Association of ventricular–arterial coupling with biomarkers involved in heart failure pathophysiology – the STANISLAS cohort
Abstract
Aims
Impaired left ventricular–arterial coupling (VAC) has been shown to correlate with worse prognosis in cardiac diseases and heart failure (HF). The extent of the relationship between VAC and circulating biomarkers associated with HF has been scarcely documented. We aimed to explore associations of VAC with proteins involved in HF pathophysiology within a large population-based cohort of middle-aged individuals.
Methods and results
In the forth visit of the STANISLAS family cohort, involving 1309 participants (mean age 48 ± 14 years; 48% male) from parent and children generations, we analysed the association of 32 HF-related proteins with non-invasively assessed VAC using pulse wave velocity (PWV)/global longitudinal strain (GLS) and arterial elastance (Ea)/ventricular end-systolic elastance (Ees). Among the 32 tested proteins, fatty acid-binding protein adipocyte 4, interleukin-6, growth differentiation factor 15, matrix metalloproteinase (MMP)-1, and MMP-9 and adrenomedullin were positively associated with PWV/GLS whereas transforming growth factor beta receptor type 3, MMP-2 and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were negatively associated. In multivariable models, only MMP-2 and NT-proBNP were significantly and inversely associated with PWV/GLS in the whole population and in the parent generation. Higher levels of NT-proBNP were also negatively associated with Ea/Ees in the whole cohort but this association did not persist in the parent subgroup.
Conclusion
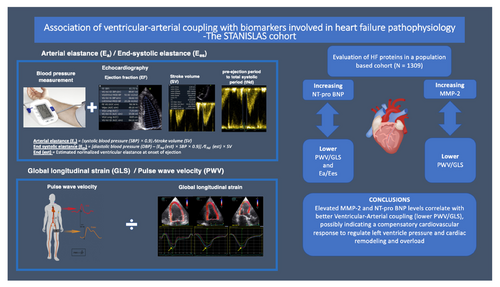
Elevated MMP-2 and NT-proBNP levels correlate with better VAC (lower PWV/GLS), possibly indicating a compensatory cardiovascular response to regulate left ventricular pressure amidst cardiac remodelling and overload.
Graphical Abstract
Introduction
The interaction between the heart and the arterial system, usually referred to as the ventricular–arterial coupling (VAC), has been targeted as an independent prognostic marker of cardiovascular disease.1 VAC has traditionally been measured via invasive intraventricular catheterization, the gold standard.2 This method, however, requires expensive equipment, specialized skills, and carries increased procedural risks. Assessed by echocardiography, VAC has been defined as the ratio between arterial (Ea) and end-systolic (Ees) elastance, where Ea is a determinant of arterial load on the left ventricle and Ees an indicator of the contractility and systolic stiffness of the left ventricle.2 Increasing Ea/Ees ratios, reflecting worse VAC, have been associated with prevalent heart failure (HF) and increased arterial stiffness, both independently associated with impaired microcirculation causing damage to the target organs.1, 3 As Ea/Ees is largely depending on the systemic vascular resistance and heart rate, together accounting for 98% of the variability of Ea,4 a new definition of VAC has recently emerged, expressed as the ratio between the pulse wave velocity (PWV) and global longitudinal strain (GLS).5, 6 The PWV/GLS ratio is considered a more informative VAC marker than Ea/Ees, as it more accurately measures arterial stiffness and left ventricular (LV) systolic function. Our research found that PWV/GLS is consistently linked to cardiovascular factors across ages, while worse Ea/Ees shows a paradoxically better cardiovascular risk profile in 30–70 year olds and neutral association in older patients.7 Higher values of PWV/GLS, characterizing impaired VAC, have also recently been directly associated with greater HF severity and worse functional capacity using combined cardiopulmonary-echocardiography exercise stress tests, especially in patients with HF with preserved ejection fraction (HFpEF).6 Accordingly, PWV/GLS offers a more comprehensive insight of VAC haemodynamics in HFpEF compared to Ea/Ees which is commonly normal in HFpEF patients due to simultaneous increase in both Ea and Ees.8 Understanding the relationship between VAC and circulating biomarkers associated with HF pathophysiology through proteomics might provide valuable insights into the underlying mechanisms of VAC impairment and potentially identify new therapeutic targets for HF. Recently, Kobayashi et al.9 were able to demonstrate that 14 out of 32 circulating biomarkers involved in inflammation, extracellular matrix (ECM) remodelling, and renal function and previously associated with HFpEF, were differently related to diverse phenotypes of diastolic dysfunction in asymptomatic middle-aged individuals derived from the STANISLAS family cohort. The exploration of whether similar distinctive biological patterns can be identified in relation to VAC remains an uncharted area of study.
As VAC impairment has been shown to be directly correlated with HF and worse functional capacity, we here aim to explore associations between the above-mentioned targeted proteins involved in HF pathophysiology, and VAC (assessed by PWV/GLS and Ea/Ees) within the STANISLAS cohort.
Methods
Study population
The STANISLAS (Suivi Temporaire Annuel Non-Invasif de la Santé des Lorrains Assuré Sociaux) cohort is designed as a single-centre familial longitudinal cohort which includes 4295 participants (1006 families) from the Nancy region of France, who were first recruited at the Center for Preventive Medicine between 1993 and 1995.10 The study recruited 1006 families, comprising 4295 subjects. The participating families were regarded as healthy, free of declared acute and/or chronic illness. During 2011–2016, 1705 participants of the original cohort participated in a fourth (STANISLAS-V4) re-examination including screening for cardiovascular risk assessment. The participants were initially healthy; however, a sizeable proportion of the cohort developed cardiovascular risk factors (mainly hypertension) during the course of the cohort and some developed cardiovascular disease. Of these individuals, we excluded 265 subjects without echocardiography at the fourth visit or with at least one missing parameter among PWV, GLS, Ea and Ees. To mitigate the potential influence of a history of cardiac disease on the association between VAC and biomarkers, participants with prevalent valvular heart disease or a LV ejection fraction (LVEF) <50% were excluded (n = 131). Conclusively, the study population composed 1309 participants (mean age 48 ± 14 years). Of these participants, 728 belonged to the parent generation (online supplementary Figure S1). The local ethics committee (Comité de Protection des Personnes Est III, Nancy, France) approved the study protocol and all study participants provided written informed consent in order to participate.
