C-type lectin SIGNR1 enhances cellular oxidative burst response against C. albicans in cooperation with Dectin-1
Abstract
We investigated the role of SIGNR1 in the recognition of Candida albicans and the subsequent cellular oxidative burst response. Soluble SIGNR1 (sSIGNR1) tetramer bound equally to zymosan and both heat-killed (HK) and live C. albicans in an EDTA-sensitive manner, whereas sDectin-1 tetramer predominantly bound to zymosan and HK-microbes in an EDTA-independent manner. In cellular response, enhanced oxidative burst was observed in RAW264.7 cells expressing SIGNR1 (RAW-SIGNR1) compared with RAW-control cells upon stimulation with HK-C. albicans and zymosan. This response was independent of TLR2 and the cytosolic portion of SIGNR1 but dependent on the recognition by SIGNR1 via carbohydrate recognition domain. Antagonistic laminarin and anti-Dectin-1 mAb cooperatively reduced the response with mannan and anti-SIGNR1 mAb, respectively, although they had no effect by themselves. Moreover, oxidative response and bactericidal activity largely relied on Syk-mediated signaling. RAW-SIGNR1 cells not only captured microbes more efficiently but also showed higher responses than RAW-control cells. Similar enhanced responses were observed in SIGNR-1-expressing resident peritoneal Mϕ. Interestingly, Dectin-1 was recruited to the phagosomal membrane upon the stimulation and physically associated with SIGNR1. These results suggest that SIGNR1 plays a significant role in inducing oxidative response to C. albicans by Syk-dependent signaling, possibly through Dectin-1.
Introduction
Innate immunity is a crucial host defense system that eliminates pathogens as they initiate an infection and leads to the subsequent initiation of the adaptive immune response 1. The system consists of germline-encoded genes, i.e. toll-like receptors (TLRs) 2, complements 3 and lectins 4, which are pattern recognition receptors (PRRs) that discriminate self from pathogen-associated molecular patterns 5. Dendritic cells (DCs) and macrophages (Mϕ) express a variety of PRRs that play important roles in both the innate and adaptive immune responses. Recent reports revealed that TLRs on DCs and Mϕ are involved in sensing various components of pathogens 2, giving rise to cellular inflammatory reactions. C-type lectin receptors (CLRs) on DCs and Mϕ also sense pathogens 4. CLRs interact with various kinds of pathogens via carbohydrate recognition domains (CRD), which lead to internalization, degradation and subsequent antigen presentation. In addition, simultaneous triggering of a different set of PRRs has been shown to induce diverse innate immune responses.
Much interest has been focused on type II transmembrane CLRs containing a single CRD. Dectin-1 6 and human (h) DC-SIGN (CD209) 7 are the most extensively studied members of this family. Dectin-1 is a major receptor for β-glucan 8, a component of the cell wall of Candida albicans, Pneumocystis carinii and Aspergillus fumigatus 8-12. Microbe-mediated stimulation of Dectin-1 results in cellular oxidative burst and cytokine production through its ITAM and the Syk kinase pathway 13, 14. In addition, Dectin-1 has been shown to function collaboratively with TLR2 to stimulate cytokine production 15 and Th17/Treg induction 16.
hDC-SIGN recognizes mannose and fucose moieties in the surface of a variety of microbes and viruses, such as Mycobacteria, Leishmania, Salmonella, Candida species, HIV, HCV, dengue virus, CMV, Ebola virus and Sindbis virus (refer to 17). However, pathogens, i.e. HIV and HCV, have also found ways to subvert and use hDC-SIGN to their advantage 18, 19. Mycobacterium tuberculosis and HIV also target hDC-SIGN in order to upregulate DC production of the immunosuppressive cytokine IL-10 through Raf-1 kinase activation, which induces acetylation of the NF-κB p65 subunit in the presence of co-signaling from TLR4 20.
Mice have eight hDC-SIGN homologues 21, 22. One of these homologues, SIGNR1, has been shown to be expressed on particular Mϕ subsets in the marginal zone of the spleen, medulla of the lymph nodes and the peritoneal cavity 23-25 and to possess mannose-binding activities like hDC-SIGN. SIGNR1 recognizes not only various polysaccharides, such as dextran and mannan, but also lipopolysaccharides (LPS) from Gram-negative bacteria (E. coli and Salmonella typhimurium) 23. The physical association of SIGNR1 with the TLR4-MD-2 complex on the cell surface accelerates TLR4 oligomerization upon recognition of the non-reductive end of LPS core on Gram-negative bacteria 26. In addition, SIGNR1 on resident peritoneal macrophages (rpMϕ) and SIGNR1-transfected RAW264.7 cells recognizes zymosan and heat-killed (HK) C. albicans together with Dectin-1 23, 24. Therefore, SIGNR1 is widely involved in immune responses to pathogens in cooperation with other PRRs.
