A machine-learning-based prediction model in patients with takotsubo syndrome: ‘You can't stop change any more than you can stop the suns from setting!’
The opinions expressed in this article are not necessarily those of the Editors of the European Journal of Heart Failure or of the European Society of Cardiology. doi: 10.1002/ejhf.2983.
This article refers to ‘Machine learning-based prediction of in-hospital death for patients with takotsubo syndrome: The InterTAK-ML model’ by O. De Filippo et al., published in this issue on pages 2299–2311.
In his Categories, the philosopher Aristotle details the kinds of beings that exist and what can be interpreted and described. For centuries, the influence of Categories applied to many domains, particularly the organization of knowledge and medicine.
In the context of a relentless quest for evidence-based personalized health systems, the organization of care pathways has radically changed in the last few decades: as patient presentations and outcomes might vary significantly, clinical decision-making models based solely on hypothetical-deductive categorization have been shown to be limited, and error-prone.
Machine learning (ML)-based clustering approaches, instead, offer the opportunity to pursue a deeper understanding of specific diseases, unveiling patterns that represent the complexity of human pathophysiology, especially in heterogeneous syndromes for which classical disease classification and prediction systems sacrifice accuracy and effectiveness.
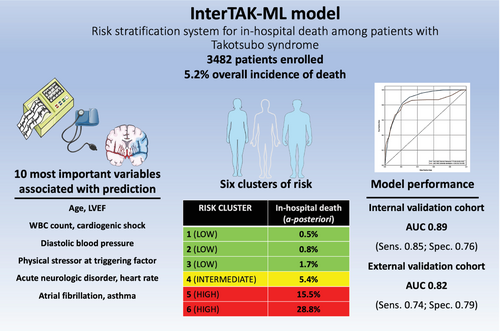
In this issue of the Journal, De Filippo et al.1 report an original manuscript aiming to design a ML-based model to predict the risk of in-hospital mortality, describing clusters of takotsubo syndrome (TTS) patients with distinctive risk profiles. TTS is indeed an acute cardiac syndrome with a complex pathophysiology denoted by transient left ventricular dysfunction.2 Approximately 2% of all patients with chest pain and suspected acute coronary syndrome suffer from TTS, highlighting its relevance in clinical practice.3 TTS is associated with a poor prognosis that portends similar short-term mortality as in patients with acute coronary syndrome, while might be even worse than in patients with reperfused ST-elevation myocardial infarction4, 5: a nationwide analysis showed that the in-hospital mortality rate among patients with TTS was 4.2% and the ones at highest risk were comorbid patients with underlying critical diseases.6 Even major cardiovascular outcomes following index TTS discharge have been demonstrated to be deeply intertwined with underlying comorbidities, such as cancer or psychological disorders.7, 8 Of importance, about 6% of all patients with TTS experience sudden cardiac death.9
The Spanish National Registry on TTS (RETAKO) indicated that physical rather than emotional triggers were associated with worse short- and long-term prognosis, along with factors such as age over 70 years, diabetes mellitus, severely reduced left ventricular ejection fraction (LVEF), and shock on admission.10 Therefore, precise identification of risk factors and clinical features associated with high risk of mortality and adverse events in TTS is of exceptional importance, as it might facilitate management improving outcomes in this vulnerable, often undertreated population.
Different scoring and prediction systems focused on short-term prognosis in TTS have been developed, such as the German and Italian Stress Cardiomyopathy (GEIST) score, providing modest prediction accuracy.11 In that sense, novel technologies and statistical algorithms facilitated by artificial intelligence and ML approaches might provide an opportunity to overcome limitations associated with classical logistic regression methods that are commonly employed for risk association and prediction of adverse outcomes. ML logistic regression models such as the ridge regression employed in the work of De Filippo et al., have the ability to create parsimonious prediction models in the presence of a large amount of variables. This technique is known as a ‘regularization’ regression as it penalizes the magnitude of coefficients of all variables in the model and thus minimizes the error between predicted and actual observations.
To strengthen these efforts, the International Takotsubo Registry (InterTAK) was founded in 2011 as an important global initiative that over time grew to become an authoritative and comprehensive worldwide systematic database capturing information on the natural history, clinical characteristics, treatment modalities, and outcomes among patients with TTS.12
In the innovative work presented in the issue, De Filippo et al.1 investigated the outcome of in-hospital death among 3482 patients with TTS and examined the impact of 31 clinically relevant variables on this outcome by utilizing a ridge logistic regression-based ML algorithm. Their InterTAK-ML model for in-hospital death prediction provides sizeable robustness as it underwent derivation, internal, and external validation processes on the cohort of >1000 patients from the Takotsubo Italian Network. Importantly, the authors found that in-hospital death among TTS patients occurred in 5 out of every 100 patients: the 10 most prominent variables associated with hospital mortality were LVEF, white blood cell count (WBC), cardiogenic shock, diastolic blood pressure, physical stressor as a trigger of TTS episode, acute neurological disorder, age, heart rate, atrial fibrillation, and asthma. The model performed very well in the internal validation cohort providing an area under the curve (AUC) of 0.89 and an AUC of 0.82 in the external validation cohort. Furthermore, the InterTAK-ML model showed comparable performance among different TTS subtypes providing the greatest AUC for focal TTS phenotype (0.93), followed by midventricular phenotype (0.91), apical phenotype (0.86) and finally, basal phenotype (0.83).
