Knowledge and application of ESC/HFA guidelines in the management of advanced heart failure
Abstract
Aims
Management of advanced heart failure (HF) remains challenging despite specific sections in the 2021 European Society of Cardiology/Heart Failure Association (ESC/HFA) guidelines, with delays in referrals exacerbating the issue. This study aimed to evaluate the awareness and implementation of these guidelines among cardiologists and identify barriers to effective referral.
Methods and results
From June to October 2023, an online survey was disseminated through the ESC mailing list, targeting cardiologists across Europe. The survey investigated four areas: guideline awareness, healthcare network organization, clinical case management, and perceptions of mechanical circulatory support (MCS) outcomes. Respondents were categorized into heart failure cardiologists (HFCs), general cardiologists (GCs), and other participants (OPs). Among 497 respondents, 25% were heart HFCs, 40% were GCs, and 35% were OPs. A total of 84% of HFCs reported a high level of guideline knowledge, compared to 57% of GCs and 62% of OPs (p < 0.001). Additionally, 76% of HFCs ‘regularly or always’ used ESC/HFA criteria to identify advanced HF, compared to 44% of GCs and 48% of OPs (p < 0.001). Correct responses regarding the recommendation class for heart transplantation were 84%, 55%, and 60% (p < 0.0001), and for MCS as a bridge to transplantation, 69%, 65%, and 55% (p = 0.018) among HFCs, GCs, and OPs, respectively. Referring patients with severe HF to a tertiary centre team was found to be ‘very difficult’ or ‘difficult’ by 8.4% of HFCs, 19.6% of GCs, and 18.2% of OPs (p = 0.0005).
Conclusion
The study highlights significant disparities in knowledge and application of advanced HF guidelines among cardiologists, revealing an opportunity for educational initiatives. The difficulty in referring patients to tertiary centres underscores the need to improve the referral pathway for advanced HF patients.
Introduction
Heart failure (HF) is common and responsible for a substantial number of hospitalizations and increased risk of death.1 Despite advancements in treatment options for HF overall, advanced HF (AdHF) still affects over 10% of patients.2 The prognosis associated with AdHF remains poor with a 1-year risk of hospitalization or death reaching 70%.3
The management of HF with reduced ejection fraction is primarily based on neurohormonal treatments, which include four therapeutic classes recommended at Class I level, and specific device therapy when indicated.4 However, severe cases often exhibit treatment resistance or intolerance, characterized by repeated hospitalizations, poor functional class, progressive multiorgan failure, arrhythmias, and an increased risk of death.5 The management of these severe forms hinges on transplantation and mechanical circulatory support (MCS). If identified too late, palliative care becomes the only option.
To assist clinicians in identifying these severe cases, the definition AdHF, which was the subject of a position paper in 2018, has been directly incorporated into the European Society of Cardiology/Heart Failure Association (ESC/HFA) recommendations for the first time in 2021.4, 6 Despite these recommendations, the referral to tertiary centres often remains unsatisfactory, with many patients being referred too late and the implementation of MCS therapies being suboptimal.7, 8 This is particularly concerning given the recent technological advancements in MCS, such as centrifugal pumps, which have significantly improved outcomes.9
The mechanisms behind these referral delays are mostly unknown, and the reasons may include insufficient knowledge of the referral criteria, disorganization within the healthcare network, or a lack of knowledge and confidence in the treatment strategy for AdHF.10
We aimed to evaluate the knowledge level of European physicians regarding the 2021 ESC/HFA HF guidelines, specifically concerning the indications for referral and the specialized management of AdHF, including the implantation of left ventricular assist devices (LVAD) and transplantation. Additionally, we will explore the knowledge of long-term outcomes and beliefs associated with MCS implantation.
Methods
To evaluate the various components involved in the referral processes, we utilized mixed methods aimed at identifying potential barriers, including (i) the level of awareness regarding AdHF guidelines and therapy outcomes, (ii) the organization of the healthcare network, and (iii) beliefs and adherence to these therapies. The survey was made available online from 16 June 2023 to 31 October 2023.