Pulse wave velocity
In accordance with the recommendations of the European Network for Non-invasive Investigation of Large Arteries, measurement of carotid to femoral PWV (cfPWV) was assessed with Complior® (Alam Medical, France) and Sphygmocor® CVMS (AtCor, Australia) devices.11 The mean value of two measurements was used to calculate the PWV. A third measurement was performed in cases when two measurements showed a difference of more than 0.5 m/s. The mean value of the three measurements was then used. Measurements were performed by four trained operators. The protocol has previously been presented in detail.10
Echocardiography
Conventional transthoracic echocardiograms were obtained by experienced sonographers using a commercially established ultrasound scanner (Vivid E9, General Electric Medical Systems, Horten, Norway) with a 2.5 MHz phased-array transducer (M5S). Parasternal long- and short-axis views as well as the standard apical views were assessed and, in every view, two-dimensional Doppler and tissue Doppler imaging with and without colour were obtained. Biplane Simpson's method was used to assess LVEF. To assess GLS, speckle tracking was performed for the LV systolic deformation in the three apical views: 4-chamber, 3-chamber and 2-chamber views. The time from the beginning of the QRS complex to the peak early positive (P) systolic (S) and global (G) strain of each segment was automatically calculated by the software. The global strain value was calculated as the mean strain value of all LV segments (n = 17). Since the definition of GLS is the change in length as a proportion to baseline length, it is presented as a percentage of LV deformation. Therefore, an improvement in LV systolic function will be seen as increasingly negative strain values. GLS below −16% is considered abnormal.12 Intra- and interobserver analyses for multilayer GLS (transmural, subendocardial, and subepicardial) have been evaluated on 50 patients within the current population. All intraobserver intraclass correlation coefficients (ICCs) were >0.75 and interobserver ICCs were >0.70 (the majority being >0.80).13
Ventricular–arterial coupling variables
The approximation of arterial load (numerator in the VAC equation) is usually derived from effective Ea: the ratio of end-systolic pressure (ESP) divided by stroke volume (SV) (ESP/SV).4 ESP is calculated as brachial systolic blood pressure ×0.9. The Ea depends on systemic vascular resistance, pulsatile load and heart rate whereas Ees is a load-independent measure of LV contractility and assessed from the principles of the classical pressure–volume relationship as the ratio of ESP and end-systolic volume normalized for body surface area (ESVi): ESP/ESVi.14 Ees has an inverse correlation with LV mass and is affected by LV chamber stiffness and geometry. In order to obtain Ees by the use of echocardiography, Chen et al.2 presented in 2001 a formula in which Ees was calculated as follows: Ees = (DBP – [End (est) × SBP × (0.9)]/End (est) × SV) where DBP and SBP are diastolic and systolic arm-cuff blood pressures, the SV is Doppler-derived SV, and End (est) is the estimated normalized ventricular elastance at the onset of ejection. VAC was assessed by two different methods: Ea/Ees and PWV/GLS. Since Ea and Ees share the same units (mmHg/ml), the ratio is unitless.
Circulatory biomarkers
Building on our group's prior methodology,9 we employed a panel of 32 circulating biomarkers involved in inflammation, ECM remodelling, and renal function. Plasma samples were analysed using the Olink Proseek® Multiplex cardiovascular disease (CVD) II, CVD III, and inflammation panel, which allow for the simultaneous quantification of 92 manually selected proteins using only 1 μl of plasma per kit. Each kit employs proximity extension assay technology featuring a dual-recognition DNA-coupled readout. This setup permits 92 oligonucleotide-labelled antibody probe pairs to bind to their corresponding targets within the sample. The amplicons are then quantified using a Fluidigm BioMark™ HD real-time polymerase chain reaction platform. The platform delivers log2 normalized protein expression (NPX) values, offering relative quantification of the proteins. A detailed description of the Olink® technology can be found on their website at https://www.olink.com. The abbreviations, full names of the 32 proteins included from the Olink® multiplex panels are described below.15
Inflammatory and adhesion biomarkers with known linkage to diastolic dysfunction in HFpEF were selected (fatty acid-binding protein [FABP]-4, growth differentiation factor [GDF]-15, galectin-3, vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, transforming growth factor beta [TGFB]-1, monocyte chemoattractant protein-1, receptor for advanced glycosylation end products, interleukin [IL]-6, IL-8, IL-1 receptor-like 2 [IL1RL2], tumour necrosis factor receptor 1 [TNF-R1], and osteopontin).16-20 Biomarkers expressing ECM remodelling and angiogenesis in HFpEF were selected as matrix metalloproteinase (MMP)-1, MMP-2, MMP-9, MMP-12, N-terminal propeptide of procollagen type III (PIIINP), C-terminal propeptide of procollagen type I (PICP), soluble ST2, N-terminal pro-B-type natriuretic peptide (NT-proBNP), B-type natriuretic peptide (BNP), C-type natriuretic peptide (CNP), troponin I, tissue inhibitor of metalloproteinase (TIMP)-1, TIMP-4, adrenomedullin (ADM), angiogenin (ANG), and vascular endothelial growth factor (VEGF)-A.9 Biomarkers associated with renal function in HFpEF were selected as blood urea nitrogen (BUN) and creatinine.21
Statistics
Proteins are depicted on a log2 scale, denoted as NPX in arbitrary units. This measurement corresponds to a twofold change in protein concentration. Continuous variables were expressed as mean ± standard deviation. Unadjusted linear regression models were carried out to explore the associations between PWV/GLS, Ea/Ees and circulating biomarkers. PWV/GLS and Ea/Ees were considered as the outcome variables, while the circulating biomarkers served as the exposure/independent variables. As PWV/GLS was not normally distributed, log-transformation was carried out. The adjustments were done in two steps: Model 1 (M1) age and sex, and Model 2 (M2) age, sex, body mass index, systolic blood pressure, diabetes and smoking. In Model 2, a p-value of p < 0.05 was considered statistically significant. The p-value of the association of each biomarker was corrected for false discovery rate (set at 1%) using the Benjamini–Hochberg method in both models M1 and M2. Beta estimates and their 95% confidence intervals are reported for the proteins with corresponding p-value. In addition, we assessed the relationship between PWV/GLS, Ea/Ees and circulating proteins in the subset of parents enrolled in the STANISLAS cohort to verify if the results were influenced by the presence of family members. All analyses were performed using SAS version 9.4.6 (SAS Institute Inc., Cary, NC, USA) and R version 3.6.1 (2019-07-05).