In this study, we investigated the roles of SIGNR1 in recognizing and inducing cellular responses to zymosan, HK- and live C. albicans. We found that SIGNR1 enhanced Syk-dependent oxidative burst response possibly in cooperation with Dectin-1.
Results
Ability of SIGNR1 to bind zymosan and HK- and live C. albicans
We first examined the binding to microbe particles using soluble forms of SIGNR1 and Dectin-1 tagged with an N-terminal Strep-tag II sequence. When tetramers were formed by preincubating with PE-Strep-Tactin at 37°C, soluble SIGNR1 (sSIGNR1) tetramer bound more to the microbes than that at 4°C, although soluble Dectin-1 (sDectin-1) bound equally to HK-C. albicans (Fig. 1A). Based on these observations, tetramers formed at 37°C were used in the subsequent experiments.
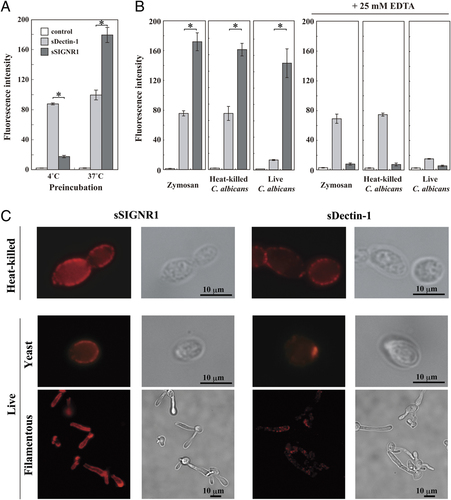
Binding of sSIGNR1 to C. albicans and zymosan. (A) Effect of thermal treatment on sSIGNR1 binding to microbes. The tetramers of sSIGNR1 and sDectin-1 were formed with PE-Strep-Tactin for 2 h at 4°C with or without 10 min of additional incubation at 37°C. Following 4 h incubation with HK-C. albicans at 4°C and washing, the fluorescence intensities were analyzed. (B) Binding of sSIGNR1 and sDectin-1 to various microbe particles. As in (A), the binding of soluble lectins to microbes was examined in the presence or absence of EDTA. (C) The binding of soluble lectins to HK- and live C. albicans was visualized by lectin staining (magnification×100). Data are expressed as the mean fluorescence intensities±SD of triplicate assays, and are representatives of three independent experiments. *p<0.01, Student's t-test.
Although both SIGNR1 and Dectin-1 recognized zymosan, as reported 23, 27, the amount of sSIGNR1 binding was much higher than that of sDectin-1 (Fig. 1B, left panels). Moreover, sDectin-1 bound comparably to zymosan and HK-microbes, but much less to live C. albicans, as reported 27. In contrast, sSIGNR1 equally bound not only to zymosan and HK-C. albicans but also live microbes (Fig. 1B, left panels). Furthermore, the binding of sSIGNR1, but not sDectin-1, was EDTA- and mannan-sensitive (Fig. 1B, right panels and data not shown).
Less binding of sDectin-1 to live microbes was also confirmed by immunofluorescence microscopy, in which sDectin-1 bound to the surface of killed microbes, but stained mainly budding scars and occasionally showed a spotty staining pattern on live microbes (Fig. 1C).
Enhanced intracellular oxidative burst in RAW-SIGNR1 was independent of TLR2
Since oxidative burst is crucial for Mϕ functions in response to microbes, we measured the oxidative burst response using RAW264.7 cells transfected with SIGNR1 cDNA (RAW-SIGNR1) or control plasmid (RAW-control). Parental RAW264.7 cells lack SIGNR1 expression. First, RAW-SIGNR1 and RAW-control cells were confirmed to express comparable levels of Dectin-1 (Fig. 2A). RAW-SIGNR1 cells showed a markedly higher response than the RAW-control cells (Fig. 2B). Although this elevated response in the RAW-SIGNR1 cells was partially reduced by depletion of zymosan, and TLR2 ligand, PAM3CSK4 was ineffective in either inducing the response by itself (Fig. 2B) or elevating the response by depleted zymosan (Fig. 2C). Antagonistic anti-TLR2 mAb (T2.5) showed no effect on the oxidative burst of RAW-SIGNR1 to zymosan or depleted zymosan (Fig. 2D). These results implied that SIGNR1 plays a role in the induction of the oxidative burst independently of TLR2, this being consistent with previous reports 13, 14.
