMP-9 activity observed in these mice. Taken together, these data imply that, in
mice (Fig. 7C). These data show that increased MMP-9 expression in injured ikkβ+/+ mice might contribute to the enhanced MMP-9 activity observed in these mice. Taken together, these data imply that, in  mice, reduced reactive nitrogen species and ROS production and MMP-9 activation might, at least in part, account for the reduced neuronal apoptosis after SCI in these mice.
mice, reduced reactive nitrogen species and ROS production and MMP-9 activation might, at least in part, account for the reduced neuronal apoptosis after SCI in these mice.
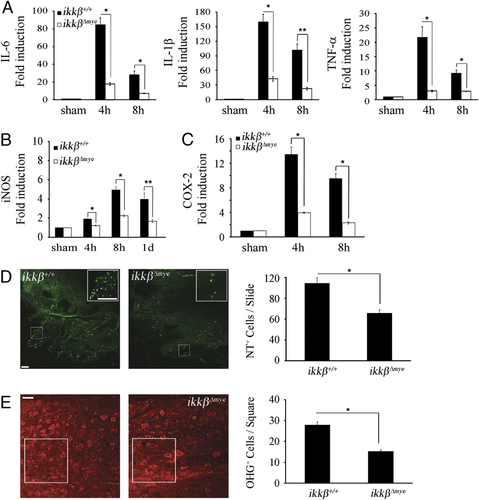
Expression of SCI-induced proinflammatory cytokines, iNOS and COX-2 are attenuated in 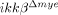 mice. (A) Spinal cords of sham-operated and SCI-injured ikkβ+/+ and
mice. (A) Spinal cords of sham-operated and SCI-injured ikkβ+/+ and 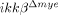 mice were isolated 4 and 8 h post-injury. Real-time RT-PCR was used to measure IL-6, IL-1β and TNF-α levels. Means±SEM of three independent experiments are shown (*p<0.05, **p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). mRNA levels of iNOS (B) and COX-2 (C) in the spinal cord at different time points after SCI, as measured by real-time RT-PCR (*p<0.05, **p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (D) Tyrosine nitrosylated-proteins in the injured spinal cords of ikkβ+/+ and
mice were isolated 4 and 8 h post-injury. Real-time RT-PCR was used to measure IL-6, IL-1β and TNF-α levels. Means±SEM of three independent experiments are shown (*p<0.05, **p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). mRNA levels of iNOS (B) and COX-2 (C) in the spinal cord at different time points after SCI, as measured by real-time RT-PCR (*p<0.05, **p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test). (D) Tyrosine nitrosylated-proteins in the injured spinal cords of ikkβ+/+ and 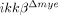 mice were detected by immunostaining using anti-nitrotyrosine antibodies. Nitrotyrosine immunoreactivity was detected as particulates in the cells surrounding the lesion site at 1 dpi in ikkβ+/+ mice. Representative figure from three independent experiments with similar results is shown. Scale bar, 20 μm. Original magnification, 100×. NT+ cells in each section were counted and are shown in a graph. Means±SEM of three independent experiments are shown (*p<0.5, one-way ANOVA followed by Tukey's post-hoc test). (E) ROS-damaged cells in the injured spinal cord were detected by immunostaining with anti-8-OHG antibodies. Representative figure from three independent experiments with similar results is shown. Scale bar, 50 μm. Original magnification, 200×. OHG+ cells in the white square area (40 000 μm2) 150 μm caudal from the spinal cord lesion site were quantified. Six slides from three animals were counted, and the means±SEM are presented (*p<0.5, one-way ANOVA followed by Tukey's post-hoc test).