Network analysis
To construct the complex network, we utilized the Fight-HF graph knowledge box22 and extracted direct interactions between the studied biomarkers from the STRING database.23 Additionally, we included other proteins that were known to directly interact with at least three biomarkers. Indirect interactions were established by linking proteins to biomarkers that belong to the same REACTOME pathway.24
Clinical assessment
Clinical evaluation included information regarding past medical history, symptoms suggestive of any cardiovascular disease (HF, myocardial infarction, valvular disease, stroke, atrial fibrillation) and anthropometric measurements (height, weight, and waist). Body mass index was calculated as kilograms per square meter, and data regarding the study participants' medication were collected. Peripheral systolic and diastolic blood pressure was measured three times with an interval of 1 min, using an electronic sphygmomanometer (Dinamap carescape V100, GE). Hypertension was defined as self-declared hypertension and/or treatment with antihypertensive agents. Pre-diabetes was defined as a fasting plasma glucose between 100 mg/dl and 125 mg/dl and/or a glycated haemoglobin between 5.7% and 6.4%. Diabetes was defined as a fasting plasma glucose ≥126 mg/dl or a glycated haemoglobin ≥6.5% or a random plasma glucose ≥200 mg/dl with symptoms of hypoglycaemia or previous diagnosis of diabetes or anti-glycaemic drug therapy.25
Results
Patient characteristics
In the current study, the population had a mean age of 48 ± 14 years, and approximately half (52%, n = 684) were female. Current smoking was reported by 23%, hypertension was identified in 19% and diabetes in 4%. The mean values of Ea/Ees and PWV/GLS were 1.06 ± 0.20 and 0.42 ± 0.12, respectively. Information on patient characteristics is summarized in Table 1. Medications for hypertension, dyslipidaemia, or diabetes in the whole population and parent generation are shown in online supplementary Table S4.
| Characteristic | n | Overall (n = 1309) | Parents only (n = 728) |
|---|---|---|---|
| Age, yearsa | 1309 | 48 ± 14 | 60 ± 5 |
| Female sex | 1309 | 684 (52.3) | 379 (52.1) |
| Weight, kg | 1309 | 73 ± 15 | 74 ± 14 |
| Height, m | 1309 | 1.69 ± 0.09 | 1.66 ± 0.09 |
| BMI, kg/m2 | 1309 | 25.6 ± 4.4 | 26.6 ± 4.3 |
| SBP, mmHg | 1309 | 125.2 ± 15.1 | 129.3 ± 16.0 |
| DBP, mmHg | 1309 | 72.2 ± 8.8 | 74.3 ± 8.9 |
| Current smoking | 1308 | 294 (22.5) | 91 (12.5) |
| Hypertension | 1306 | 252 (19.3) | 229 (31.5) |
| Pre-diabetes | 1295 | 436 (33.7) | 351 (48.8) |
| Diabetes | 1295 | 52 (4.0) | 48 (6.7) |
| Antihypertensive treatment | 1309 | 216 (16.5) | 202 (27.8) |
| Lipid-lowering therapy | 1309 | 177 (13.5) | 176 (24.2) |
| Diabetes treatment | 1309 | 39 (3.0) | 35 (4.8) |
| EF, % | 1309 | 65.3 ± 6.1 | 66.3 ± 5.9 |
| LVMi, g/m2 | 1304 | 75 ± 18 | 79 ± 19 |
| NT-proBNPb | 1218 | 3.55 ± 0.98 | 3.81 ± 0.92 |
| Total cholesterol, g/L | 1308 | 2.13 ± 0.40 | 2.25 ± 0.38 |
| Triglycerides, g/La | 1308 | 1.03 ± 0.54 | 1.11 ± 0.55 |
| HDL cholesterol, g/L | 1308 | 0.58 ± 0.14 | 0.60 ± 0.14 |
| LDL cholesterol, g/L | 1306 | 1.34 ± 0.34 | 1.43 ± 0.34 |
| Creatinine, mmol/L | 1306 | 0.07 ± 0.01 | 0.07 ± 0.01 |
| eGFR (CKD-EPI formula), ml/min/1.73 m2a | 1308 | 97 ± 15 | 88 ± 12 |
| Urine albumin/creatininea | 1299 | 0.98 ± 1.94 | 1.10 ± 1.90 |
| PWV/GLS, m/s % | 1309 | 0.42 ± 0.12 | 0.46 ± 0.13 |
| Ea/Ees | 1309 | 1.06 ± 0.20 | 1.02 ± 0.19 |
- Values are given as mean ± standard deviation, or n (%).
- BMI, body mass index; DBP, diastolic blood pressure; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; Ea, arterial elastance; Eas, end-systolic elastance; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVMi, left ventricular mass index; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PWV, pulse wave velocity; SBP, systolic blood pressure.
- a Age, triglycerides, urine albumin/creatinine and eGFR have skewed distributions (mean ± standard deviation).
- b As it is an Olink biomarker, it is measured in normalized protein expression and hence no unit to be precised.
Association of ventricular–arterial coupling with circulating biomarkers
In the whole population, linear regression analysis adjusted for age and sex revealed that higher levels of FABP4, IL-6, GDF-15, ADM, MMP-1 and MMP-9 were positively associated with ratios of PWV/GLS, whereas higher levels of transforming growth factor beta receptor type 3 (TGFBR-3), MMP-2 and NT-proBNP were inversely associated with PWV/GLS. After performing regression analysis adjusted for multiple variables, only MMP-2 and NT-proBNP remained significantly inversely associated with PWV/GLS (Table 2).