Survey design
The survey was designed to last 15–20 min and assess four components of the referral process: awareness of guidelines, organization and modalities of the healthcare network, inclusion of a clinical case, and knowledge of long-term outcomes of MCS and perceptions of these therapies (questionnaire is available as online supporting information). The initial version of the questionnaire was drafted by G.B. and revised by F.G., N.G., and C.D., and further reviewed by the ESC guideline board.
Target population
The survey was distributed through the ESC mailing list to cardiologists who had previously expressed interest in the following areas: ‘Device therapy’, ‘Chronic HF’, ‘Acute HF’, ‘Cardiovascular surgery’. These criteria were selected to ensure the inclusion of a representative sample of cardiologists engaged in HF management and targeted by the ESC guidelines. This approach is also congruent with the demographic identified for forthcoming educational initiatives.
To tailor results according to the initial level of knowledge and to separate potential populations for future training, respondents were categorized by their field of activity, distinguishing between HF cardiologists (HFCs), general cardiologists (GCs), and other participants (OPs), including interventional cardiologists, electrophysiologists, imaging cardiologists, cardiac surgeons and acute cardiac care physicians.
Statistical analysis
Categorical variables were described as frequency and percentage. Missing data were excluded from the percentage calculations. Characteristics were compared across centre levels and professions using the chi-square test or Fisher's exact test as appropriate.
All analyses were conducted using R software (version 4.1.2), with a two-tailed significance level set at p < 0.05.
Results
Respondent demographics
A total of 498 respondents completed at least one question of the survey, with 497 specifying their specialty and included in the final analysis (online supplementary Table S1 and Figure S1). Among these, 200 (40%) were GCs, 124 (25%) were HFCs, and 173 (35%) were classified as OPs (online supplementary Figure S2). HFCs were more experienced, with 66% being seniors and 6.5% fellows/interns/residents, compared to a more balanced distribution in the other groups, with 60% seniors and an even split between juniors and fellows/interns/residents (p = 0.0002). A mirrored distribution was observed between HFCs, more than half of whom were in tertiary centres, and GCs, who were primarily in primary centres, while OPs were more evenly distributed across all three levels of centres (p < 0.0001).
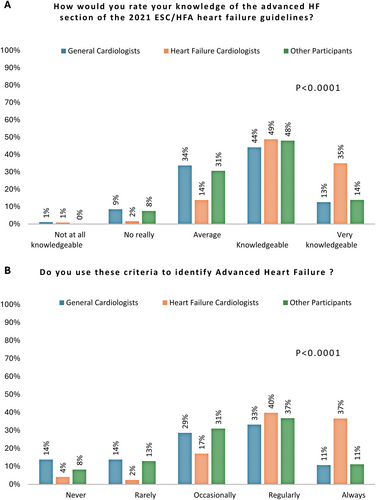
Guideline awareness
The primary findings regarding guideline awareness are illustrated in Figures 1 and 2. Our survey indicated that HFCs are more familiar with the guidelines compared to GCs and OPs. Specifically, 84% of HFCs described themselves as ‘knowledgeable or very knowledgeable’, compared to 57% of GCs and 62% of OPs (p < 0.001), and 76% ‘regularly or always’ use ESC/HFA criteria to identify AdHF, compared to 44% of GCs and 48% of OPs (p < 0.001). Conversely, 43% of GCs and 38% of OPs rated their guideline knowledge as ‘average’ or lower, leading to the occasional or rarer use of the ESC/HFA definition for identifying AdHF patients in 56% and 52% for GCs and OPs, respectively.
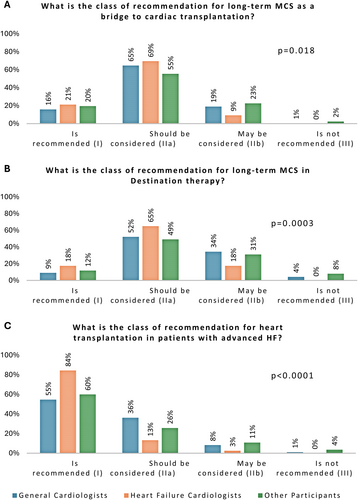
The evaluation of the recommendation class associated with AdHF therapy in the latest guidelines showed a similar distribution. Adequate response rates concerning the recommendation class were 84%, 55%, and 60% for heart transplantation (p < 0.0001); 69%, 65%, and 55% for MCS as a bridge to transplantation (p = 0.018); and 65%, 52%, and 49% for MCS as destination therapy (p = 0.0003) among HFCs, GCs, and OPs, respectively (Figure 2).
