Incidence and predictors of weaning failure from veno-arterial extracorporeal membrane oxygenation therapy in patients with cardiogenic shock
Abstract
Aims
This study aimed to investigate incidence and predictors of weaning failure and in-hospital death after successful weaning from veno-arterial extracorporeal membrane oxygenation (VA-ECMO) in patients with cardiogenic shock (CS).
Methods and results
Overall, 685 patients with CS treated with VA-ECMO from 23 tertiary care centres in 7 countries were analysed (median age 57 [interquartile range 49–66] years, 542 [79.1%] male, median lactate 7.6 [interquartile range 4.1–12.7] mmol/L). The cause of CS was acute myocardial infarction in 438 (63.9%) patients, and 431 (62.9%) patients presented with cardiac arrest. A total of 410 patients (59.9%) were successfully weaned from VA-ECMO, whereas in 275 patients (40.1%) weaning failed (i.e. patients died on or within 48 h after VA-ECMO support). Of the successfully weaned patients, 150 (36.6%) died before hospital discharge. On multivariable logistic regression, predictors for both patient groups varied: age (per 10 years, odds ratio [OR] 1.49, 95% confidence interval [CI] 1.25–1.76; p < 0.001) and cardiac arrest before VA-ECMO implantation (OR 1.64, 95% CI 1.01–2.64; p = 0.04) were associated with weaning failure, whereas lactate clearance within 24 h after VA-ECMO initiation was associated with successful weaning (OR 0.21, 95% CI 0.1–0.44; p < 0.001). In-hospital death after successful weaning was more likely with higher age (per 10 years, OR 1.56, 95% CI 1.24–1.97; p < 0.001), renal replacement therapy (OR 2.56, 95% CI 1.4–4.68; p = 0.002) and bleeding events (OR 2.93, 95% CI 1.4–6.14; p = 0.004).
Conclusion
Weaning from VA-ECMO fails in 40% of patients treated with VA-ECMO for CS. When successful, survival after VA-ECMO weaning mostly depends on age and the incidence of device- and shock-related complications.
Introduction
Cardiogenic shock (CS) remains a clinical challenge with a high mortality despite significant advances in overall cardiovascular medicine.1 Early culprit vessel revascularization can improve survival when CS is caused by acute myocardial infarction (AMI).2 Recent clinical and research efforts have focused on the role of mechanical circulatory support (MCS) devices, highlighted by a significant increase in the use of veno-arterial extracorporeal membrane oxygenation (VA-ECMO).3 However, until the recently published DanGer Shock trial,4 thus far available randomized controlled trials addressing MCS have shown neutral results.5, 6 DanGer Shock is the first trial which demonstrated a survival benefit with the use of microaxial flow pump (Impella®) in patients with ST-elevation myocardial infarction-related CS without risk of hypoxic brain injury.4 It is proposed that rigorous patient selection with clear guidelines for standard of care and device management contributed to the positive outcome of this trial. This highlights the notion that there is an unmet need to develop clear standard operating procedures for MCS device management, specifically VA-ECMO.
Successful weaning from VA-ECMO is generally defined as survival after complete removal of the extracorporeal circuit without requirement for further mechanical support or heart replacement therapies.7-10 The timing of weaning is crucial; premature removal may lead to recurrence of shock and resulting in secondary organ injury. Conversely, prolonged VA-ECMO support is associated with increasing complications including haemorrhagic and thrombotic events, limb ischaemia, haemolysis and sepsis and increased in-hospital mortality.11-13 Reported weaning success (WS) rates range from 30% to 75%.7, 9, 12 Despite successful weaning, a substantial portion of patients do not survive to hospital discharge.8, 14 Underlying comorbidities, adverse complications during VA-ECMO support and the patients' condition after weaning may affect the clinical outcome.