| Biomarker | Whole cohort | Parents | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| Exp (Beta)a | FDR adjusted p-value | Exp (Beta) | FDR adjusted p-value | Exp (Beta) | FDR adjusted p-value | Exp (Beta) | FDR adjusted p-value | |
| Fatty acid-binding protein adipocyte (FABP4) | 1.02 (1.01; 1.04) | 0.008 | 1.00 (0.99; 1.02) | 0.79 | 1.03 (1.01; 1.06) | 0.013 | 1.01 (0.98; 1.03) | 0.84 |
| Receptor for advanced glycosylation end products (RAGE) | 0.99 (0.97; 0.99) | 0.069 | 0.99 (0.98; 1.01) | 0.55 | 0.98 (0.96; 0.99) | 0.072 | 0.99 (0.98; 1.01) | 0.76 |
| Interleukin-6 (IL-6) | 1.02 (1.01; 1.04) | 0.004 | 1.01 (0.99; 1.02) | 0.29 | 1.03 (1.01; 1.04) | 0.025 | 1.01 (0.99; 1.03) | 0.65 |
| Interleukin-8 (IL-8) | 0.99 (0.98; 1.01) | 0.57 | 0.99 (0.99; 1.01) | 0.55 | 0.99 (0.98; 1.01) | 0.73 | 0.99 (0.98; 1.01) | 0.83 |
| Interleukin-1 receptor-like 2 (IL1RL2) | 0.99 (0.99; 1.003) | 0.22 | 0.99 (0.98; 1.01) | 0.56 | 0.99 (0.98; 1.02) | 0.73 | 0.99 (0.98; 1.02) | 0.94 |
| Osteopontin (OPN) | 0.99 (0.98; 1.003) | 0.24 | 0.99 (0.99; 1.01) | 0.79 | 0.99 (0.97; 1.01) | 0.35 | 0.99 (0.98; 1.02) | 0.94 |
| Tumour necrosis factor receptor 1 (TNF-R1) | 1.01 (0.99; 1.02) | 0.36 | 0.99 (0.99; 1.01) | 0.79 | 1.01 (0.99; 1.03) | 0.35 | 0.99 (0.98; 1.02) | 0.92 |
| Transforming growth factor beta receptor type 3 (TGFBR-3) | 0.98 (0.97; 0.99) | 0.014 | 0.99 (0.97; 0.99) | 0.087 | 0.98 (0.96; 0.99) | 0.045 | 0.99 (0.97; 1.00) | 0.39 |
| Vascular cell adhesion molecule 1 (VCAM-1) | 0.99 (0.98; 1.01) | 0.39 | 0.99 (0.98; 1.01) | 0.55 | 0.99 (0.98; 1.01) | 0.71 | 0.99 (0.98; 1.01) | 0.76 |
| Intercellular adhesion molecule 1 (ICAM-1) | 1.01 (0.99; 1.02) | 0.24 | 1.00 (0.99; 1.01) | 0.83 | 1.01 (0.99; 1.03) | 0.55 | 1.00 (0.98; 1.02) | 0.95 |
| Monocyte chemotactic protein 1 (MCP-1) | 0.99 (0.99; 1.01) | 0.78 | 0.99 (0.99; 1.01) | 0.79 | 0.99 (0.98; 1.01) | 0.58 | 0.99 (0.98; 1.01) | 0.84 |
| Growth differentiation factor 15 (GDF-15) | 1.03 (1.02; 1.05) | 0.0003 | 1.01 (0.99; 1.03) | 0.29 | 1.04 (1.02; 1.06) | 0.003 | 1.01 (0.99; 1.03) | 0.65 |
| Galectin-3 (Gal-3) | 1.01 (0.99; 1.02) | 0.39 | 0.99 (0.99; 1.01) | 0.87 | 1.01 (0.99; 1.03) | 0.44 | 0.99 (0.98; 1.01) | 0.85 |
| Matrix metalloproteinase-1 (MMP-1) | 1.02 (1.01; 1.03) | 0.015 | 1.01 (1.00; 1.03) | 0.16 | 1.03 (1.01; 1.04) | 0.018 | 1.02 (1.00; 1.03) | 0.27 |
| Matrix metalloproteinase-2 (MMP-2) | 0.98 (0.97; 0.99) | 0.009 | 0.98 (0.97; 0.99) | 0.014 | 0.98 (0.96; 0.99) | 0.028 | 0.97 (0.96; 0.99) | 0.047 |
| Matrix metalloproteinase-9 (MMP-9) | 1.02 (1.01; 1.03) | 0.018 | 1.01 (0.99; 1.02) | 0.29 | 1.03 (1.01; 1.04) | 0.029 | 1.02 (1.00; 1.04) | 0.27 |
| Matrix metalloproteinase-12 (MMP-12) | 1.01 (1.00; 1.03) | 0.11 | 1.01 (0.99; 1.02) | 0.40 | 1.02 (0.99; 1.03) | 0.19 | 1.02 (0.99; 1.03) | 0.41 |
| C-terminal of type 1 collagen propeptide (C1CP) | 0.99 (0.98; 1.01) | 0.55 | 0.99 (0.99; 1.01) | 0.77 | 0.99 (0.97; 1.01) | 0.44 | 0.99 (0.98; 1.01) | 0.76 |
| N-terminal propeptide of procollagen type III (PIIINP) | 0.99 (0.99; 1.01) | 0.89 | 0.99 (0.98; 1.01) | 0.56 | 1.01 (0.99; 1.03) | 0.72 | 0.99 (0.98; 1.02) | 0.85 |
| ST2 protein (ST2) | 1.01 (0.99; 1.03) | 0.22 | 1.01 (0.99; 1.02) | 0.73 | 1.02 (0.99; 1.04) | 0.18 | 1.01 (0.99; 1.02) | 0.84 |
| N-terminal pro-B-type natriuretic peptide (NT-proBNP) | 0.98 (0.97; 0.99) | 0.015 | 0.98 (0.97; 0.99) | 0.014 | 0.98 (0.96; 1.00) | 0.23 | 0.98 (0.96; 0.99) | 0.27 |
| B-type natriuretic peptide (BNP) | 0.99 (0.97; 1.00) | 0.14 | 0.98 (0.97; 0.99) | 0.084 | 0.99 (0.97; 1.01) | 0.44 | 0.99 (0.97; 1.00) | 0.48 |
| Troponin I cardiac muscle (TNNI3) | 1.01 (0.99; 1.02) | 0.59 | 0.99 (0.99; 1.01) | 0.79 | 1.01 (0.99; 1.03) | 0.44 | 0.99 (0.98; 1.01) | 0.84 |
| C-type natriuretic peptide (NPPC) | 1.00 (0.99; 1.02) | 0.69 | 1.00 (0.99; 1.02) | 0.79 | 1.00 (0.99; 1.02) | 0.73 | 1.00 (0.99; 1.02) | 0.92 |
| Metalloproteinase inhibitor 1 (TIMP1) | 0.99 (0.98; 1.01) | 0.57 | 0.99 (0.98; 1.00) | 0.29 | 0.99 (0.98; 1.01) | 0.73 | 0.99 (0.97; 1.00) | 0.48 |
| Metalloproteinase inhibitor 4 (TIMP4) | 0.99 (0.99; 1.01) | 0.82 | 0.99 (0.98; 1.00) | 0.25 | 1.00 (0.99; 1.02) | 0.73 | 0.99 (0.97; 1.01) | 0.76 |
| Adrenomedullin (ADM) | 1.02 (1.01; 1.04) | 0.008 | 1.01 (0.99; 1.02) | 0.55 | 1.04 (1.02; 1.06) | 0.008 | 1.01 (0.99; 1.03) | 0.65 |
| Angiogenin (ANG) | 1.00 (0.99; 1.02) | 0.78 | 0.99 (0.99; 1.01) | 0.79 | 1.00 (0.98; 1.02) | 0.99 | 0.99 (0.98; 1.01) | 0.85 |
| Vascular endothelial growth factor A (VEGF-A) | 1.01 (0.99; 1.02) | 0.18 | 1.01 (0.99; 1.02) | 0.62 | 1.01 (0.99; 1.03) | 0.35 | 1.01 (0.99; 1.02) | 0.76 |
- FDR, false discovery rate.