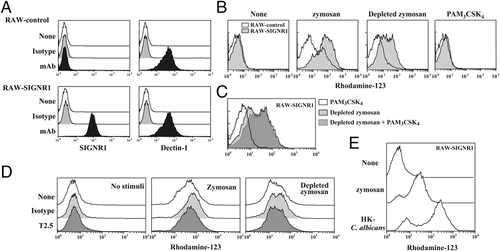
TLR2-independent augmented oxidative burst of RAW-SIGNR1 cells in response to zymosan and HK-C. albicans. (A) Expression of SIGNR1 and Dectin-1 on RAW-SIGNR1 and RAW-control cells. (B) Induction of cellular oxidative burst in RAW-SIGNR1 cells by TLR ligand-depleted zymosan. RAW264.7 transfectants (2.5×105) were stimulated with depleted and intact zymosan (5×106) or PAM3CSK4 (100 ng/mL) for 3 h and oxidative burst was measured by flow cytometry. (C) Effect of PAM3CSK4 on the augmented oxidative burst by depleted zymosan in RAW-SIGNR1 cells. (D) Effect of anti-TLR2 (50 μg/mL) on the oxidative burst. (E) Comparison of the oxidative burst in RAW-SIGNR1 cells upon stimulation with zymosan or HK-C. albicans (5×106). All data are representatives of at least three independent experiments.
Role of Dectin-1 in the enhanced oxidative response by microbe recognition via SIGNR1
Considering the role of Dectin-1 in oxidative burst 13, 14, it is possible that SIGNR1 utilizes the Dectin-1-dependent pathway, although both of these lectins can independently recognize zymosan/HK-C. albicans.
To confirm this possibility, the effects of various inhibitors were examined in response to HK-C. albicans, since HK-C. albicans much more potently induced an oxidative burst than zymosan (Fig. 2E). In RAW-control cells, laminarin, but not mannan, almost completely inhibited the oxidative burst (Fig. 3A), suggesting that Dectin-1 is a major element in eliciting the oxidative burst in the RAW-control cells. In contrast, laminarin had little effect on the oxidative burst in RAW-SIGNR1 cells, whereas mannan significantly decreased it, and it was further reduced with the simultaneous addition of laminarin. Such a cooperative action between SIGNR1 and Dectin-1 was also proven using respective specific mAbs (Fig. 3B). These results strengthen the possibility that SIGNR1 and Dectin-1 cooperate to induce an oxidative burst in the RAW-SIGNR1 cells.
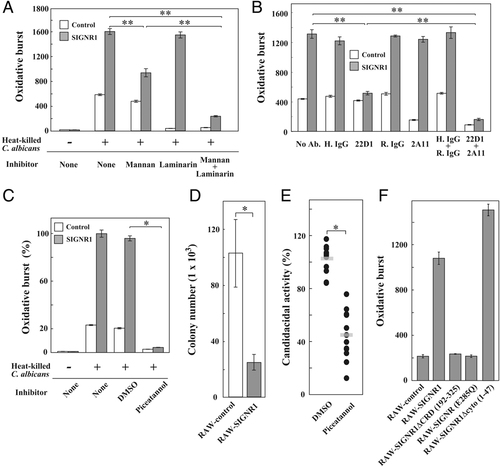
Dependency of the augmented oxidative burst of RAW-SIGNR1 on Dectin-1, Syk-mediated signaling and the recognition via CRD of SIGNR1. (A) The transfectants (2.5×105) were stimulated in the presence of mannan and/or laminarin (both 1000 μg/mL) for 1 h, and the oxidative burst stimulated by HK-C. albicans (5×106) was determined 90 min later by measuring fluorescence intensities of cell lysates using a microplate reader. Fluorescence intensities are shown as arbitrary units. (B) Transfectants were cultured in the presence of 25 μg/mL of anti-SIGNR1 (22D1) and/or anti-Dectin-1 (2A11) mAbs for 1 h and then stimulated with HK-C. albicans as in (A). (C) Effect of the Syk inhibitor, piceatannol. After 1 h incubation with 40 μM piceatannol, the transfectants were stimulated with HK-C. albicans as in (A). The results are represented as the percent activity of the oxidative burst relative to that without inhibitors. (D) Candidacidal activity of RAW-SIGNR1 cells. After pretreating with IFN-γ, RAW-control and -SIGNR1 cells were infected with live C. albicans at MOI=1 for 4 h. Colony counts were determined after 24 h cultivation. (E) Syk-dependent candidacidal activity in RAW-SIGNR1 cells. Cells were pre-treated with vehicle or piceatannol at 40 μM for 1 h before infection. Colony counts were performed as in (D). Results are shown as a summary of ten separate experiments. Horizontal bars represent the mean of each group. (F) Various RAW-transfectants stimulated by HK-C. albicans were analyzed for oxidative response as in (A). All data show mean±SD of triplicate assays and are representative of at least three independent experiments. *p<0.01, Student's t-test; and **p<0.05, Tukey's test.