mice were detected by immunostaining using anti-nitrotyrosine antibodies. Nitrotyrosine immunoreactivity was detected as particulates in the cells surrounding the lesion site at 1 dpi in ikkβ+/+ mice. Representative figure from three independent experiments with similar results is shown. Scale bar, 20 μm. Original magnification, 100×. NT+ cells in each section were counted and are shown in a graph. Means±SEM of three independent experiments are shown (*p<0.5, one-way ANOVA followed by Tukey's post-hoc test). (E) ROS-damaged cells in the injured spinal cord were detected by immunostaining with anti-8-OHG antibodies. Representative figure from three independent experiments with similar results is shown. Scale bar, 50 μm. Original magnification, 200×. OHG+ cells in the white square area (40 000 μm2) 150 μm caudal from the spinal cord lesion site were quantified. Six slides from three animals were counted, and the means±SEM are presented (*p<0.5, one-way ANOVA followed by Tukey's post-hoc test).
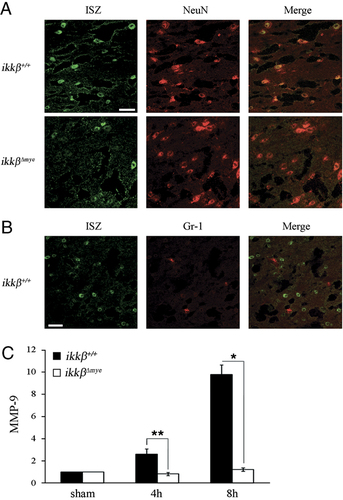
Localization of gelatinolytic activity in neurons and MMP expression after SCI. (A) Cryostat tissue sections at 6 h post-SCI were used for in situ zymography (ISZ) and NeuN immunostaining and number of cells with gelatinolytic activity was measured in 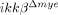 mice (lower panels) and ikkβ+/+ mice (upper panels). Representative figure from three independent experiments with similar results is shown. Scale bar, 20 μm. Original magnification, 400×. Gelatinolytic activity co-localized with NeuN+ neurons (A) but not Gr-1+ neutrophils (B). (C) Real-time RT-PCR of MMP-9 from injured spinal cord tissue of ikkβ+/+ and
mice (lower panels) and ikkβ+/+ mice (upper panels). Representative figure from three independent experiments with similar results is shown. Scale bar, 20 μm. Original magnification, 400×. Gelatinolytic activity co-localized with NeuN+ neurons (A) but not Gr-1+ neutrophils (B). (C) Real-time RT-PCR of MMP-9 from injured spinal cord tissue of ikkβ+/+ and 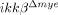 mice at 4 and 8 h after injury. The data are means±SEM of three independent experiments (*p<0.05, **p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test).
mice at 4 and 8 h after injury. The data are means±SEM of three independent experiments (*p<0.05, **p<0.5, one-way ANOVA followed by independent Tukey's post-hoc test).
Discussion
In this study, we tested the in vivo role of IKK-β activation in neutrophils responding to SCI. Previously, conflicting results suggested both beneficial and detrimental roles of neutrophil activation and infiltration after SCI. Our results showing that ablating IKK-β reduced morphological and behavioral deficits after SCI are consistent with previous studies that suggest a detrimental role for neutrophils in SCI secondary damage. Our study is distinct from a recent study that used a Ly6G/Gr-1 antibody to deplete blood neutrophils 9. In that study, Gr-1+ cells consisting of mainly, but not exclusively, neutrophils were depleted from the circulatory system. In contrast, 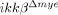 mice did not lose blood neutrophils (data not shown), and only IKK/NF-κB-mediated responses were compromised. Although it is speculative, neutrophils in a resting state might possibly have a neuroprotective role, so depleting the total neutrophil population worsens the neurological outcome after SCI.
mice did not lose blood neutrophils (data not shown), and only IKK/NF-κB-mediated responses were compromised. Although it is speculative, neutrophils in a resting state might possibly have a neuroprotective role, so depleting the total neutrophil population worsens the neurological outcome after SCI.
