Effect of long-term tafamidis treatment on health-related quality of life in patients with transthyretin amyloid cardiomyopathy
Abstract
Aims
To evaluate the effect of long-term tafamidis treatment on health-related quality of life (HRQoL) in patients with transthyretin amyloid cardiomyopathy (ATTR-CM) enrolled in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension (LTE) study.
Methods and results
We examined change from baseline in Kansas City Cardiomyopathy Questionnaire overall summary (KCCQ-OS) and clinical summary (KCCQ-CS) scores in patients who received tafamidis meglumine 80 mg for 30 months in ATTR-ACT and tafamidis (meglumine 80 mg or bioequivalent free acid 61 mg) for 30 months in the LTE study, and in patients who received placebo for 30 months in ATTR-ACT and tafamidis for 30 months in the LTE study. In ATTR-ACT, 176 and 177 patients were randomized to tafamidis 80 mg and placebo, respectively. Patients who continuously received tafamidis had a 6- to 7-point reduction in least squares (LS) mean (standard error) KCCQ-OS and KCCQ-CS scores at month 30 (−6.25 [1.53] and −7.48 [1.39]), with little or no further decline over the next 30 months (−5.92 [1.77] and −9.21 [1.88] at month 60). Patients who received placebo in ATTR-ACT had a 20-point reduction in LS mean KCCQ-OS and KCCQ-CS scores at month 30 (−19.60 [1.94] and −19.90 [2.01]), but the decline slowed after initiating tafamidis (−24.70 [3.04] and −25.30 [3.36] at month 60).
Conclusion
Tafamidis reduced HRQoL decline in patients with ATTR-CM. Patients continuously treated with tafamidis for 60 months demonstrated stabilized HRQoL. In patients who initially received placebo in ATTR-ACT, tafamidis reduced the decline in HRQoL during the LTE study.
Transthyretin amyloid cardiomyopathy (ATTR-CM) is caused by the accumulation of variant or wild-type transthyretin amyloid in the myocardium and is associated with progressive heart failure and declining quality of life (QoL).1 In the Phase 3 Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT; NCT01994889), tafamidis reduced all-cause mortality and cardiovascular-related hospitalizations after 30 months in patients with ATTR-CM compared with placebo.2 Tafamidis also reduced decline in Kansas City Cardiomyopathy Questionnaire overall summary (KCCQ-OS) scores starting from month 6, and in all four of its subdomains at month 30.2, 3 A greater proportion of tafamidis-treated patients reported improvement or no change in the KCCQ-OS at month 30 (41.8% vs. 21.4% with placebo).3 Patients completing ATTR-ACT could enrol in the ongoing long-term extension (LTE) study (NCT02791230) wherein all patients received open-label tafamidis for 60 months. This interim analysis of the LTE study examined the impact of long-term tafamidis treatment on health-related QoL (HRQoL), measured by KCCQ-OS and Kansas City Cardiomyopathy Questionnaire clinical summary (KCCQ-CS) scores.
Details of ATTR-ACT have been reported.2 Briefly, patients aged 18–90 years with biopsy-confirmed ATTR-CM and a history of heart failure were randomized 2:1:2 to once daily tafamidis meglumine 80 or 20 mg or placebo for 30 months. In the LTE study, patients receiving tafamidis in ATTR-ACT continued this dose (continuous tafamidis group) and patients receiving placebo in ATTR-ACT were randomized 2:1 to tafamidis meglumine 80 or 20 mg (placebo to tafamidis group). Following a protocol amendment, all patients in the LTE study transitioned to tafamidis free acid 61 mg (bioequivalent to tafamidis meglumine 80 mg). Patients receiving tafamidis meglumine 20 mg in ATTR-ACT were excluded from this analysis as this dose is not approved for the treatment of ATTR-CM. Both studies were approved by the independent review board or ethics committee at each participating site and were conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization Good Clinical Practice guidelines. All patients provided written informed consent.
The KCCQ is a 23-item patient-reported questionnaire assessing patients' HRQoL.4, 5 The KCCQ-OS score is the mean of the physical limitation, symptom frequency, symptom burden, QoL, and social limitation domains. The KCCQ-CS score is the mean of the physical limitation, symptom frequency, and symptom burden domains. Scores range from 0 to 100; higher scores indicate better health status. Scores were collected at baseline and every 6 months thereafter. We examined change from baseline in KCCQ-OS and KCCQ-CS scores over 60 months in the continuous tafamidis and placebo to tafamidis groups. The least squares (LS) mean change from baseline analysis was based on a mixed model for repeated measures with an unstructured covariance matrix. Baseline scores were included as a covariate; centre and patient-within-centre were included as random effects; and treatment, visit, genotype, and visit-by-treatment interaction were included as fixed effects.