Since Dectin-1 transduces intracellular signaling using Syk kinase 14, the effects of a specific Syk kinase inhibitor, piceatannol, were examined. As expected, piceatannol effectively and totally abolished the oxidative burst in the RAW-control as well as RAW-SIGNR1 cells (Fig. 3C). Moreover, live microbes cultured with RAW-SIGNR1 cells formed fewer colonies than those with RAW-control cells (Fig. 3D). This enhanced candidacidal activity in RAW-SIGNR1 cells was again markedly inhibited by piceatannol (Fig. 3E). Furthermore, the deletion of most of the carbohydrate recognition domain (ΔCRD) as well as the substitution of Glu with Gln (E285Q) in the EPN motif of CRD in the SIGNR1 gene diminished the augmented oxidative response (Fig. 3F), indicating that CRD-mediated recognition of microbes by SIGNR1 is crucial for the enhanced response. In contrast, cytosolic portion was dispensable in the activity (Fig. 3F). Taken together, these results suggest that efficient recognition of the microbes by SIGNR1 facilitates Dectin-1-mediated signaling possibly through Syk, leading to an enhanced intracellular oxidative burst against HK-C. albicans.
Significance of SIGNR1 in cellular response in addition to contact efficiency
In order to define any impact of SIGNR1 more directly, we titrated the dose of microbes during the culture with RAW-SIGNR1 and RAW-control cells using fluoresceinated HK-C. albicans. Results showed that RAW-SIGNR1 more efficiently captured microbes (Fig. 4A and B) and produced higher levels of response than RAW-control cells (Fig. 4A). When the oxidative burst of RAW-SIGNR1 was compared with control cells under equivalent capturing efficiency conditions, e.g. RAW-SIGNR1 with 1.25×105 microbes (7.93%) versus RAW-control with 5×105 microbes (7.98%), a higher oxidative response was evident in the former (Fig. 4C left panel) and a larger number of the former showed strong oxidative response than the latter (Fig. 4C right panel). These results support the hypothesis that SIGNR1 not only plays a role in capturing microbes with high contact efficiency but also facilitates the induction of the oxidative response.
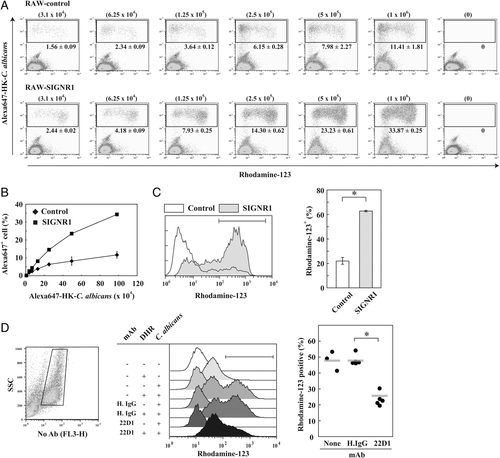
Correlation between SIGNR1-mediated capture of microbes and oxidative burst responses. (A) RAW-SIGNR1 and RAW-control cells (2.5×105) were cultured with graded doses of Alexa647-labeled HK-C. albicans for 90 min, and oxidative burst was analyzed as in Fig. 2. Numbers of microbes are shown in parentheses. Values represent the mean percentages of Alexa647+ cells±SD of triplicate assays. Data are representatives of two independent experiments. (B) Cells binding microbes were determined by gating on Alexa647+ cells as shown in (A). (C) Comparison of oxidative burst of RAW-SIGNR1 with that of RAW-control cells stimulated with 1.25×105 and 5×105 of Alexa647-labeled HK-C. albicans, respectively (left panel). Quantification of rhodamine-123-positive cells in Alexa647+ cells (right panel). Data show the mean±SD of triplicate assays and are representatives of three independent experiments. (D) Effect of anti-SIGNR1 mAb on the oxidative burst of rpMϕ. Mice pre-treated with 22D1 or control IgG 24 h before were injected i.p. with 4×106 HK-C. albicans. One hour later, peritoneal cells obtained were cultured with DHR-123 for 2 h, and oxidative burst of rpMϕ (high auto-fluorescent cells in left panel) was analyzed by flow cytometry (middle panel). Data from two independent experiments are summarized (right panel). Closed circles represent individual mice. *p<0.01, Student's t-test.