- Model 1 adjusted for age and sex. Model 2 adjusted for age, sex, body mass index, systolic blood pressure, diabetes and smoking. In Model 1 and 2, a p-value of p < 0.05 was considered statistically significant.
- a Exp (Beta) are provided as the linear regression was performed with a log transformation of pulse wave velocity/global longitudinal strain. Each 1 unit increase in biomarker translates into multiplying pulse wave velocity/global longitudinal strain by Exp (beta).
In the parent generation, higher levels of FABP4, IL-6, GDF-15, MMP-1, MMP-9 and ADM were associated with higher ratios of PWV/GLS in regression models adjusted for age and sex, whereas higher levels of MMP-2 and TGFBR-3 were significantly associated with lower PWV/GLS. In multivariable adjusted models, only MMP-2 remained significantly inversely associated with PWV/GLS in the parent generation (Table 2). In the M2 adjusted regression, higher levels of NT-proBNP were associated with decreasing ratios of Ea/Ees in the whole population (Table 3). In the parent generation, no associations between proteins and Ea/Ees where observed. In regression models exploring the relationships among parameters included in VAC assessment (Ea, Ees, PWV, GLS), it was found that higher levels of both MMP-2 and NT-proBNP were significantly associated with higher GLS within both the entire population and the parent generation. On the contrary, elevated levels of MMP-2 and NT-proBNP were correlated with lower Ea. Notably, Ees and PWV displayed no associations with MMP-2 and NT-proBNP (online supplementary Table S1). When including participants with prevalent valvular heart disease or LVEF <50%, higher MMP-2 and NT-proBNP remained associated with lower PWV/GLS; however, this association did not persist in the multivariate model (online supplementary Table S2). Ea/Ees showed no relationship to biomarkers when including those with prevalent valvular heart disease or LVEF <50% (online supplementary Table S3). In correlation analysis, MMP-2 was positively correlated with NT-proBNP (online supplementary Figure S1).
| Biomarker | Whole cohort | Parents | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| Beta (95% CI) | FDR adjusted p-value | Beta (95% CI) | FDR adjusted p-value | Beta (95% CI) | FDR adjusted p-value | Beta (95% CI) | FDR adjusted p-value | |
| Fatty acid-binding protein adipocyte (FABP4) | 0.001 (−0.01; 0.01) | 0.99 | −0.008 (−0.02; 0.01) | 0.59 | −0.007 (−0.02; 0.01) | 0.73 | −0.015 (−0.04; 0.01) | 0.51 |
| Receptor for advanced glycosylation end products (RAGE) | −0.011 (−0.02; −0.00) | 0.16 | −0.011 (−0.02; 0.00) | 0.23 | −0.016 (−0.03; −0.001) | 0.32 | −0.015 (−0.03; 0.00) | 0.45 |
| Interleukin-6 (IL-6) | −0.000 (−0.01; 0.01) | 0.99 | −0.005 (−0.02; 0.01) | 0.63 | −0.006 (−0.02; 0.01) | 0.73 | −0.009 (−0.02; 0.01) | 0.53 |
| Interleukin-8 (IL-8) | −0.003 (−0.01; 0.02) | 0.89 | −0.004 (−0.02; 0.01) | 0.69 | −0.002 (−0.02; 0.01) | 0.91 | −0.002 (−0.02; 0.01) | 0.88 |
| Interleukin-1 receptor-like 2 (IL1RL2) | 0.004 (−0.01; 0.02) | 0.85 | 0.005 (−0.01; 0.02) | 0.63 | −0.012 (−0.03; 0.01) | 0.73 | −0.010 (−0.03; 0.01) | 0.53 |
| Osteopontin (OPN) | 0.000 (−0.01; 0.01) | 0.99 | 0.002 (−0.01; 0.01) | 0.84 | −0.008 (−0.02; 0.01) | 0.73 | −0.006 (−0.02; 0.01) | 0.66 |
| Tumor necrosis factor receptor 1 (TNF-R1) | −0.005 (−0.02; 0.01) | 0.84 | −0.009 (−0.02; 0.00) | 0.36 | −0.008 (−0.02; 0.01) | 0.73 | −0.011 (−0.03; 0.00) | 0.51 |
| Transforming growth factor beta receptor type 3 (TGFBR-3) | −0.012 (−0.02; −0.001) | 0.16 | −0.011 (−0.02; −0.00) | 0.18 | −0.007 (−0.02; 0.01) | 0.73 | −0.006 (−0.02; 0.01) | 0.64 |
| Vascular cell adhesion molecule 1 (VCAM-1) | −0.002 (−0.01; 0.01) | 0.95 | −0.001 (−0.01; 0.01) | 0.94 | 0.003 (−0.01; 0.02) | 0.91 | 0.003 (−0.01; 0.02) | 0.87 |
| Intercellular adhesion molecule 1 (ICAM-1) | −0.001 (−0.01; 0.01) | 0.99 | −0.004 (−0.02; 0.01) | 0.69 | 0.002 (−0.01; 0.02) | 0.91 | 0.000 (−0.01; 0.02) | 0.98 |