In 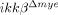 mice, the IKK-β gene was deleted not only in neutrophils but also in a majority of monocytes/macrophages. Therefore, the phenotype observed in these mice cannot be attributed solely to IKK-β deficiency in neutrophils. In our study, however, leukocytes infiltrating the lesion at early time points were mostly neutrophils, and we did not detect any macrophage infiltration within 24 h post-injury. Neuronal apoptosis was clear at 24 h post-injury in ikkβ+/+ but not
mice, the IKK-β gene was deleted not only in neutrophils but also in a majority of monocytes/macrophages. Therefore, the phenotype observed in these mice cannot be attributed solely to IKK-β deficiency in neutrophils. In our study, however, leukocytes infiltrating the lesion at early time points were mostly neutrophils, and we did not detect any macrophage infiltration within 24 h post-injury. Neuronal apoptosis was clear at 24 h post-injury in ikkβ+/+ but not 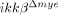 mice. These findings argue that the early effects on neuronal apoptosis were most likely due to neutrophil activation and infiltration, not macrophages. Therefore, we deduced that IKK-β-dependent neutrophil activation contributed to neuronal damage at early time points. In
mice. These findings argue that the early effects on neuronal apoptosis were most likely due to neutrophil activation and infiltration, not macrophages. Therefore, we deduced that IKK-β-dependent neutrophil activation contributed to neuronal damage at early time points. In 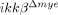 mice, macrophage infiltration was also reduced at later time points. It has been reported that macrophage infiltration impedes functional recovery after SCI 5. Therefore, the relative improvement in behavioral recovery observed in
mice, macrophage infiltration was also reduced at later time points. It has been reported that macrophage infiltration impedes functional recovery after SCI 5. Therefore, the relative improvement in behavioral recovery observed in 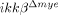 mice cannot be totally attributed to inhibiting neutrophil activation and infiltration. Rather, it might be the result of combined neutrophil and macrophage activation. Thus far, we have not been able to dissect the relative contributions of each cell type to behavioral deficits. Of note, an approximately 50% reduction in neuronal cell number was observed in the injured spinal cords of
mice cannot be totally attributed to inhibiting neutrophil activation and infiltration. Rather, it might be the result of combined neutrophil and macrophage activation. Thus far, we have not been able to dissect the relative contributions of each cell type to behavioral deficits. Of note, an approximately 50% reduction in neuronal cell number was observed in the injured spinal cords of 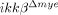 mice compared to sham-operated mice, as measured by NeuN-immunostaining, but very few TUNEL-positive cells were detected in
mice compared to sham-operated mice, as measured by NeuN-immunostaining, but very few TUNEL-positive cells were detected in 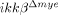 mice at 1 dpi. These data imply that neuronal cells also underwent non-apoptotic cell death in these mice, and this cell death was not greatly affected by neutrophil infiltration and activation.
mice at 1 dpi. These data imply that neuronal cells also underwent non-apoptotic cell death in these mice, and this cell death was not greatly affected by neutrophil infiltration and activation.
To elucidate the molecular mechanisms underlying the reduced neutrophil and macrophage infiltrations in 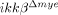 mice, we tested the innate migratory activities of neutrophils and monocytes from
mice, we tested the innate migratory activities of neutrophils and monocytes from 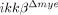 mice. Our data showed that IKK-β deficiency did not alter the migratory activities of these cells. Therefore, neutrophil- and macrophage-recruitment are more likely impaired in
mice. Our data showed that IKK-β deficiency did not alter the migratory activities of these cells. Therefore, neutrophil- and macrophage-recruitment are more likely impaired in 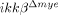 mice due to defective production of neutrophil- and macrophage-attracting chemokines after SCI. Indeed we found that SCI-induced CXCL1 expression is severely compromised in
mice due to defective production of neutrophil- and macrophage-attracting chemokines after SCI. Indeed we found that SCI-induced CXCL1 expression is severely compromised in 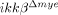 mice and LPS-mediated CXCL1 expression in vitro was eliminated in
mice and LPS-mediated CXCL1 expression in vitro was eliminated in 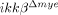 neutrophils. Based on these data, we speculated that neutrophils that initially infiltrated the spinal cord of injured
neutrophils. Based on these data, we speculated that neutrophils that initially infiltrated the spinal cord of injured 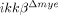 mice did not express CXCL1, so they did not further augment neutrophil infiltration. This might explain the observed differences in the post-SCI neutrophil infiltration rate between ikkβ+/+ and
mice did not express CXCL1, so they did not further augment neutrophil infiltration. This might explain the observed differences in the post-SCI neutrophil infiltration rate between ikkβ+/+ and 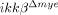 mice.
mice.