Current consensus regarding a practical weaning approach is based on gradual flow reduction, however standardized guidance on timing and weaning criteria are poorly defined.10 Further, data describing predictors of weaning failure (WF) and in-hospital death after successful weaning from VA-ECMO in CS patients remain scarce. The aim of the present study was to describe the incidence of WS in patients with CS supported with VA-ECMO and to identify predictors of WF and in-hospital mortality.
Methods
Study design
This is a retrospective analysis based on a combination of two previously reported databases: (1) the non-ischaemic CS registry13 and (2) the ECMO/ECMELLA database.3, 11 For both databases comprehensive documentation regarding the data entry process, definition of CS, as well as inclusion and exclusion criteria have been described previously. In short, in (1) patients with non-ischaemic CS, treated with or without MCS, were retrospectively enrolled between 2010 and 2021 from 16 centres across five countries (NCT03313687). The definition of CS was based on the criteria outlined by Society for Cardiovascular Angiography and Interventions (SCAI).15 In (2) patients with CS unselected for its aetiology treated with VA-ECMO were retrospectively enrolled from 16 centres in four countries (NCT03313687). CS was defined using local criteria. Only patients with post-cardiotomy CS and those <18 years were excluded. Data were retrospectively collected by the local investigators after review of the available case records. Patients were followed up until death or discharge from hospital. For this analysis, only patients treated with VA-ECMO from (1) the non-ischaemic CS cohort and only patients with AMI-CS from (2) the ECMO/ECMELLA database (to avoid duplicates) were included, resulting in a concise all-aetiology CS cohort treated with VA-ECMO from 23 centres from seven countries. All participating centres were experienced in the management of VA-ECMO.
Weaning failure was defined as death during VA-ECMO support and death within the first 48 h after VA-ECMO explanation. The following complications during VA-ECMO were collected: (1) bleeding complications, including moderate and life-threatening bleeding events defined using the GUSTO bleeding classification, as well as bleeding events that required interventional or surgical intervention; (2) ischaemic complications, which included cases of peripheral vascular complications requiring an intervention and cases of abdominal compartment syndrome with the need for a laparotomy; (3) systemic inflammatory response syndrome (SIRS)/sepsis, defined using clinical criteria. Inotropic score was calculated as: dopamine dose (μg/kg/min) + dobutamine dose (μg/kg/min) + 100 × epinephrine dose (μg/kg/min) + 10 × milrinone dose (μg/kg/min) + 10 000 × vasopressin dose (U/kg/min) + 100 × norepinephrine dose (μg/kg/min).
This study conforms with the principles outlined in the Declarations of Helsinki, and was approved by each local ethics committee. Due to the retrospective study design, all patient data were anonymized, hence the need to give informed consent was waived.
Statistical analyses
Baseline characteristics were generated for the whole cohort, as well as the WF and WS subgroups. Continuous variables are expressed as median with interquartile range (IQR, 25th–75th percentile). Categorical variables are shown as number and percentage. To visualize mortality risk over time, Kaplan–Meier method was applied and a violin plot was generated. To compare characteristics and identify risk factors for patients with WF versus WS, continuous variables were analysed using Kruskal–Wallis test, whereas categorical variables were compared using the Fisher's exact test. To evaluate whether these risk factors and simultaneously patient or treatment specific characteristics could serve as predictors for WS or WF, multivariable logistic regressions models were fitted. Analyses were adjusted for the following potential confounders: age per 10 years, sex, AMI, prior cardiac arrest (CA), renal replacement therapy (RRT), lactate clearance within 24 h, left ventricular (LV) unloading via Impella®, bleeding events, ischaemic complications, cerebrovascular complications and sepsis/SIRS. Odds ratios (ORs) are reported for each variable with 95% confidence intervals (CIs). A p-value <0.05 was considered statistically significant. Analyses were performed using R statistical software version 4.3.1.