Involvement of SIGNR1 in the enhanced oxidative burst in vivo
To clarify functions of SIGNR1 in situ, rpMϕ with high autofluorescence intensity (Fig. 4D left panel) were employed. SIGNR1 on rpMϕ was successfully downregulated by 1 day after i.v. injection with anti-SIGNR1 mAb (22D1) without eliminating rpMϕ (data not shown), as reported in marginal zone Mϕ 28.
When mice treated with 22D1 mAb were inoculated i.p. with HK-C. albicans, oxidative burst by rpMϕ was significantly reduced (Fig. 4D middle and right panels), demonstrating that SIGNR1 plays a role in oxidative burst at least in rpMϕ.
Association/co-localization of SIGNR1 and Dectin-1 in rpMϕ upon stimulation
To confirm the interaction of SIGNR1 with Dectin-1 in rpMϕ, we stained the cells with specific Ab before and after the addition of HK- or live C. albicans. Co-localization of SIGNR1 and Dectin-1 was very limited without microbes, but their accumulation at the contact site with HK- and live microbes on phagosomal membrane was observed (Fig. 5A). Physical association of these two molecules was also detected only when rpMϕ were stimulated (Fig. 5B), and such an association was shown to be induced rapidly (Fig. 5C).
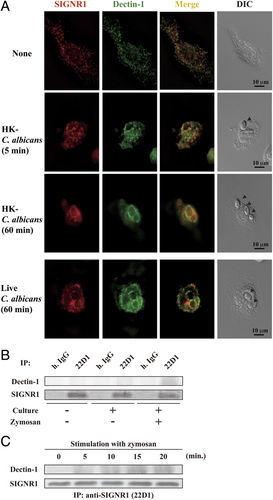
Co-localization of SIGNR1 and Dectin-1 and their physical association in rpMϕ in response to microbes. (A) Distribution of SIGNR1 and Dectin-1 in rpMϕ cells with or without stimulation with HK- and live C. albicans. Following the washing off of non-adherent peritoneal cells after 2 h culture on coverslips at 1.5×105 cells, adherent Mϕ incubated with HK- or live C. albicans (1×105) for 5 min or 1 h were fix-permeabilized and stained by anti-SIGNR1 (red) and anti-Dectin-1 (green) Abs. Arrow heads indicate the microbes in the right panels (magnification×100). (B and C) Physical association between SIGNR1 and Dectin-1 upon the stimulation with zymosan. Purified rpMϕ cells (1×107/lane) were lysed immediately (Culture (−)) or after stimulation with or without zymosan (Culture (+)) for 10 min (B) or the indicated time periods (C), and subjected to immunoprecipitation using 22D1, followed by Western blotting with anti-Dectin-1. Amounts of SIGNR1 in the 1% of individual immunoprecipitants were monitored using polyclonal anti-SIGNR1 Ab as controls. (lower panels of (B) and (C)). Representative results in at least two independent experiments are shown.
Discussion
To explore the role of SIGNR1 in C. albicans recognition, we prepared sSIGNR1 and sDectin-1 tetramers, instead of the previously formed Dectin1-Ig-fusion proteins 9, 24. Thermal treatment of sSIGNR1 with Strep-Tactin at 37°C enhanced binding activity. This result may be due to the aggregation of SIGNR1 via its long neck domain (116 amino acids), which contains a heptad-repeat sequence, leading to increased ligand affinity and specificity, as previously reported 22, 25.