Care network and referral structure
A total of 43.5% of HFCs described themselves as referrals for AdHF, with 93% of the remaining HFCs referring patients to a cardiologist or a heart team; 16% of GCs and 7% of OPs do not have referrals and direct to an AdHF cardiologist or a heart team, respectively, with 62% and 72% (p < 0.0001) as outlined in Table 1. Less than 12% and 6% of respondents refer patients to an intermediate provider or a surgeon, respectively.
| Variable | General cardiologists (n = 200) | HF cardiologists (n = 124) | Other participants (n = 173) | p-value* |
|---|---|---|---|---|
| Do you have direct access to a referent (or dedicated team) in a tertiary centre, to discuss the implementation of ventricular assistance or transplantation? | ||||
| Refer to an advanced HF cardiologist | 30.5% | 20.2% | 30.1% | <0.0001 |
| Refer to a surgeon | 6.0% | 1.6% | 4.6% | |
| Refer to a heart team | 32.5% | 32.3% | 42.2% | |
| Go via an intermediate provider | 11.5% | 1.6% | 7.5% | |
| No referent for the discussion of assistance and transplantation | 16.5% | 0.8% | 11.0% | |
| I am myself a referent | 3.0% | 43.5% | 4.6% | |
| Do you have access to measuring pVO2 for HF patients? | ||||
| Yes, easy access for most HF patients | 13.8% | 53.7% | 36.6% | <0.0001 |
| Yes, access limited to selected HF patients | 30.3% | 26.8% | 24.4% | |
| Yes, access restricted to pre-transplant/LVAD patients | 5.6% | 7.3% | 4.1% | |
| No access | 50.3% | 12.2% | 34.9% | |
| For patients with ESC/HFA AdHF criteria, what proportion did you refer to an AdHF team? | ||||
| <25% | 39.3% | 23.1% | 29.9% | 0.005 |
| 25%–49% | 14.3% | 9.4% | 13.8% | |
| 50%–74% | 19.9% | 18.8% | 16.2% | |
| 75%–99% | 15.3% | 20.5% | 18.0% | |
| 100% | 11.2% | 28.2% | 22.2% | |
| How would you rate the difficulty of discussing a severe HF patient with a specialized team? | ||||
| Very difficult | 7.5% | 0.0% | 5.2% | <0.0001 |
| Difficult | 11.0% | 6.5% | 9.8% | |
| Average | 30.5% | 17.1% | 26.0% | |
| Easy | 32.5% | 32.5% | 24.9% | |
| Very easy | 18.5% | 43.9% | 34.1% | |
| How would you rate the difficulty of referring a severe HF patient to a tertiary structure? | ||||
| Very difficult | 8.0% | 0.8% | 9.3% | 0.0005 |
| Difficult | 11.6% | 7.6% | 9.9% | |
| Average | 28.1% | 18.6% | 24.4% | |
| Easy | 33.2% | 30.5% | 26.2% | |
| Very easy | 19.1% | 42.4% | 30.2% | |
- AdHF, advanced heart failure; ESC, European Society of Cardiology; HF, heart failure; HFA, Heart Failure Association; LVAD, left ventricular assist device; pVO2, peak oxygen consumption.
- * p-value from chi-square test or Fisher's exact test.
Additionally, 50% of GCs, 35% of OPs, and 12% of HFCs lacked access to exercise testing with peak oxygen consumption measurement for assessing patient severity, showing heterogeneous access to this fundamental examination (p < 0.0001).
Heart failure cardiologists, GCs, and OPs found it ‘very difficult’ or ‘difficult’ to refer patients with severe HF to a tertiary centre team in 8.4%, 19.6%, and 18.2% of cases, respectively (p = 0.0005). The results were broadly similar for discussing these patients with important intergroups variability (p < 0.0001). The proportion of referred patients varied significantly between groups, with HFCs > OPs > GCs (p = 0.005).