Results
Baseline characteristics of the study population
The study cohort consisted of 731 patients with CS unselected for its aetiology supported with VA-ECMO. Of these, 46 patients without information on WF were excluded, such that 685 patients were included in the final analysis. Baseline characteristics of the overall cohort as well as stratified by WS versus WF are shown in Table 1.
| All (n = 685) | Weaning success (n = 410) | Weaning failure (n = 275) | p-value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 57.0 (49.0–66.0) | 55.0 (47.0–64.0) | 61.0 (53.0–68.0) | <0.001 |
| Male sex, n (%) | 542 (79.1) | 321 (78.3) | 221 (80.4) | 0.57 |
| Index event | ||||
| Cardiac arrest, n (%) | 431 (62.9) | 227 (55.4) | 204 (74.2) | <0.001 |
| Duration of CPR (min) | 20 (0–45) | 10 (0–30) | 35 (7–60.2) | <0.001 |
| Shockable rhythm at index event, n (%) | 181 (44.8) | 93 (42.5) | 88 (47.6) | 0.32 |
| Index event out of hospital, n (%) | 364 (55.2) | 198 (51.0) | 116 (61.3) | 0.011 |
| Cause of cardiogenic shock, n | ||||
| Acute myocardial infarction | 438 (63.9) | 240 (58.5) | 198 (72.0) | <0.001 |
| STEMI | 331 (48.6) | 186 (45.6) | 145 (53.1) | 0.06 |
| Successful revascularization, n (%) | 376 (92.8) | 203 (94.0) | 173 (91.5) | 0.44 |
| Clinical presentation | ||||
| Mechanical ventilation, n (%) | 562 (93.0) | 330 (91.2) | 232 (95.9) | 0.033 |
| Haemodynamics | ||||
| Systolic blood pressure (mmHg) | 76 (60–90) | 80 (65–90.3) | 70 (23–85) | <0.001 |
| Diastolic blood pressure (mmHg) | 47 (34–58) | 50 (38.4–60) | 40 (19.7–53) | <0.001 |
| Mean arterial pressure (mmHg) | 53 (43.8–65) | 55 (45–68.1) | 50 (35–60.8) | <0.001 |
| Heart rate (bpm) | 100 (76–125) | 98 (78–120) | 101 (72–132.7) | 0.16 |
| Inotropes and vasoconstrictors | ||||
| Inotropic score day 1 | 35 (11–130) | 29.2 (10–100) | 56.7 (15–202.9) | <0.001 |
| Inotropic score day 3 | 15 (3.4–51.5) | 10.6 (2.6–40) | 31 (9.7–91.7) | <0.001 |
| Inotropic score day 5 | 8 (0–30.3) | 5 (0–27.4) | 20 (2–50.9) | 0.004 |
| Laboratory | ||||
| pH | 7.2 (7.1–7.4) | 7.3 (7.1–7.4) | 7.2 (7.0–7.3) | <0.001 |
| Lactate (first measurement) (mmol/L) | 7.6 (4.1–12.7) | 6.1 (3.6–11) | 9.5 (5.9–14) | <0.001 |
| Lactate clearance within 24 h, n (%) | 112 (18.9) | 100 (28.6) | 12 (4.9) | <0.001 |
| Lactate day 1 (mmol/L) | 6.2 (3.1–10.8) | 4.3 (2.3–8.5) | 9.5 (5.9–14.8) | <0.001 |
| Lactate day 3 (mmol/L) | 2.4 (1.6–3.5) | 2.1 (1.4–2.9) | 3.5 (2.4–7.5) | <0.001 |
| Lactate day 5 (mmol/L) | 1.8 (1.2–2.5) | 1.7 (1.2–2.2) | 2.5 (1.7–3.6) | <0.001 |
| Creatinine (mg/dl) | 1.6 (1.2–2.3) | 1.5 (1.2–2.3) | 1.7 (1.3–2.4) | 0.017 |
| Mechanical circulatory support | ||||
| VA-ECMO only, n (%) | 389 (56.8) | 230 (56.1) | 159 (57.8) | 0.69 |