We also found that the expression levels of proinflammatory cytokines (IL-1β, IL-6 and TNF-α), iNOS, COX-2 and MMP-9 were lower in injured spinal cords of 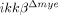 mice than in WT mice. All of these proinflammatory cytokines are implicated in neuronal apoptosis 25. NO and prostaglandin E (PGE), which are produced by iNOS and COX-2, respectively, induce neuronal apoptosis by nitrotyrosylation of proteins or DNA damage 23, 26. We found reduced nitrotyrosylation and DNA damage in the injured spinal cords of
mice than in WT mice. All of these proinflammatory cytokines are implicated in neuronal apoptosis 25. NO and prostaglandin E (PGE), which are produced by iNOS and COX-2, respectively, induce neuronal apoptosis by nitrotyrosylation of proteins or DNA damage 23, 26. We found reduced nitrotyrosylation and DNA damage in the injured spinal cords of 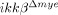 mice compared to ikkβ+/+ mice. In addition, neuronal gelatinase activation was attenuated in
mice compared to ikkβ+/+ mice. In addition, neuronal gelatinase activation was attenuated in 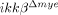 mice, probably because of the combined effects of reduced MMP-9 expression and MMP-9 nitrotyrosylation. MMP-9 activation is a key event in neuronal apoptosis 27, although the underlying molecular mechanisms are not completely understood. Our data imply that reduced proinflammatory cytokines, NO and prostaglandin E in
mice, probably because of the combined effects of reduced MMP-9 expression and MMP-9 nitrotyrosylation. MMP-9 activation is a key event in neuronal apoptosis 27, although the underlying molecular mechanisms are not completely understood. Our data imply that reduced proinflammatory cytokines, NO and prostaglandin E in 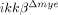 mice might contribute to reduced neuronal apoptosis at 24 h post-injury. Again, since IKK-β is deficient only in neutrophils and macrophages, and only neutrophils infiltrate at 24 h post-injury, the differences in the above genes are likely in neutrophils. This is supported by in vitro studies using neutrophils cultured from ikkβ+/+ and
mice might contribute to reduced neuronal apoptosis at 24 h post-injury. Again, since IKK-β is deficient only in neutrophils and macrophages, and only neutrophils infiltrate at 24 h post-injury, the differences in the above genes are likely in neutrophils. This is supported by in vitro studies using neutrophils cultured from ikkβ+/+ and 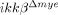 mice. Of note, we found that LPS-induced inflammatory gene expression was completely blocked in
mice. Of note, we found that LPS-induced inflammatory gene expression was completely blocked in 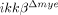 neutrophils, although expression of these genes was only partially reduced in vivo. This implies that cells other than the tissue-infiltrating neutrophils express these genes in the spinal cord after SCI, although we did not demonstrate this experimentally.
neutrophils, although expression of these genes was only partially reduced in vivo. This implies that cells other than the tissue-infiltrating neutrophils express these genes in the spinal cord after SCI, although we did not demonstrate this experimentally.
In conclusion, the results of our study demonstrate that IKK-β-dependent neutrophil activation and infiltration in the injured spinal cord potentiated inflammation and neuronal damage and impeded functional recovery after SCI.
Materials and methods
Mice and SCI
Myeloid cell type-specific IKK-β-deficient (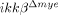 ) mice were generated by crossing floxed-ikkβ (ikkβF/F) mice and LysM-Cre knock-in mice expressing Cre under the control of the endogenous lysozyme M promoter, as described previously 28, 29. Both mouse lines were of C57BL/6 background. Adult male mice (8–10 wk old) were anesthetized with sodium pentobarbital (30 mg/kg body weight, i.p.) and laminectomized between T8 and T10. The exposed spinal cord was subjected to lateral hemisection at the T9 level with mini-Vannas scissors under microscope visualization.