One of the potential pitfalls when addressing the prognosis of patients with TTS so far has been to consider the co-existence of important clinical conditions such as coronary artery disease, cardiogenic shock at presentation, and patient gender. The authors provided sensitivity analysis that showed similar performance of the InterTAK-ML model regardless of the presence of these conditions and nearly equal performance in both sexes. Previous research suggested that male sex was associated with higher short-term and long-term mortality in TTS and this was also true for physical triggers compared to emotional triggers with unclear effect of age on mortality.6, 13 It is important to note that there is a palpable lack of studies conducted in the clinical context of TTS focused on short-term and in-hospital outcomes. However, an important study conducted among patients with TTS hospitalized in the intensive care unit showed that several variables (i.e. serum potassium, prothrombin time, age, myocardial infarction, WBC, haematocrit, anion gap, and Sequential Organ Failure Assessment score) derived from LASSO regression are independently associated with in-hospital mortality,14 confirming, at least in part, the findings of De Filippo et al. The previously mentioned GEIST score identified male sex, history of neurologic disorder, right ventricular involvement, and LVEF as independent predictors of the main outcome in patients hospitalized for TTS. However, it is crucial to distinguish that the composite outcome in the GEIST study included in-hospital death, pulmonary oedema, need for invasive ventilation, and cardiogenic shock.11 Interestingly, sex as an independent variable did not make it to the top 10 variables derived by the ML algorithm in the InterTAK-ML, although male sex seems to be consistently associated with higher morbidity and mortality rates in TTS despite women having a vastly greater incidence of this syndrome.15
The authors could differentiate six unique risk clusters of patients with TTS using 10 ML-derived variables that showed the highest association with in-hospital mortality. For example, patients in the highest risk cluster had an in-hospital mortality rate of a staggering 28.8% while only 0.5% of patients stratified to the lowest risk cluster experienced in-hospital death. The creators of the InterTAK-ML score have to be commended for also providing a myriad of subanalyses that validate their model against classical modes of logistic regression such as standard and penalized logistic regression and for explaining the rationale on how six TTS risk clusters were developed. The InterTAK-ML model over performed the predictive abilities of two previously published risk scores such as those derived from smaller sub-cohorts of the InterTAK and GEIST registries.
Since collaboration is fundamental to delivering outcomes that expand the boundaries of human knowledge, the authors were kind enough to provide a straightforward, free link (https://compbiomed.hpc4ai.unito.it/intertako/) through which a health professional might insert all the required numbers, receiving risk calculation and cluster assignment reflecting the probability that a given patient belongs to one of six potential incremental group stratified for the risk of in-hospital death (Figure 1). It is important to note that the algorithm will be able to estimate risk even if not all of the variables are entered into the model, although a more accurate prediction is provided if all clinical pieces of information are available. It is worth acknowledging that most of the variables required for risk calculation can be immediately gathered from physical examination and patient history while the remaining others (e.g. WBC) are easily available through routine laboratory work-up tests or through a glance with a bedside ultrasound probe. The interTAK-ML model appears to be a valuable, simple, pragmatic, and readily available tool for the physicians involved in the care of TTS.
However, some inherent limitations are present due to the retrospective nature of the data. The inclusion of other ethnicities and perhaps minority groups would have likely increased the scalability of the findings. As pharmacological and device management make the cornerstone of the clinical approach in TTS, the lack of these data should be perceived as a notable drawback, as it could greatly modify the short-term outcome analyses.
The accelerating use of artificial intelligence and ML technologies introduces vulnerabilities and risks: ML systems learn from certain source data, introducing challenges in how these systems interact with their environments since they were developed in testbeds isolated from real-world scenarios, not taking into consideration the changing clinical landscape and the progress of knowledge. Moreover, the models are not able to adjust their performance according to the user acceptance and the experience acquired.
Going back to Aristotle, scientific knowledge, in the end, is based mainly on the analysis of human actions and behaviours. As contemporary physicians dealing with novel ML-derived approaches, we should keep in mind that clinical assessment is not only the most important ingredient for undertaking a coherent therapeutic process but also for deploying a new framework for diagnosis and risk stratification, scaling ML-based clustering-derived tools from an intricate, secluded ‘ugly duckling’ into a striking, sympathetic ‘swan’.
Conflict of interest: none declared.