Our study and several other reports indicate that Dectin-1 and TLR2 recognize microbial components and induce inflammatory responses in either a cooperative 15, 29, 30 or independent manner 13, 14. In RAW-control cells, zymosan induced weak oxidative burst, but TLR ligand-depleted zymosan and PAM3CSK4 did not. By contrast, TLR ligand-depleted zymosan induced a significant oxidative burst in RAW-SIGNR1 cells, and this response was not enhanced by PAM3CSK4. In addition, TLR2 blocking mAb had no effect on their oxidative burst in RAW-SIGNR1 cells. Based on these results, TLR2 is not largely involved in the oxidative burst response.
SIGNR1 was shown to enhance the intracellular oxidative burst of rpMϕ in response to HK-C. albicans. Such an enhancement was due to the recognition of microbes via CRD, since RAW-SIGNR1 cells lacking CRD function were unable to elevate the response. In addition, binding/capture of microbes by SIGNR1 was demonstrated to be crucial for the enhanced oxidative response by the experiment titrating the number of microbes during the culture.
Dectin-1-specific inhibitors, such as laminarin and anti-Dectin-1 mAb, blocked the oxidative response in RAW-control cells, whereas these reagents by themselves showed no effect on the response in RAW-SIGNR1 cells. However, they were able to inhibit the response in cooperation with reagents to SIGNR1, as previously reported in the case of zymosan binding in rpMϕ 23. In addition, piceatannol, a Syk-specific inhibitor, totally blocked the response in not only the RAW-control but also RAW-SIGNR1 cells, demonstrating that the SIGNR1-dependent enhanced response relies on the Syk-mediated signaling pathway. Since recognition of β-glucan by Dectin-1 was shown to induce significant oxidative response through the Syk-dependent signaling pathway 13, we considered the possibility that SIGNR1 might utilize the Dectin-1-dependent Syk-mediated signaling to promote the oxidative response. This possibility seemed to be strengthened by the observation that SIGNR1 physically associates with Dectin-1 constitutively in cells over-expressing SIGNR1 and Dectin-1 (data not shown). Moreover, SIGNR1 and Dectin-1 co-localized to part of the phagosomal membrane in RAW-SIGNR1/Dectin-1 cells (data not shown).
This is not the case in rpMϕ, where association/co-localization of SIGNR1 and Dectin-1 was not observed without stimulation, as reported in the case of TNF-α production by collaboration between TLR2 and Dectin-1 8. However, Dectin-1 was recruited to the phagosomal membrane where SIGNR1 captures microbes, and both molecules were detected to physically associate with each other in a time-dependent manner after stimulation.
The oxidative burst of RAW-SIGNR1 cells in response to live C. albicans was too weak to detect (data not shown). This may be due to the fact that the cell wall in the live microorganism is covered with mannoproteins, preventing Dectin-1 from accessing the β-glucan ligand. However, RAW-SIGNR1 cells showed significant candidacidal activity, and this activity was substantially dependent on Syk-mediated signaling. When RAW-SIGNR1/Dectin-1 cells (data not shown) and rpMϕ were exposed to live microbes, β-glucan appeared to be accessible to Dectin-1, and SIGNR1 and Dectin-1 co-localized to part of the phagosomal membrane. Therefore, it is feasible that such cellular events effectively induce candidacidal activity.
It is not clear how SIGNR1 utilizes Syk-mediated signaling though Dectin-1. It has been reported that cross-linking of SIGNR1 by neo-glycoprotein containing mannose residues and specific antibody induces the activation of JNK and NF-κB, leading to the production of TNF-α 31, IL-12 32 and IL-10 33. Therefore, it is plausible that SIGNR1 transduces the signal by itself. However, RAW264.7 cells expressing the SIGNR1 truncated cytosolic portion were still able to facilitate the oxidative response, suggesting that it is unlikely that there is any direct involvement of the cytosolic portion of SIGNR1 in signal transduction. SIGNR1 in RAW264.7 transfectants is reported to co-localize in lipid rafts with several Src family kinases 31. Therefore, cross-linking of SIGNR1 by ligand/microbes possibly induces activation of the kinases. Alternatively, SIGNR1 might also cooperate with other unidentified molecules than Dectin-1 to induce the Syk-dependent signaling. These possibilities remain to be elucidated in future experiments.