| Monocyte chemotactic protein 1 (MCP-1) | −0.006 (−0.02; 0.01) | 0.8 | −0.008 (−0.02; 0.00) | 0.42 | −0.008 (−0.02; 0.01) | 0.73 | −0.008 (−0.02; 0.01) | 0.57 |
| Growth differentiation factor 15 (GDF-15) | −0.005 (−0.02; 0.01) | 0.84 | −0.013 (−0.03; 0.00) | 0.23 | −0.002 (−0.02; 0.02) | 0.91 | −0.008 (−0.03; 0.01) | 0.64 |
| Galectin-3 (Gal-3) | −0.001 (−0.01; 0.01) | 0.99 | −0.003 (−0.01; 0.01) | 0.81 | 0.005 (−0.01; 0.02) | 0.74 | 0.003 (−0.01; 0.02) | 0.87 |
| Matrix metalloproteinase-1 (MMP-1) | −0.005 (−0.02; 0.01) | 0.84 | −0.007 (−0.02; 0.00) | 0.49 | 0.001 (−0.01; 0.02) | 0.92 | −0.000 (−0.01; 0.01) | 0.98 |
| Matrix metalloproteinase-2 (MMP-2) | −0.013 (−0.02; −0.002) | 0.15 | −0.013 (−0.02; −0.00) | 0.14 | −0.015 (−0.03; −0.00) | 0.32 | −0.016 (−0.03; −0.00) | 0.39 |
| Matrix metalloproteinase-9 (MMP-9) | −0.007 (−0.02; 0.01) | 0.77 | −0.011 (−0.02; −0.00) | 0.18 | −0.006 (−0.02; 0.01) | 0.73 | −0.008 (−0.02; 0.01) | 0.57 |
| Matrix metalloproteinase-12 (MMP-12) | 0.005 (−0.01; 0.02) | 0.84 | 0.002 (−0.01; 0.01) | 0.87 | −0.005 (−0.02; 0.01) | 0.74 | −0.006 (−0.02; 0.01) | 0.64 |
| C-terminal of type 1 collagen propeptide (C1CP) | 0.001 (−0.01; 0.01) | 0.99 | 0.001 (−0.01; 0.01) | 0.94 | −0.005 (−0.02; 0.01) | 0.73 | −0.006 (−0.02; 0.01) | 0.64 |
| N-terminal propeptide of procollagen type III (PIIINP) | −0.003 (−0.01; 0.01) | 0.89 | −0.004 (−0.01; 0.01) | 0.69 | −0.000 (−0.02; 0.02) | 0.97 | −0.000 (−0.02; 0.02) | 0.98 |
| ST2 protein (ST2) | −0.000 (−0.01; 0.01) | 0.99 | −0.000 (−0.01; 0.01) | 0.94 | −0.010 (−0.03; 0.01) | 0.73 | −0.013 (−0.03; 0.00) | 0.51 |
| N-terminal pro-B-type natriuretic peptide (NT-proBNP) | −0.021 (−0.03; −0.01) | 0.021 | −0.021 (−0.03; −0.01) | 0.019 | −0.019 (−0.04; −0.00) | 0.32 | −0.020 (−0.04; −0.00) | 0.39 |
| B-type natriuretic peptide (BNP) | −0.016 (−0.03; −0.004) | 0.13 | −0.016 (−0.03; −0.01) | 0.074 | −0.014 (−0.03; −0.00) | 0.32 | −0.014 (−0.03; −0.00) | 0.39 |
| Troponin I cardiac muscle (TNNI3) | −0.003 (−0.02; 0.01) | 0.85 | −0.005 (−0.02; 0.01) | 0.63 | −0.006 (−0.02; 0.01) | 0.73 | −0.010 (−0.03; 0.01) | 0.53 |
| C-type natriuretic peptide (NPPC) | −0.004 (−0.02; 0.01) | 0.84 | −0.004 (−0.02; 0.01) | 0.69 | 0.002 (−0.01; 0.02) | 0.91 | 0.002 (−0.01; 0.02) | 0.91 |
| Metalloproteinase inhibitor 1 (TIMP1) | −0.014 (−0.03; −0.003) | 0.15 | −0.015 (−0.03; −0.00) | 0.074 | −0.007 (−0.02; 0.01) | 0.73 | −0.009 (−0.02; 0.01) | 0.53 |
| Metalloproteinase inhibitor 4 (TIMP4) | −0.012 (−0.02; −0.00) | 0.16 | −0.014 (−0.03; −0.00) | 0.088 | −0.011 (−0.03; 0.00) | 0.73 | −0.014 (−0.03; 0.00) | 0.45 |
| Adrenomedullin (ADM) | 0.004 (−0.01; 0.02) | 0.85 | −0.004 (−0.02; 0.009) | 0.69 | −0.000 (−0.02; 0.02) | 0.97 | −0.005 (−0.02; 0.01) | 0.81 |
| Angiogenin (ANG) | −0.006 (−0.02; 0.01) | 0.8 | −0.008 (−0.02; 0.003) | 0.4 | −0.003 (−0.02; 0.01) | 0.91 | −0.004 (−0.02; 0.01) | 0.81 |
| Vascular endothelial growth factor A (VEGF-A) | −0.011 (−0.02; −0.00) | 0.16 | −0.015 (−0.03; −0.003) | 0.075 | −0.008 (−0.02; 0.01) | 0.73 | −0.010 (−0.02; 0.00) | 0.53 |
- CI, confidence interval; FDR, false discovery rate.
- Model 1 adjusted for age and sex. Model 2 adjusted for age, sex, body mass index, systolic blood pressure, diabetes and smoking. In Model 1 and 2, a p-value of < 0.05 was considered statistically significant.
Network analysis
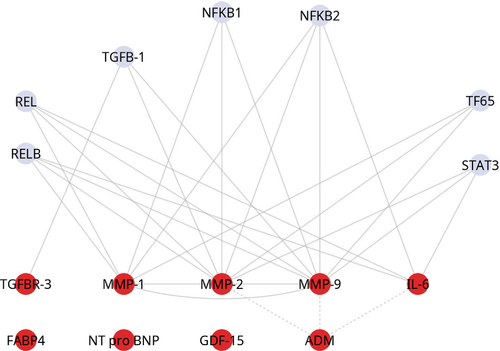
In the network analysis based on the nine proteins (FABP4, IL-6, GDF-15, ADM, MMP-1, MMP-9, TGFBR-3, MMP-2 and NT-proBNP) associated with VAC, v-rel avian reticuloendotheliosis viral oncogene homolog A and B (RELA and RELB) expressed an interaction with MMP-1, MMP-2, MMP-9 and IL-6. Similarly, nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 and 2 (NFKB1 and NFKB2) showed an interaction with MMP-1, MMP-2, and MMP-9, with the additional finding that NFKB also interacted with IL-6. T65 interacted with MMP-1, 2, 9 while signal transducer and activator of transcription 3 (STAT3) interacted with MMP-2, MMP-9 and IL-6. Transforming growth factor beta 1 (TGFB-1) interacted with TGFBR-3 (Figure 1).