Evaluation of a stereotypical advanced heart failure clinical case
The first two questions of the clinical case assessed the risk of hospitalization or death within 1 year. Across all groups, approximately two-thirds of respondents accurately assessed the risk of hospitalization (p = 0.77), while around 60% accurately estimated the 1-year mortality risk (p = 0.41) (Table 2). Over 75% of cardiologists across groups believed the patient had AdHF; the primary differential diagnosis was worsening HF (p = 0.069), and the majority thought referral to a tertiary centre was necessary (p = 0.51).
| Variable | General cardiologists (n = 200) | HF cardiologists (n = 124) | Other participants (n = 173) | p-value* |
|---|---|---|---|---|
| What is the probability of 1-year hospitalization? | ||||
| ≤30% | 10.9% | 10.6% | 10.4% | 0.77 |
| 30%–40% | 23.4% | 18.7% | 18.4% | |
| >40% | 65.6% | 70.7% | 71.2% | |
| What is the probability of 1-year mortality (according to the MAGGIC or BCN-Bio-HF score)? | ||||
| ≤20% | 20.7% | 18.6% | 24.0% | 0.41 |
| 20%–25% | 19.0% | 13.6% | 19.5% | |
| >25% | 60.3% | 67.8% | 56.5% | |
| At this point, how would you describe the patient's situation? | ||||
| Advanced HF | 74.9% | 87.0% | 78.5% | 0.069 |
| Worsening HF | 13.6% | 9.8% | 11.0% | |
| Acute HF/decompensated HF | 10.6% | 2.4% | 9.9% | |
| Stable HF | 1.0% | 0.8% | 0.6% | |
| At this stage, which strategy do you think is the most appropriate? | ||||
| Refer him to an advanced HF team | 84.3% | 92.7% | 86.6% | 0.51 |
| Initiate inotropic treatment | 4.6% | 3.3% | 5.2% | |
| Getting closer to a palliative team, this patient is too old for a transplant | 4.1% | 2.4% | 4.1% | |
| Organize hospitalization at home to increase intravenous diuretics | 5.1% | 1.6% | 2.3% | |
| Increase beta-blockers | 2.0% | 0.0% | 1.7% | |
| Would you consider a short-term mechanical assistance device? | ||||
| Not indicated in this patient | 47.1% | 61.9% | 61.3% | 0.001 |
| Solution to be discussed in this patient, regardless of the type of assistance | 39.7% | 35.6% | 33.7% | |
| Indicated | 13.2% | 2.5% | 4.9% | |
| What is your perception of long-term LVAD in ‘destination therapy’ for this patient? | ||||
| Too early | 23.5% | 7.4% | 15.0% | 0.0002 |
| Not indicated | 7.5% | 2.5% | 9.0% | |
| It is a solution to discuss | 69.0% | 90.2% | 76.0% | |
- Correct answers according to panel experts or scores are highlighted in bold.
- HF, heart failure; LVAD, left ventricular assist device.
- * p-value from chi-square test or Fisher's exact test.
While the assessment of the patient's condition and 1-year risk was similar across groups, 52.9% of GCs, 38.7% of OPs, and 38.1% of HFCs believed short-term MCS was indicated or should be considered (p = 0.001), whereas 69.0%, 76.0%, and 90.2% considered long-term MCS, such as LVAD, should be considered (p = 0.0002).
Knowledge and perception of long-term outcomes of mechanical circulatory support devices
Among the five options for 5-year survival rates with centrifugal flow LVADs, ranging from 38% to 78% in 10% increments, only 42.3% of HFCs, 38.5% of OPs, and 28.3% of GCs selected the correct answer of 58% (p < 0.0001). Interestingly, GCs tended to underestimate actual survival whereas HFCs tended to overestimate it (Table 3). When considering complication rates of this device type, adequate estimation of risk was very limited, with only 35.4% of HFCs aware of the stroke rate (p = 0.036 for groups comparison) and 20.3% aware of bleeding rates (p = 0.017 for groups comparison), compared to much lower awareness in other groups. Respondents tended to overestimate (sometimes significantly) the risk of complications related to haemocompatibility.