| ECMELLA, n (%) | 296 (43.2) | 180 (43.9) | 116 (42.2) | 0.69 |
| Duration of VA-ECMO support (days) | 5 (3–7.2) | 6 (4–8) | 3 (1–6) | <0.001 |
| Complications, n (%) | ||||
| Renal replacement therapy | 324 (47.6) | 196 (47.8) | 128 (47.2) | 0.94 |
| Sepsis/SIRS | 143 (23.8) | 97 (26.7) | 46 (19.4) | 0.04 |
| Haemolysis | 143 (24.7) | 78 (22.2) | 65 (28.5) | 0.094 |
| Bleeding complications | 424 (73.1) | 248 (72.1) | 176 (74.6) | 0.57 |
| Moderate bleeding | 312 (54.1) | 201 (58.9) | 111 (47) | 0.005 |
| Life-threatening bleeding | 200 (29.3) | 104 (25.4) | 96 (35.2) | 0.008 |
| Surgical or radiographic intervention due to bleeding | 113 (18.7) | 72 (19.8) | 41 (17.1) | 0.46 |
| Cerebrovascular complications | 87 (16) | 55 (16.3) | 32 (15.5) | 0.9 |
| Intracerebral bleeding | 39 (7.3) | 22 (6.6) | 17 (8.3) | 0.5 |
| Ischaemic stroke | 56 (8.7) | 39 (9.8) | 17 (7) | 0.25 |
| Ischaemic complications | 126 (19.1) | 64 (16.4) | 62 (23.0) | 0.044 |
| Peripheral vascular complication requiring an intervention | 103 (15.1) | 56 (13.7) | 47 (17.2) | 0.23 |
| Laparotomy | 44 (6.7) | 15 (3.9) | 29 (10.8) | <0.001 |
- Values are given as n (%), or median (interquartile range).
- CPR, cardiopulmonary resuscitation; ECMELLA, combination of VA-ECMO and Impella® support; SIRS, systemic inflammatory response syndrome; STEMI, ST-elevation myocardial infarction; VA-ECMO, veno-arterial extracorporeal membrane oxygenation.
Median age across the entire cohort was 57 (IQR 49–66) years and 542 (79.1%) were male. More than half of patients presented with CS due to AMI (n = 438, 63.9%) and prior CA (n = 431, 62.9%) of whom 181 patients (44.8%) had a shockable rhythm, either ventricular fibrillation or tachycardia. Median pH upon admission was 7.2 (IQR 7.1–7.4), lactate 7.6 (IQR 4.1–12.7) mmol/L and creatinine 1.6 (IQR 1.2–2.3) mg/dl. Median mean arterial pressure prior to initiation of VA-ECMO was 53 (IQR 44–65) mmHg and median heart rate was 100 (IQR 76–125) bpm. LV unloading (via Impella®) was implemented in 296 patients (43.2%), while the remaining were treated with VA-ECMO only. Median duration of VA-ECMO support was 5 days. Median length of intensive care unit stay was 11 days.
Weaning failure and mortality
Weaning from VA-ECMO failed in 275 patients (40.1%), while the majority, 410 patients (59.9%), were successfully weaned. Among those successfully weaned, 150 patients (36.6%) died before hospital discharge. The mortality rate of the overall cohort was 63.8%, with 248 patients (36.2%) surviving to hospital discharge following liberation from VA-ECMO. For a graphical presentation of patients with WF, death after WS and survival to hospital discharge see Figure 1.