) mice were generated by crossing floxed-ikkβ (ikkβF/F) mice and LysM-Cre knock-in mice expressing Cre under the control of the endogenous lysozyme M promoter, as described previously 28, 29. Both mouse lines were of C57BL/6 background. Adult male mice (8–10 wk old) were anesthetized with sodium pentobarbital (30 mg/kg body weight, i.p.) and laminectomized between T8 and T10. The exposed spinal cord was subjected to lateral hemisection at the T9 level with mini-Vannas scissors under microscope visualization.
All surgical and experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Seoul National University.
Immunohistochemistry
Immunohistochemistry was performed as previously described 30. Information on the antibodies used in this study is provided in Table 1.
| Antibody | Titer | Company |
|---|---|---|
| Mouse anti-NeuN | 1:1000 | Chemicon |
| Rat anti-CD68 | 1:500 | Serotec (Oxford, UK) |
| Rat anti-Gr-1 | 1:1000 | Invitrogen (Carlsbad, CA, USA) |
| Rabbit anti-caspase-3 | 1:200 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| Mouse anti-iNOS | 1:100 | Santa Cruz Biotechnology |
| Rabbit anti-arginase I | 1:100 | Santa Cruz Biotechnology |
| Goat anti-CXCL1 | 1:10 | R&D Systems (Minneapolis, MN, USA) |
| Rabbit anti-nitrotyrosine | 1:500 | Upstate (Temecula, CA, USA) |
| Mouse anti-8-hydroxyguanosine | 1:1000 | Abcam (Cambridge, MA, USA) |
Luxol fast blue staining and lesion volume measurement
Luxol fast blue staining was performed to calculate lesion volume as previously described 31. Images of the stained sections were taken under microscope (Axiovert200, Carl Zeiss, Munchen, Germany) and the lesion size (x–y stage) was calculated using the Axiovision 4.8 image program. The total lesion volume was calculated by summing individual subvolumes following the Cavalieri method 32.
TUNEL staining
Apoptotic cells were detected using an Apoptaq Plus Fluorescein in situ cell apoptosis detection kit (Chemicon, Temecula, CA, USA) according to the manufacturer's instructions.
Real-time RT-PCR
Real-time RT-PCR was performed as previously described 33. Relative mRNA levels were calculated according to the 2−ΔΔCt method 34. All Ct values were normalized to GAPDH. All experiments were performed at least three times. The PCR primer sequences used in this study are listed in Table 2.
| Gene | Forward primer | Reverse primer | GenBank no. |
|---|---|---|---|
| Mouse GAPDH | 5′-AGG TCA TCC CAG AGC TGA ACG-3′ | 5′-CAC CCT GTT GCT GTA GCC GTA T-3′ | NM_008084 |
| Mouse IL-6 | 5′-GCC CTT CAG GAA CAG CTA TG-3′ | 5′-CAG AAT TGC CAT TGC ACA AC-3′ | NM_012589 |
| Mouse IL-1β | 5′-TTG TGG CTG TGG AGA AGC TGT-3′ | 5′-AAC GTC ACA CAC CAG CAG GTT-3′ | NM_008361 |
| Mouse TNF-α | 5′-AGC AAA CCA CCA AGT GGA GGA-3′ | 5′-GCT GGC ACC ACT AGT TGG TTG T-3′ | NM_013693 |
| Mouse iNOS | 5′-TCT GTG CCT TTG CTC ATG ACA-3′ | 5′-TGC TTC GAA CAT CGA ACG TC-3′ | NM_012611 |
| Mouse COX-2 | 5′-CAG TAT CAG AAC CGC ATT GCC-3′ | 5′-GAG CAA GTC CGT GTT CAA GGA-3′ | U03389 |
| Mouse CXCL1 | 5′-CCG AAG TCA TAG CCA CAC TCA A-3′ | 5′-GCA GTC TGT CTT CTT TCT CCG TTA C-3′ | NM_008176 |