| Variable | General cardiologists (n = 200) | HF cardiologists (n = 124) | Other participants (n = 173) | p-value* |
|---|---|---|---|---|
| What is the average 5-year survival of patients implanted with latest generation of LVAD (centrifugal pump from MOMENTUM 3 trial)? | ||||
| 38% | 17.9% | 1.9% | 11.5% | <0.0001 |
| 48% | 26.9% | 14.4% | 17.7% | |
| 58% | 28.3% | 42.3% | 38.5% | |
| 68% | 17.9% | 19.2% | 22.3% | |
| 78% | 9.0% | 22.1% | 10.0% | |
| What is the stroke rate of patients implanted with latest generation of LVAD (centrifugal pump from MOMENTUM 3 trial)? | ||||
| 0.05 Events/patient-years | 15.5% | 35.4% | 17.8% | 0.036 |
| 0.20 Events/patient-years | 30.2% | 22.0% | 26.2% | |
| 0.30 Events/patient-years | 24.1% | 13.4% | 21.5% | |
| 0.40 Events/patient-years | 13.8% | 11.0% | 10.3% | |
| 0.50 Events/patient-years | 16.4% | 18.3% | 24.3% | |
| What is the rate of digestive bleeding of patients implanted with latest generation of LVAD (centrifugal pump from MOMENTUM 3 trial)? | ||||
| 0.25 Events/patient-years | 10.3% | 20.3% | 7.6% | 0.017 |
| 0.35 Events/patient-years | 13.9% | 13.0% | 15.1% | |
| 0.45 Events/patient-years | 14.4% | 14.6% | 15.1% | |
| 0.55 Events/patient-years | 12.4% | 3.3% | 14.0% | |
| 0.65 Events/patient-years | 5.7% | 9.8% | 8.1% | |
| Don't know | 43.3% | 39.0% | 40.1% | |
| To what extent do you agree/disagree with the following statements describing the benefit–risk balance with long-term mechanical support? | ||||
| It improves quality of life | 69.1% | 80.5% | 74.4% | 0.082 |
| It deteriorates quality of life | 3.7% | 3.4% | 2.4% | 0.80 |
| Survival at 2 years is much lower than transplantation | 9.9% | 2.5% | 10.1% | 0.023 |
| Survival at 2 years is comparable to transplantation | 46.6% | 67.8% | 51.2% | 0.0010 |
| Survival at 5 years is much lower than transplantation | 27.7% | 22.0% | 33.9% | 0.084 |
| Survival at 5 years is comparable to transplantation | 15.7% | 36.4% | 20.2% | 0.0001 |
| Benefits are limited due to complications | 34.6% | 28.0% | 33.9% | 0.45 |
| In which case would you accept a long-term mechanical support (multiple answers possible) for yourself (or your family)? | ||||
| Life expectancy related to HF <1 year | 63.4% | 76.9% | 64.7% | 0.031 |
| Life expectancy related to HF <2 years | 34.6% | 45.3% | 34.1% | 0.11 |
| Dyspnoea NYHA class IV | 71.2% | 79.5% | 76.0% | 0.25 |
| Two hospitalizations in the year for HF | 23.6% | 41.9% | 32.9% | 0.003 |
| Contraindications to heart transplant | 69.6% | 82.9% | 71.3% | 0.023 |
| Creatine clearance <45 ml/min/1.73 m2 related to cardiorenal syndrome | 9.9% | 21.4% | 12.0% | 0.017 |
| Creatine clearance <30 ml/min/1.73 m2 related to cardiorenal syndrome | 32.5% | 40.2% | 32.9% | 0.34 |
| Categorical refusal regardless of my situation | 9.9% | 6.0% | 6.0% | 0.33 |
| Already on anticoagulant for atrial fibrillation | 5.8% | 12.0% | 7.8% | 0.16 |
- Correct answers according to the 5-year results of the MOMENTUM 3 trial are highlighted in bold.24
- HF, heart failure; LVAD, left ventricular assist device NYHA, New York Heart Association.
- * p-value from chi-square test or Fisher's exact test.
We evaluated the perception of survival with long-term MCS compared to standard treatment. The outcomes with MCS were comparable to transplantation at 2 and 5 years for 67.8% and 36.4% of HFCs, 46.6% and 15.7% of GCs, and 51.2% and 20.2% of OPs (p = 0.001 and p = 0.0001), respectively. Thirty percent of respondents believed the benefits were limited by complications, yet 70% or more still thought MCS improved quality of life.