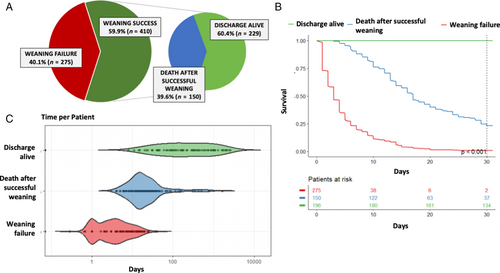
Comparative analysis of patient characteristics based on weaning failure and success from VA-ECMO
The study groups WF and WS showed several significant differences in patient and clinical characteristics on admission and during clinical course (Table 1). As compared to the WS group, patients with WF were older (WF: 61 (53–68) vs. WS: 55 (47–64) years; p < 0.001) with higher prevalence of CS due to AMI (WF: n = 198, 72% vs. WS: n = 240, 58.5%; p < 0.001). More patients in the WF group presented with CA (WF: n = 204, 74.2% vs. WS: n = 227, 55.4%; p < 0.001) and a had longer duration of cardiopulmonary resuscitation (WF: 35 [7–60.2] vs. WS: 10 (0–30) min; p < 0.001), but there was no difference in cardiac rhythm at index event between groups. Patients with WF had a lower median mean arterial pressure (WF: 50 [35–60.8] vs. WS: 55 [45–68.1] mmHg; p < 0.001), median pH was more acidotic (WF: 7.2 [7.0–7.3] vs. WS: 7.3 [7.1–7.4]; p < 0.001) and inotropic score and lactate on subsequent days 1, 3 and 5 were higher. Lactate clearance within 24 h post VA-ECMO implantation was less common in the group of patients who failed to be weaned as it was only observed in 12 patients (4.9%) compared to 100 patients (28.6%) in the WS group. The need for RRT was equally distributed between groups. SIRS/sepsis was less common in patients with WF (WF: n = 46, 19.4% vs. WS: n = 97, 26.7%; p = 0.04). Regarding common complications associated with MCS, moderate bleeding events were less common (WF: n = 111, 47% vs. WS: n = 201, 58.9%; p = 0.005), whereas life-threatening bleeding events were more common in patients with WF (WF: n = 96, 35.2% vs. WS: n = 104, 25.4%; p = 0.008). As for ischaemic limb complications, cerebrovascular events or haemolysis, no difference was observed. A laparotomy for suspected bowl ischaemia was more often performed in patients with WF (WF: n = 29, 10.8% vs. WS: n = 15, 3.9%; p < 0.001). The overall duration of VA-ECMO support was shorter in patients with WF (WF: 3 [1–6] vs. WS: 6 [4–8] days; p < 0.001).
Prognostic factors for failed weaning from VA-ECMO
Multivariable logistic regression analysis identified two predictors of WF and two predictors of WS (Figure 2): age (per 10 years; OR 1.49, 95% CI 1.25–1.76; p < 0.001) and CA before VA-ECMO implantation (OR 1.64, 95% CI 1.01–2.64; p = 0.043) were the only baseline parameters associated with WF. Lactate clearance within 24 h after VA-ECMO initiation (OR 0.21, 95% CI 0.10–0.44; p < 0.001) and the prevalence of SIRS/sepsis (OR 0.55, 95% CI 0.33–0.91; p = 0.02) were associated with WS. For RRT, LV unloading via Impella® or MCS associated complications, no significant difference was observed.
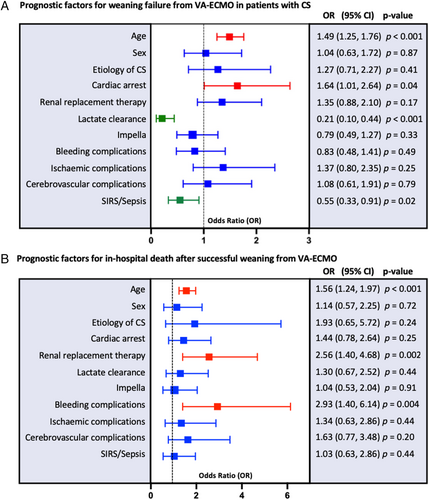
Prognostic factors for in-hospital death after successful weaning from VA-ECMO
After successful weaning from VA-ECMO, multivariable logistic regression analysis revealed different predictors for in-hospital death (Figure 2): age (per 10 years; OR 1.56, 95% CI 1.24–1.97; p < 0.001), RRT (OR 2.56, 95% CI 1.40–4.68; p = 0.002) and the occurrence of any bleeding event (OR 2.93, 95% CI 1.40–6.14; p = 0.004) were associated with in-hospital death after successful weaning from VA-ECMO. For CA, lactate clearance, SIRS/sepsis and ischaemic complications associated with MCS, no difference was observed.