| Mouse CXCR2 | 5-GGT GGG GAG TTC GTG TAG AA-3 | 5-CGA GGT GCT AGG ATT TGA GC-3 | BC051677.1 |
| Mouse CD11a | 5′-AGA TCG AGT CCG GAC CCA CAG-3′ | 5′-GGC AGT GAT AGA GGC CTC-3′ | NM_008400.2 |
| Mouse CCL2 | 5′-TCA GCC AGA TGC AGT TAA CG-3′ | 5′-GAT CCT CTT GTA GCT CTC CAG C-3′ | BC05507 |
| Mouse CCL3 | 5′-ACT GCC TGC TGC TTC TCC TAC A-3′ | 5′-AGG AAA ATG ACA CCT GGC TGG-3′ | BC111443 |
| Mouse CCL4 | 5′-TCC CAC TTC CTG CTG TTT CTC T-3′ | 5′-GAA TAC CAC AGC TGG CTT GGA-3′ | NM_013652.2 |
| Mouse MMP-9 | 5′-TGT ACG GAC CCG AAG C-3′ | 5′-CCG TCC TTA TCG TAG TCA G-3′ | NM_013599 |
Behavioral tests
Hind limb motor function was assessed by open field locomotion using BMS 19. All behavioral tests were performed blind.
Neutrophil and monocyte isolation and migration assay
Neutrophils and monocytes were prepared as described previously 35, 36. Isolated neutrophil or monocyte cells (5×104 cells/well) were seeded onto a 5-μm pore-size Transwell plate (Costar, Corning, NY, USA) and incubated for 2 h in culture medium, in the presence or absence of CXCL1 (100 ng/mL, Bio-Research Products, South Lancaster, MA, USA) or CCL2 (50 ng/mL, Bio-Research Products) in the lower chamber. Neutrophils or monocytes that had migrated into the lower chamber were counted under magnification.
In situ zymography
In situ gelatinolytic activity was detected as described previously 37.
Flow cytometry
Flow cytometry was performed as previously described 30. PE-conjugated anti-CD11a (eBioscience, San Diego, CA, USA), FITC-conjugated anti-CD11b (BD Pharmingen, San Diego, CA, USA), PE-conjugated anti-CD45 (BD Pharmingen), FITC-conjugated anti-Gr-1 (BioLegend, San Diego, CA, USA) or Cy5.5-conjugated anti-CXCR2 (BioLegend) antibodies were used for flow cytometry.
ELISA
Spinal cord tissues (±0.25 cm to the injury site) were dissected from the spinal cord and proteins were extracted using tissue lysis buffer (137 mM NaCl, 20 mM Tris-HCl, 1% NP40, and protease inhibitor cocktail set III (Calbiochem, La Jolla, CA, USA). The KC/CXCL1 in the tissue lysates was measured using ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Statistical analysis
Data are presented as mean±SEM. Statistical analyses for real-time PCR data, flow cytometry data, cell migration assay data, ELISA data and image analysis were performed using a one-way ANOVA for measurements, followed by an independent Tukey's post-hoc test to compare the procedures. A p<0.5 was considered statistically significant. Statistical analysis for motor behavioral tests was performed using two-way ANOVA for measurements, followed by Tukey's post-hoc test to compare procedures.
Acknowledgements
This work was supported by the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (MEST) (No. 2009-0081467, 2008-0062413, 2010-0026575), by a Korea Student Aid Foundation (KOSAF) grant funded by the MEST (No. S2-2009-000-00972-1), and by the Korea Science & Engineering Foundation through the Infection Signaling Network Research Center (R13-2007-020-01000-0) at Chungnam National University.
Conflict of interest: The authors declare no financial or commercial conflict of interest.