Regarding quality of life under long-term MCS, HFCs had the best perception, with 49.2% finding it good or excellent, compared to 26.4% of GCs and 26.3% of OPs. Conversely, only 10% of HFCs considered it poor or worse, against 24.9% of GCs and 21.1% of OPs (p < 0.0001).
Acceptability of long-term mechanical circulatory support
Categorical refusal of MCS was under 10% across groups (p = 0.33). Only three severity criteria were associated with more than 50% acceptance: survival related to HF less than 1 year, New York Heart Association dyspnoea class IV, and contraindication to transplantation (Table 3).
Heart failure cardiologists were consistently more favourable towards receiving long-term MCS, significantly so in cases of survival less than 1 year (p = 0.031), two hospitalizations in the year for HF (p = 0.003), contraindication to heart transplantation (p = 0.023), and creatinine clearance <45 ml/min/1.73 m2 related to cardiorenal syndrome (p = 0.017).
Discussion
This survey is the first to assess barriers to referral by evaluating its various components, including knowledge of ESC/HFA guidelines and the adoption of the newly introduced definition of AdHF; network structure; knowledge of outcomes for heart replacement therapies and their perception by healthcare professionals. Analysis by professional activity allowed for a better understanding and the consideration of targeted educational efforts. The survey results highlighted that half of the non-HFCs self-reported low level of knowledge regarding HF guidelines and infrequent use of ESC/HFA criteria to identify AdHF. This self-assessment was corroborated by questionnaire results, indicating a need to enhance education for cardiologists and other clinicians about the prognosis and treatment of AdHF. Furthermore, our findings confirmed that GCs face challenges in discussing or referring patients, highlighting a suboptimal structure in the HF care network (Graphical Abstract).
Improving knowledge of heart failure guidelines
General cardiologists play a fundamental role in the initial diagnosis and management of patients with HF.1, 11 Enhancing the understanding of HF guidelines is crucial to optimize prognosis and reduce therapeutic inertia by optimizing medical treatment and enabling referral of patients intolerant to treatment or presenting with high-risk criteria.12, 13 Thus, knowledge dissemination serves a dual purpose of reducing the progression to AdHF and more quickly identifying at-risk patients to decrease the risk of hospitalization and death.
Our study showed that half of the non-HF specialists had modest knowledge of the HF guidelines and regularly used ESC/HFA criteria to identify AdHF patients, demonstrating the low current penetration among the cardiology community. The use of the acronym ‘I NEED HELP’ has proven effective in identifying and assessing patients at risk and is complementary to the ESC/HFA criteria.14 Regarding the management of AdHF, despite the therapies being classified as Class I or IIa, only half of the cardiologists were aware of the recommendation levels for AdHF therapies, including transplantation and MCS. This was confirmed in a clinical case where the identification of AdHF was uniform between all groups, although non-HF specialists were more inclined to propose short-term MCS and more frequently found MCS use in this context too premature.
Several strategies to improve AdHF knowledge are already proposed by the ESC and HFA, including the ‘postgraduate course in HF’15 featuring a module specifically on AdHF, the ‘HFA Advanced Heart Failure Course’,16 and a substantial amount of content on this topic available on ESC365 website.17 Additionally, an ‘HFA Certification in Heart Failure’ has been established to assess knowledge in HF.18 There is evidence showing that continuing medical education is associated with improved physician performance and patient health outcomes.19 One of the limitations of these teachings is their specialization targeting cardiologists with a strong interest in HF. Teaching and certification strategies targeting GCs could help improve knowledge levels in this population more specifically.
Improving the network structure
Our results suggest that referrals are predominantly made to a heart team or an AdHF cardiologist, and in a minority of cases, to an intermediate provider or a cardiac surgeon. Numerous advocacy efforts for early referral of severe HF patients have focused on the identification of severity signs by cardiologists.5, 12, 20 However, our results show that once the diagnosis is established, barriers to referral persist.21 It is interesting to note that half of the GCs face significant challenges in accessing cardiopulmonary exercise testing, as well as difficulties in discussing or referring patients to AdHF units. It is crucial for tertiary centres to offer solutions to address this issue and improve patient referral processes. In a recent study, HFCs referred half of the patients to tertiary centres despite their limited number compared to other cardiologists.22 Moreover, patients referred by HFCs were more often offered a proposal for cardiac transplantation or LVAD.22 In this paper, the authors did not formulate a difficulty in referral as a hypothesis to explain these differences, which seems consistent with our results.22 In its position statement on AdHF, the HFA emphasized the importance of network organization for managing patients with three functioning levels (community, specialized, and AdHF units) interacting synergistically.