Discussion
In this study we investigated patient characteristics and predictors associated with WF from VA-ECMO support as well as predictors for in-hospital mortality in CS patients who were initially successfully weaned. The key findings were: firstly, about 40% of patients on VA-ECMO have WF defined as death during VA-ECMO treatment or death within the first 48 h after VA-ECMO explantation, and of those with WS, in-hospital mortality is about 37%. Secondly, different factors appear to impact mortality in the cohort with WF compared to those with in-hospital mortality after successful weaning; prior CA and persistent malperfusion (e.g. absence of lactate clearance) were strong drivers of WF whilst in-hospital mortality after successful weaning was largely dependent on the occurrence of (bleeding) complications and extracardiac organ failure (Graphical Abstract).
Weaning failure versus in-hospital death after successful weaning from VA-ECMO
Within the study cohort, 60% of all patients who received VA-ECMO support were successfully weaned from VA-ECMO. This WS rate is consistent with reported rates between 35% and 70%,7-9, 12, 16 allowing for differing populations and definitions of WS. In older studies, success rate was lower (40%),9, 12 whereas recent studies with selected patients suitable for weaning report WS rates comparable to the data herein (60–70%).7, 16 Despite successful weaning, 40% of patients die in hospital. The results of the DanGer Shock trial highlighted the importance of patient selection combined with standard operating procedures for MCS management to optimize clinical outcomes.4 There is an unmet need for evidence-based practice for patient selection and best practice guidance for VA-ECMO management in CS.
Predictors of weaning failure and in-hospital death after successful weaning from VA-ECMO
Illness severity and organ perfusion
The initial severity of CS is known to impact the odds of successful weaning from VA-ECMO.17 An inability to rapidly restore macro- and microcirculation within the first 24 to 72 h is associated with WF.7, 18, 19 Early correction of biomarkers reflecting tissue hypoperfusion appear to predict WS.20 In accordance with literature, in this study patients with WF presented with lower mean arterial pressure and higher baseline lactate compared to patients with WS, as well as a higher demand of inotropic support during VA-ECMO support.7, 19, 20 Lactate clearance within the first 24 h of VA-ECMO support was the strongest predictor of WS. These parameters did not predict in-hospital death in those patients successfully weaned. Aissaoui et al.9 noted that these predictors reflected severity and progression of multiorgan failure at the time of implantation and should be evaluated prior to any weaning attempt. It seems that initial shock severity and early recovery of systemic perfusion are more relevant in terms of short-term WF compared to long-term survival once patients are successfully weaned from VA-ECMO.
Previous studies have also highlighted underlying demographic risk factors such as age, as well as an ischaemic cause of CS as predictors of WF and mortality.12, 14, 21 Among patients with CS, those with CA before VA-ECMO implantation are considered at even higher risk with mortality rates up to 80%.22 In this study, age was a predictor of both WF and in-hospital death after successful weaning. Aetiology of CS was not associated with successful weaning. CA before VA-ECMO implantation was a strong predictor of WF; however, this was not observed for in-hospital mortality after successful weaning. Although this may not directly guide weaning readiness it may help set expectations and improve our prognostic fidelity.