In ATTR-ACT, 176 patients received tafamidis 80 mg and 177 received placebo: 89.8% and 88.7% were male, 76.1% and 75.7% had wild-type disease, and median age was 76 and 74 years, respectively.6 In the tafamidis 80 mg and placebo groups, respectively, 110 and 82 patients enrolled and received tafamidis in the LTE study. Characteristics at month 30 have been reported.7
Mean (standard deviation [SD]) KCCQ-OS scores at baseline were 67.12 (21.29) for patients treated with tafamidis 80 mg in ATTR-ACT and 65.90 (21.74) for patients treated with placebo in ATTR-ACT. The continuous tafamidis group versus placebo to tafamidis group had a significantly smaller decline in KCCQ-OS score at months 30 (LS mean difference [95% confidence interval, CI] in change between groups: 13.38 [9.21–17.54], p < 0.0001) and 60 (18.83 [12.64–25.03], p < 0.0001) (Figure 1, online supplementary Table S1). The continuous tafamidis group had a 6-point reduction in LS mean (standard error [SE]) KCCQ-OS scores at month 30 (−6.25 [1.53]), with no further decline over the next 30 months (−5.92 [1.77] at month 60). The placebo to tafamidis group had a 20-point reduction in LS mean KCCQ-OS scores at month 30 (−19.60 [1.94]), but the decline slowed after initiating tafamidis in the LTE study (−24.70 [3.04] at month 60).
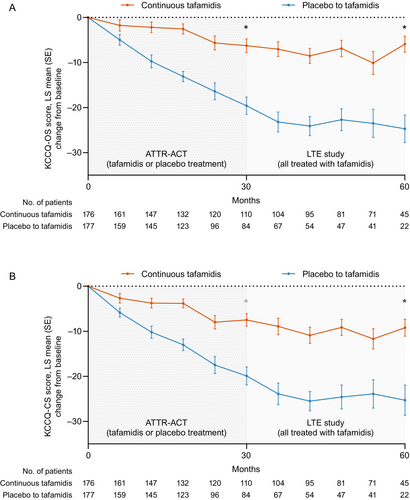
Mean (SD) KCCQ-CS scores at baseline were 71.12 (20.11) in the tafamidis 80 mg group and 70.15 (20.51) in the placebo group. Change from baseline in KCCQ-CS scores followed a similar pattern to KCCQ-OS scores (Figure 1, online supplementary Table S1). In the LTE study, KCCQ-CS scores stabilized in the continuous tafamidis group (LS mean change [SE] from baseline, −7.48 [1.39] at month 30 and −9.21 [1.88] at month 60), whereas tafamidis slowed the decline in KCCQ-CS scores in the placebo to tafamidis group (LS mean change [SE] from baseline, −19.90 [2.01] at month 30 and −25.30 [3.36] at month 60).
Results were similar in the subgroups of patients with wild-type and variant disease (online supplementary Tables S2 and S3).
In ATTR-ACT, tafamidis was associated with significant attenuation in the decline of KCCQ-OS scores over 30 months compared with placebo. This analysis found that KCCQ-OS and KCCQ-CS scores stabilized after 60 months in patients who received continuous tafamidis. Additionally, patients who received continuous tafamidis had less pronounced decline from baseline in KCCQ-OS and KCCQ-CS scores versus patients who received placebo then tafamidis. Despite this, tafamidis slowed the decline in KCCQ-OS and KCCQ-CS scores when patients who received placebo in ATTR-ACT transitioned to tafamidis in the LTE study. A similar effect with survival was reported in the LTE study.7 Together, these findings emphasize the importance of early treatment and demonstrate that tafamidis fulfils two primary goals of heart failure treatment: reducing QoL decline and improving survival.8 The stabilization of HRQoL after month 30 in the continuous tafamidis group suggests that a period of time is required after initial interruption of amyloid fibril deposition to achieve a full response. These long-term results are encouraging, especially for patients treated early in the disease course.
Study limitations include missing patient data during follow-up and the impracticality of conducting reliable stratified sub-analyses based on New York Heart Association class given the sample size.
In summary, HRQoL stabilized in patients continuously treated with tafamidis meglumine 80 mg or free acid 61 mg for 60 months in ATTR-ACT and its LTE study. In patients initially treated with placebo in ATTR-ACT, the decline in HRQoL was reduced after transitioning to tafamidis.
Funding
This study was sponsored by Pfizer. Medical writing support was provided by Emily Balevich, PhD, of Engage Scientific Solutions and was funded by Pfizer.
Conflict of interest: M.G. reports grants, participation in advisory boards, and consultancy fees paid to her institution from Alnylam, Eidos, Janssen, Novo Nordisk, and Pfizer. M.K.D. reports honoraria for advisory board participation from Akcea, Alnylam, AstraZeneca, Bayer, Boehringer Ingelheim, Ferring, Ionis, Janssen, and Pfizer; consulting fees from Janssen and Novo Nordisk; speaking fees from Bayer, Ferring, Janssen, and Pfizer; and research funding from Pfizer. M.G.C.L. reports grants from Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV) and personal fees from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Novartis, and Vifor Pharma. M.B.S., B.G. and F.S.A. report current or former full-time employment with Pfizer and stock and/or stock options at Pfizer. M.H. reports honoraria for advisory board participation from Alnylam, Akcea, Eidos, and Pfizer, and served as a speaker for a scientific meeting session funded by Alnylam.