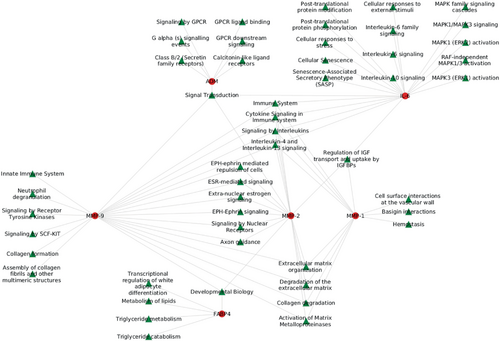
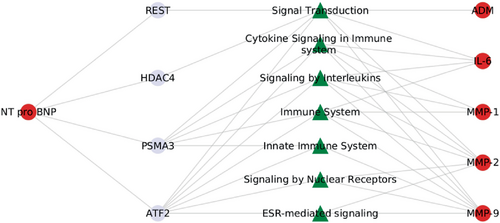
When examining the interactions of proteins with biological pathways, we found that several pathways were associated with two or more proteins (Figure 2). These core pathways were primarily related to interleukin signalling, ephrin signalling, and remodelling. Further investigation into the pathways involving NT-proBNP revealed its association with cytokine/interleukin signalling and immune response, particularly in the context of VAC-related proteins (Figure 3).
Discussion
In this comprehensive population-based cohort study, we have demonstrated a significant association between elevated levels of MMP-2 and NT-proBNP and improved VAC, as evidenced by reduced PWV/GLS ratios. Furthermore, we observed that higher NT-proBNP levels correlated with a decrease in the Ea/Ees ratio. The involvement of MMP-2 in extracellular matrix turnover and vascular remodelling, coupled with the role of NT-proBNP in modulating LV load, suggests the presence of a sophisticated compensatory cardiovascular mechanism. This mechanism appears to actively regulate LV pressure during the early stages of cardiac remodelling and overload, which are pivotal in the progression of cardiovascular diseases. This insight provides a deeper understanding of the complex interplay between biomarkers and cardiac function in the early phases of cardiovascular pathologies (Graphical Abstract).
Protein association with ventricular–arterial coupling
In the current study, higher levels of FABP4, IL-6, GDF-15, ADM, MMP-1 and MMP-9 were positively associated with PWV/GLS adjusted for age and sex. High plasma levels of these biomarkers have been associated with different aspects of HF.9 Given that previous studies have linked increased PWV/GLS to the advancement of HF and atherosclerotic disease, it is understandable that higher levels of these cardiovascular-related biomarkers in plasma are associated with higher PWV/GLS.6 However, when all relevant cardiovascular variables were considered, these associations ceased to persist, indicating that they are more likely attributable to underlying clinical conditions associated with VAC, rather than being intrinsic to VAC itself.
Contrary to expectations, higher levels of MMP-2 and NT-proBNP were significantly inversely associated with PWV/GLS, both in initial analyses and after adjusting for all pertinent cardiovascular variables. This inverse relationship, especially for MMP-2 with PWV/GLS, persisted even in the parent generation subgroup, underscoring the consistency of our findings across various age groups. Several factors could contribute to this initially counterintuitive result. MMP-2 is a zinc-dependent protease enzyme involved in the degradation of the ECM and basement membrane.26 In HF, MMP-2 has been shown to play an important role in ECM degradation which leads to myocardial fibrosis, ventricular remodelling, and impaired cardiac function.27 Elevated levels of MMP-2 have been detected in patients with HF, and increased MMP-2 activity has been linked to the development and progression of HF, as well as poor outcomes.28, 29 The mechanisms by which MMP-2 contributes to HF are not fully understood but may include its effects on the ECM, its ability to influence the expression of other MMPs, growth factors, and cytokines, and its involvement in the activation of inflammatory pathways and the remodelling of the vasculature.30 Since higher MMP-2 has previously been associated with arterial stiffness, it is possible that the inverse relationship between levels of MMP-2 and PWV/GLS might reflect cardiac compensatory mechanisms expressed as increasing GLS in order to offset some of the negative effects of increased MMP-2 activity on the arteries.31 In support to this hypothesis, our study showed that increasing levels of MMP-2 and NT-proBNP were both associated with higher values of GLS. In mice with cardiac-specific overexpression of tumour necrosis factor (TNF)-α, deficiency of MMP-2 was associated with reduced survival rates, worsening LV contractile dysfunction, heightened LV end-diastolic pressure, pleural effusion, and an increased number of myocardial infiltrating inflammatory cells, including macrophages in the myocardium. These inflammatory cells impaired cardiac function and contributed to early death in TNF-α-overexpressing transgenic mice that lacked MMP-2.32 Since we showed that low plasma levels of MMP-2 were associated with worse VAC reflected by higher ratios of PWV/GLS, our findings underscore that MMP-2 deficiency may contribute to an unfavourable cardiovascular state, emphasizing the significance of MMP-2 in maintaining optimal VAC.
The second biomarker that remained significant in the multivariable model was NT-proBNP which is produced in the heart in response to ventricular stretch and volume overload, and released into the bloodstream where it promotes natriuresis and vasodilatation. Elevated NT-proBNP levels are known to indicate poorer outcomes in HF and, combined with echocardiography, are crucial in HF diagnosis.33 While B-type natriuretic peptide (BNP) is the biologically active hormone and an effector in the regulation of blood pressure, volume, and salt balance, NT-proBNP is a precursor that is broken down to produce BNP, making NT-proBNP essential for the formation of the active hormone despite not being biologically active itself. A recent study has demonstrated that elevated levels of NT-proBNP were positively associated with PWV/GLS in patients with HFpEF and HF with reduced ejection fraction (HFrEF).6 This is thought to relate to increased LV filling pressure and myocardial stretching, triggering natriuretic peptide release. However, these findings primarily concern patients with manifest HF.
In the broader population of the STANISLAS cohort, an inverse relationship between PWV/GLS and NT-proBNP was consistently observed. In our cohort, NT-proBNP levels are much lower than those observed in HF cohorts. Interestingly, it is these higher levels within the normal range that are associated with improved VAC in our analysis. This intriguing outcome may highlight the beneficial effects of natriuretic peptides. NT-proBNP, once converted to BNP, induces arterial vasodilatation, which lowers systemic vascular resistance, reducing cardiac afterload and improving LV function.34 However, NT-proBNP variations, even when within the normal range, are associated with long-term cardiovascular outcomes.35 As the role of NT-proBNP (or rather of BNP, broken down from NT-proBNP, its biological effector) is yet insufficiently explored in the setting of population science, further research should better delineate the early interactions between VAC, neurohormonal pathways, and clinical trajectory. Notably, NT-proBNP levels have been repeatedly shown to exhibit variations in different populations and pre-disease settings.36, 37
This is aligned with findings that acute afterload reduction, like with sildenafil, decreases Ea without affecting Ees, thereby improving VAC.38 This theory is bolstered by our findings showing that higher NT-proBNP levels correlate with lower Ea but not with Ees. This intriguing outcome may highlight the beneficial effects of natriuretic peptides. Such positive aspects are already leveraged in managing HFrEF through medications like sacubitril/valsartan. Following this idea, evaluating the effect of sacubitril/valsartan on changes in VAC would be extremely insightful. In essence, elevated natriuretic peptides, even in the absence of increased LV pressures, may be associated with various positive outcomes, including potential improvements in VAC.