Organ dysfunction and complications
In CS, circulatory failure is often accompanied by ischaemic injury to organs such as brain, liver and kidney. The presence of extracardiac organ failure is a known prognostic factor for increased mortality in patients receiving VA-ECMO support.12, 21, 23 In fact, it is difficult to distinguish whether organ dysfunction remaining after weaning from VA-ECMO is a result of ischaemic injury during initial circulatory failure or a complication arising during VA-ECMO support. RRT has been shown to be negatively linked to WF as well as survival after WS.7, 8, 14 In this study, the need for RRT was equally distributed between patients with WF as well as WS and showed no predictive value for WF from VA-ECMO. For patients after successful weaning, RRT was a strong predictor for in-hospital death. It seems that kidney dysfunction regardless of cause or onset is more closely related to the clinical outcome in patients who survived VA-ECMO rather than the short-term weaning process itself. Another possible explanation for this observation could be the indication for RRT, which often is for metabolic reasons in the acute setting whereas renal failure and uremic complications become more important after the acute phase.
The timing of weaning is crucial; premature removal may lead to recurrence of shock. In this study, patients with WF had a shorter duration of VA-ECMO support, which may suggest that attempting to wean patients too early may correlate with poorer outcomes. Another factor to consider is survival bias. WF includes patients with death on the device, translating into shorter overall time of VA-ECMO support.
Conversely, during VA-ECMO support there is a risk of developing circuit-related adverse complications that can become life-threatening and significantly worsen the prognosis.11
Infections have been reported to occur in 10–50% of patients on VA-ECMO support and septic shock, but also systemic inflammatory response (SIRS) is a significant risk factor for mortality in patients with CS treated with VA-ECMO.24, 25 Kim et al.8 identified that patients who developed an infection during VA-ECMO support had a poorer prognosis even after successful weaning. Contrary to this, in the present study sepsis/SIRS was an independent predictor of WS from VA-ECMO, but showed no significant difference in regard to in-hospital death after WS. It seems that restoring circulation in the early stages of shock via VA-ECMO might be beneficial for patients in which SIRS leads to additional hypoperfusion. In a prior study, we demonstrated that systemic inflammation was only associated with mortality in patients treated conventionally, not in those managed with VA-ECMO. It is plausible that VA-ECMO support might modify the host inflammatory response, potentially through restoration of end-organ perfusion.26
Patients with CS and specifically those supported with VA-ECMO are at high risk for either early thrombotic or bleeding events with the associated increased mortality risk.13, 27, 28 They also may impact the expediency of removal of VA-ECMO to mitigate MCS-related complications and drive WF.29 In the present study, we observed more severe bleeding events in the group with WF whilst patients with WS demonstrated more moderate bleeding events. For WF, bleeding events in general as well as ischaemic events were not predictive of WF. Importantly, after successful weaning any bleeding event was a strong predictor for in-hospital mortality. Overall, these results highlight the need to reduce such complications as they interfere with patient management even after patients are weaned from VA-ECMO.
In attempt to improve MCS therapy, active LV unloading in addition to VA-ECMO has been considered to improve weaning from VA-ECMO and mortality rates in patients with CS.30 In this study no difference was observed which may be attributed to the small and heterogeneous population. Further randomized controlled trials are underway to address this (ANCHOR [Assessment of ECMO in Acute Myocardial Infarction Cardiogenic Shock, NCT04184635], REVERSE [Impella CP With VA ECMO for Cardiogenic Shock, NCT03431467] and UNLOAD ECMO [Left Ventricular Unloading to Improve Outcome in Cardiogenic Shock Patients on VA-ECMO, NCT05577195]).
Limitations
The major limitation of this study is its retrospective and observational study design. First, there is a potential risk of selection bias. Secondly, although each centre is skilled in VA-ECMO therapy and broadly follows the latest recommendations and protocols for weaning, in each case the attending physicians made the final decision to remove the VA-ECMO based on the patient's clinical presentation. We cannot exclude the possibility that the decision to wean patients from VA-ECMO was highly variable across institutions. Detailed haemodynamic profiles including data from echocardiographic and pulmonary artery catheter assessment have not been collected. Additionally, the circumstances that lead to removal of VA-ECMO are not reported. Therefore, the state of haemodynamic recovery of the patients is not known. It is possible that patients had expeditious weaning due to complications in the context of incomplete haemodynamic recovery, which in turn could be considered underlying cause of WF. Also, the rationale for why some patients received LV unloading is not available. Thirdly, although is remains a large multicentre analysis, the potential of missing characteristics beyond those reported and unmeasured or unknown confounders preclude from establishing causal relationships. Larger prospective multicentre studies are necessary to confirm our findings and to further establish criteria and treatment guidelines that can be used to guide VA-ECMO therapy.