In the systemic infection or stimulation, SIGNR1 may not be a major player in the host defense, since SIGNR1 is expressed in limited populations of DCs and Mϕ. However, their distributions are strategic for sensing polysaccharides/antigens from pathogens. SIGNR1 resides in the spleen marginal zone 28 and lymph node medulla 34 captures antigens from distal infection sites via blood and lymph, respectively. Therefore, SIGNR1 in confined parts of the body in vivo plays a role as the first sensing machinery against infection. For instance, it is known that SIGNR1 in the spleen marginal zone is involved in systemic complement activation by sensing blood-borne CPS of S. pneumoniae 35. Likewise, rpMϕ are also the first interceptors for peritoneal infection and a major source of oxidative burst in peritoneal cells, as shown Fig. 4D, possibly leading to subsequent inflammatory responses in the cavity.
The host innate immune system simultaneously recognizes various types of ligands on microbes via a variety of receptors on the various types of cells. Recently, Dectin-2 36, 37 has been shown to also be important for host response to C. albicans. Nevertheless, our finding sheds light on the cooperation of different and/or similar types of PRRs in innate responses. Like the intracellular crosstalk of distinct PRR-mediated signaling pathways, PRRs also collaborate to recognize and capture microbes and to transduce signals for enhancing cellular responses.
Collectively, although the cooperative action pathway between SIGNR1 and Dectin-1 in the oxidative response is not entirely definitive, our results suggest that the anti-microbial activity/oxidative burst induction is due to efficient recognition of cell wall mannoproteins via SIGNR1 and their subsequent internalization, possibly along with the association with Dectin-1, allowing Dectin-1 to access the limited β-glucans and leading to the activation of Syk-mediated signaling.
Materials and methods
Mice
Female BALB/c mice were purchased from Japan SLC (Hamamatsu, Shizuoka, Japan). The mice were maintained under specific pathogen-free conditions, and used at 8–12 wk of age. All experiments were conducted according to our institutional guidelines.
Cells and microbes
HEK293T cells, the mouse monocytic cell line RAW264.7 cells and RAW-transfectants (RAW-SIGNR1, RAW-control and RAW-SIGNR1Δcyto cells) were maintained as described previously 26. Expression levels of SIGNR1 and Dectin-1 of these transfectants were analyzed with biotinylated anti-SIGNR1 clone 22D1 28 with PE-streptavidine and anti-Dectin-1 clone 2A11 (AbD Serotec, Oxford, UK) with PE-anti-rat IgG, respectively. Substitutions of glutamic acid 285 with glutamine (E285Q) in SIGNR1 were introduced by overlapping PCR. cDNA fragments of SIGNR1ΔCRD (192–325) was PCR amplified using forward primer 5′-GATCGAATTCATGAGTGACTCCACAGAAGCC-3′ in combination with reverse primer 5′-GATCCTCGAGCTACAGGCGGAAGAGTTCAGTCTTC-3′. pcDNA4/HisMax-SIGNR1 23 was used as a template, and the resulting PCR products were cloned into the EcoRI-XhoI site of pcDNA4/HisMax (Invitrogen, Carlsbad, CA). Surface expression of these mutant proteins was confirmed by flow cytometry with polyclonal anti-SIGNR1 (R&D Systems, Minneapolis, MN). Peritoneal cells were obtained by washing the peritoneal cavity with ice-cold 5 mM EDTA in PBS. For Western blot analysis, rpMϕ were negatively enriched by depleting CD3ε, B220, CD19, Gr-1 and CD49b-expressing cells using biotinylated mAbs with avidin-IMAg (BD Pharmingen, San Diego, CA).
C. albicans (JCM 1542: Riken Bioresource Center, Saitama, Japan) was cultured overnight in Sabouraud dextrose broth (Sigma-Aldrich, Irvine, CA) at 28°C. HK-C. albicans were obtained by treating at 95°C for 30 min in PBS. In some experiments, HK-C. albicans were labeled by Alexa Fluor 647 carboxylic acid, succinimidyl ester (Invitrogen) according to the manufacturer's protocol. In some experiments, zymosan (Sigma-Aldrich) was depleted of TLR ligands by boiling in 10 N NaOH for 30 min 15.
Preparations of sSIGNR1 and Dectin-1 and the microbe particle-binding assay
cDNA fragments encoding the extracellular domains of SIGNR1 and Dectin-1 were cloned into pEXPR-IBA44 (IBA, Göttingen, Germany) to add the N-terminal BM40 secretion signal and Strep-tag II sequence, and then transferred into pEF6/V5-His (Invitrogen). HEK293T cells transfected with each plasmid 38 were maintained in serum-free medium 293 SFM II (Invitrogen) for the last 48 h of culture. sSIGNR1 and sDectin-1 were purified using Strep-Tactin Sepharose (IBA) in accordance with the manufacturer's protocol (>95% purity by SDS-PAGE).