Improving knowledge and perception of outcomes of advanced heart failure therapies
Less than one cardiologist in two (regardless of specialty) knew the 5-year survival rate for the latest generation of centrifugal pump MCS. The knowledge level of complication rates was even lower. However, HFCs had better level of knowledge compared with non-specialists. Regarding the perception of benefits, a majority of cardiologists agree that MCS improves quality of life with survival comparable to transplantation at 2 years. Nonetheless, significant differences between the groups exist, with HFCs evaluating the outcomes of MCS more positively in terms of survival, quality of life, and risk of events. Logically, HFCs were often more inclined to accept a long-term MCS than other cardiologists. Studies show that a significant part of resource use depends on organizational belief but especially on the belief of physicians.23 Indeed, some authors indicate that the use of health resources is primarily dependent on physician belief and less so on patient demand, which could account for up to 35% of resource use at the end of life.23 Our results suggest that a higher level of knowledge was associated with greater acceptability of the device and argues for significant educational plans across the cardiologic community.
Limitations
There are limitations secondary to the methodology used, relying on a voluntary response to the questionnaire with a risk of selecting respondents interested in the topic and overestimating the actual knowledge level of cardiologists. We did not collect data on the sex of respondents, prior participation in educational programmes, or the potential association between age or sex and programme participation, all of which could influence the findings. Future studies should consider incorporating these variables to assess any sex-specific differences in knowledge, as well as the impact of age on engagement with educational initiatives. To help address these limitations, future efforts could incorporate the questionnaire at both the beginning and end of structured educational programmes, such as the HFA Advanced Heart Failure Course. This approach would provide a means to assess the impact of training on physician knowledge and behaviour and offer insights into whether such interventions could help reduce disparities in HF management across regions.
Additionally, despite distribution through the ESC mailing list, country-specific and regional disparities in HF management cannot be excluded. Moreover, the design of the questionnaire did not allow for identification of the underlying causes of the observed disparities. Factors such as the heterogeneity of care, availability of resources, differences in healthcare structures, the absence of expert hub-and-spoke systems, and governmental resource challenges were not taken into account. Furthermore, there are no prior studies on this topic in Europe or globally to assess whether these disparities are better or worse than before.
Conclusion
In conclusion, our survey shows that there are numerous educational needs to improve the management of patients with AdHF. These needs include better identification of AdHF, the recognition and knowledge of level of recommendation associated with MCS and transplantation, and the outcomes of these therapies. Insufficient network structuring and the perception of MCS are other points that could limit referrals, on which clinicians can leverage for future educational programmes.
Conflict of interest: G.B. reports consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim and Novartis, outside the submitted work. N.G. reports consulting fees from AstraZeneca, Bayer, Boehringer, Novartis and Vifor, outside the submitted work. M.C. reports institutional research grants from Abbott, Novartis, Pfizer; clinical study contracts with institution: NovoNordisk, CorVia; advisory role, speaker honoraria, travel grants: Abbott, Abiomed, Amgen, Amicus Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Meyers Squibb, GE Healthcare, Krka Pharma, Novartis, NovoNordisk, Pfizer, Pulsify Medical, Roche, Swixx, Takeda, Teva Pharmaceutical Industries, Viatris, outside the submitted work. M.G.C.L. reports institutional research grants or contracts from AstraZeneca and Vifor Pharma; travel grants or honoraria for lectures, from Boehringer Ingelheim, AstraZeneca, Vifor Pharma, CareDx, Astellas, Abbott, Medtronic, Bayer or Pfizer, all outside the submitted work. The employer of K.D. receives speaker/consultancy fees by Abbott, Boehringer Ingelheim, AstraZeneca, FIRE1 and Echosense outside the submitted work. C.D. reports consulting fees from Abiomed and Abbott, outside the submitted work. F.G. reports consulting fees from Abbott, AstraZeneca, Alnylam, Corwave, FineHeart, Ionis and Pfizer, outside the submitted work. All other authors have nothing to disclose.