Insights from the network analysis
In all, the network analysis showed that key regulatory proteins, including NFKB1, NFKB2, REL, RELB, T65, STAT3 and TGFB-1, physically interacted with multiple biomarkers associated with VAC, such as MMP-1, MMP-2, MMP-9, IL-6 and TGFBR-3. This suggests a complex molecular interplay involving these proteins, potentially influencing the pathways related to VAC and contributing to the observed associations in the study. As anticipated, the protein-signalling pathways revealed that interleukin/immune signalling and remodelling pathways predominantly interacted with MMP-1, MMP-2, and MMP-9. This is primarily because metalloproteinases play crucial roles in the degradation of ECM components, which is essential for tissue remodelling and repair. Additionally, these MMPs are known to be involved in the regulation of inflammatory processes through their ability to modulate cytokines and chemokines, further linking them to interleukin and immune signalling pathways.39 Further, we believe that the association present in this cohort between NT-proBNP and PWV/GLS highlights a mechanism different from what is occurring in established HF. The network analysis also suggested that NT-proBNP interacted with MMPs and interleukins, primarily through modulation of immune response, cytokine, and interleukin signalling. This suggests that natriuretic peptides, known for their anti-inflammatory effects, may act as a counter-regulatory mechanism in the presence of inflammation and tissue remodelling, highlighting their potential role in mitigating inflammatory processes associated with VAC.40
The interaction between NFKB1 and NFKB2 with MMP-1, MMP-2, MMP-9, and IL-6 suggests that these proteins may be involved in regulating the expression of genes involved in inflammation, tissue remodelling, and immune responses. RELA and RELB are also subunits of the transcription factor complex nuclear factor-κB, and like NFKB1 and NFKB2, they play important roles in regulating the expression of genes involved in inflammation, immune responses, and other cellular processes.41 The interaction of RELA and RELB with MMP-1, MMP-2, and MMP-9 implies that these proteins might participate in the regulation of gene expression associated with tissue and ECM remodelling. The interaction observed among T65, STAT3, MMP-2 and MMP-9, implies their potential mutual role in controlling the expression of genes related to immune responses, tissue remodelling, and inflammation. As anticipated, the interaction between TGFB-1 and TGFBR-3 occurred, and this can be attributed to the role of TGFBR-3 as a co-receptor in the TGF-β superfamily. Higher concentration of TGFB-1 has been linked to increased arterial stiffness in hypertension subjects42 which potentially influence the pathways related to VAC and contributing to the observed associations in the study. In summary, the network analyses suggest that biomarker expression and their correlation with VAC could be influenced by factors affecting inflammation and tissue remodelling.
Strengths and limitations
To the best of our knowledge, this is the first study to compare two different methods of VAC assessment in regard to biomarkers involved in HF pathophysiology. Nevertheless, due to the cross-sectional nature of this study, the establishment of a causal relationship between the biomarkers under consideration and VAC remains unattainable. Additionally, no information was available regarding the duration of hypertension, diabetes and smoking. Finally, the study was undertaken in a defined region with a rather homogeneous genetic background, and other studies should be conducted to ensure the generalizability of our results. Analysing the parent group separately highlights generation-specific risk factors for cardiovascular diseases that could be influenced by differences in lifestyle, environmental exposures, and medical history across age groups. This separation also helps to identify changes specific to an aging population, where the prevalence of cardiovascular risk factors increased in the parent group. Invasive intraventricular catheterization remains the gold standard method to assess VAC (Ea/Ees). However, this requires expensive specialized equipment and expertise but also comes with an increased procedure risk. Even if Ea/Ees still is regarded as the conventional methods to assess VAC, PWV/GLS now offers a surrogate reliable and clinically accepted index of VAC. Given that the study participants were selected from a healthy population and individuals with pre-existing HF were excluded, it should be acknowledged that these findings may not fully capture the association between VAC and the biomarkers in the context of manifest cardiovascular disease.
Conclusion
In a middle-aged population cohort free from known cardiovascular disease, we here demonstrate that higher concentrations of several proteins linked to inflammation, cell growth and adhesion were associated with impaired VAC (higher PWV/GLS). However, when accounting for important surrounding CVD parameters in the multivariable model, only two proteins, namely MMP-2 and NT-proBNP exhibited a significant inverse association with PWV/GLS, whereas higher levels of NT-proBNP were also negatively associated with Ea/Ees. Among individuals without cardiovascular disease, the outcomes might indicate a compensatory cardiovascular mechanism to sustain low LV filling pressure despite fibrotic remodelling and cardiac pressure overload. Further studies are warranted to determine the pathophysiological role of circulating biomarkers for VAC.
Acknowledgements
We are highly grateful to the Vandoeuvre-Lès-Nancy Center de Médecine Préventive staff and Institut National de la Santé et de la Recherche Médicale (INSERM) U1122 who managed the STANISLAS (Suivi Temporaire Annuel Non-Invasif de la Santé des Lorrains Assurés Sociaux) cohort for the first 3 visits. The authors deeply thank the staff of the Clinical Investigation Center and other personnel involved in the STANISLAS cohort management: biostatisticians, computer scientists, co-investigators, data managers, data entry operators, echocardiographists, echographists, imaging and laboratory engineer/technicians, project managers, quality engineers, nurses, secretaries, study coordinators and all persons who helped to the funding, initiation, accrual, management, and analysis of the fourth visit of the STANISLAS cohort. The authors also thank the Centre de Ressources Biologiques (CRB) Lorrain of the Nancy Center Hospitalier Régional Universitaire of Nancy (CHRU) for management of the biobank as well as the steering, technical and scientific committees.
Funding
M.M. was supported by grants from the Swedish Medical Research Council, the Swedish Heart and Lung Foundation, Skåne University Hospital, the Crafoord Foundation, Knut and Alice Wallenberg Foundation and the Marianne and Marcus Wallenberg Foundation and by Wallenberg Centre for Molecular Medicine at Lund University. N.G. and P.R. are supported by public grants overseen by the European Commission (EUFP7) and the French National Research Agency (ANR) as part of the second ‘Investissements d'Avenir’ programme FIGHT-HF (ANR-15-RHU-0004); programme HOMAGE under grant agreement No. 305507; and project FIBROTARGETS grant HEALTH-2013-602904.
Conflict of interest: none declared.