Tetramers were formed by mixing soluble lectins and PE-labeled Strep-Tactin in HBSS (pH 8.3) at 4°C for 2 h, and then incubated for another 10 min at 37°C. The tetramers were incubated with 5×106 of microbe particles at 4°C for 4 h in HBSS containing 1% BSA with or without 25 mM EDTA. The amount of PE-Strep-Tactin bound to the particles was measured using a Gemini EM fluorescence plate reader (Molecular Devices, Sunnyvale, CA).
To visualize the binding to microbes, the bound soluble lectins were labeled with an anti-Strep-tag mAb (IBA) for 2 h at 4°C in HBSS, followed by staining with a Cy3-anti-mouse IgG (Jackson Immuno Research, West Grove, PA). They were then analyzed by deconvolution microscopy (BX51-FL: Olympus, Tokyo, Japan) using imaging software, SlideBook (Intelligent Imaging Innovation, Denver, CO).
Cellular responses to microbes
Oxidative burst after culture of RAW264.7 transfectants with microbes for indicated time periods was measured by quantitating the intracellular conversion of DHR (dihydrorhodamine)-123 to rhodamine-123 39 for time indicated using a flow cytometer and a Gemini EM fluorescence plate reader for cells and cell lysates, respectively. For inhibition assays, the mAbs and inhibitors were added at the indicated concentrations 1 h before the stimulation. Antagonistic anti-TLR2 mAb clone T2.5 was from Hycult Biotechnology (Uden, The Netherlands). To detect contact and/or capture efficiency, Alexa 647-labeled HK-C. albicans was used.
In primary Mϕ, mice were i.v. injected with 150 μg of 22D1 or control Armenian hamster IgG 24 h prior to i.p. injection of 4×105 HK-C. albicans. One hour later, peritoneal cells were obtained, and the oxidative burst of rpMϕ gated by high autofluorescence (Fig. 4D) was measured after 2 h of culture as described above.
To determine the candidacidal activity, RAW264.7 transfectants at 3×105 cells/well in a 24-well plate were preactivated with 100 U/mL IFN-γ for 4 h and then infected with live C. albicans (2.5×105) for another 4 h. The microbes obtained by lysing the cells were seeded on Sabouraud dextrose agar plates, and the total number of live C. albicans in each well of triplicate cultures was counted after 24 h incubation at 28°C. The effect of piceatannol on candidacidal activity was calculated as the percent of (colony number in RAW-SIGNR1−that in RAW-SIGNR1 experimental group)/(colony number in RAW-control−that in RAW-SIGNR1).
Distribution and physical association of SIGNR1 with Dectin-1 in peritoneal Mϕ
Following 2 h culture of peritoneal cells (1.5×105) on coverslips, adherent Mϕ were incubated with HK- or live C. albicans (1×105 microbes) for the time indicated, then fixed-permeabilized, followed by staining with anti-SIGNR1 (22D1) and polyclonal goat anti-Dectin-1 (R&D Systems).
Purified rpMϕ cells (1×107) were pre-cultured for 30 min, followed by stimulation with zymosan (200 μg/mL) for the periods indicated. For Western blot analysis, cell lysates were clarified extensively by centrifugation (two times at 16 000×g for 30 min) and then treated with 25 mM EDTA to remove microbial materials, followed by the immunoprecipitation with 22D1 or control IgG. Western blot analyses were performed as described previously 23 using polyclonal anti-Dectin-1 and HRP-anti-goat IgG (Goat TrueBlot, eBioscience). Immunoprecipitation of SIGNR1 was confirmed separately using anti-SIGNR1 polyclonal antibody with HRP-anti-goat IgG.
Statistical analysis
Data are expressed as the mean±SD of triplicate analyses. Statistical significance was determined by the two-tailed Student's t-test. In some cases, multiple comparisons were performed by ANOVA with Tukey's test. All experiments were performed at least two times and representative results are shown.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research (19590389 to K. T. and 18390121 to K. I.), a Grant-in-Aid for Scientific Research on Priority Area (19041936) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and Core Research for Evolutional Science and Technology, Japan Science and Technology Agency. K. N. is also supported by a Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists.
Conflict of interest: The authors declare no financial or commercial conflict of interest.