Conclusion
Patients undergoing VA-ECMO therapy continue to have a high mortality with high rates of WF. Further, even in those patients successfully weaned from VA-ECMO 37% of patients died during the index hospital stay. Age and CA were predictors of WF, whereas early lactate clearance was an independent predictor of WS. RRT and bleeding complications were independent predictors of in-hospital death after successful weaning. This emphasized the need for clinicians caring for VA-ECMO patients to be cognizant of the importance of early restoration of systemic perfusion and recovery of the cardiac function to maximize survival to hospital discharge. Further prospective multicentre studies are required to establish weaning criteria and best practices for patients supported with VA-ECMO as well as the management of those patients who are successfully weaned.
Acknowledgement
Open Access funding enabled and organized by Projekt DEAL.
Conflict of interest: J.D. received speaker fees from AstraZeneca, Boehringer Ingelheim and Bayer. P.K. was partially supported by European Union AFFECT-AF (grant agreement 847 770), and MAESTRIA (grant agreement 965 286), British Heart Foundation (PG/17/30/32961; PG/20/22/35093; AA/18/2/34218), German Center for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK), Deutsche Forschungsgemeinschaft (Ki 509 167 694), and Leducq Foundation; he receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Center for Cardiovascular Research, from several drug and device companies active in atrial fibrillation, and has received honoraria from several such companies in the past, but not in the last 3 years; he is listed as inventor on two issued patents held by University of Hamburg (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). S.K. received research support from Cytosorbents and Daiichi Sankyo; he also received lecture fees from ADVITOS, Biotest, CSL Behring, Daiichi Sankyo, Fresenius Medical Care, Gilead, Mitsubishi Tanabe Pharma, MSD, Pfizer, Shionogi and Zoll; and consultant fees from ADVITOS, Fresenius, Gilead, MSD and Pfizer. T.R. received speaker fees from AstraZeneca, Bayer, Daiichi Sankyo, CVRx, BMS, Novartis; he is co-founder of Bimyo GmbH, a company focussing on protection of mitochondria and is speaker of a Graduate Research School funded by the German Research Foundation (GRK2989). C.S. received speaker honoraria from AstraZeneca. P.C.S. received honoraria and travel support from Bayer, AstraZeneca, Daiichi Sankyo, Novartis, Actelion, Roche, Sanofi Aventis, Pharmacosmos, Medtronic, Thoratec, Boehringer Ingelheim, Heartware, Coronus, Abbott, Edwards, Boston Scientific, St. Jude Medical, Abiomed, DGK, ESC; and received research support to his institution from the National Institute of Health, German Research Foundation, Else Kröner Fresenius Foundation, American Heart Association, European Society of Cardiology, Actelion, Medtronic, BMBF, Abiomed, Boehringer Ingelheim, Boston Sci. N.M. reports personal fees from Edwards Lifesciences, Medtronic, Biotronik, Novartis, Sanofi Genzyme, AstraZeneca, Pfizer, Bayer, Abbott, Abiomed, B. Braun, and Boston Scientific, outside the submitted work. E.B.W. reports personal fees from Amgen, AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, CVRx, Daiichi Sankyo, Novartis, Novo Nordisk, and Pfizer, outside the submitted work. B.S. received speaker fees from Abiomed, AstraZeneca and Abbott; and research support from Abiomed, the German Research Foundation and the Else Kröner-Fresenius-Stiftung. All other authors have nothing to disclose.




