Predictors of outcome in a contemporary cardiac sarcoidosis population: Role of brain natriuretic peptide, left ventricular function and myocardial inflammation
Abstract
Aims
Cardiac sarcoidosis (CS) is a potentially fatal condition that varies in its clinical presentation. Here, we describe baseline characteristics at presentation along with prognosis and predictors of outcome in a sizable and deeply phenotyped contemporary cohort of CS patients.
Methods and results
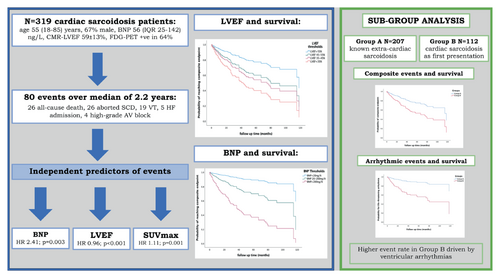
Consecutive CS patients seen at one institution were retrospectively enrolled after undergoing laboratory testing, electrocardiogram, echocardiography, cardiac magnetic resonance (CMR) imaging and 18F-flourodeoxyglucose positron emission tomography (FDG-PET) at baseline. The composite endpoint consisted of all-cause mortality, aborted sudden cardiac death, major ventricular arrhythmic events, heart failure hospitalization and heart transplantation. A total of 319 CS patients were studied (67% male, 55.4 ± 12 years). During a median follow-up of 2.2 years (range: 1 month–11 years), 8% of patients died, while 33% reached the composite endpoint. The annualized mortality rate was 2.7% and the 5- and 10-year mortality rates were 6.2% and 7.5%, respectively. Multivariate analysis showed serum brain natriuretic peptide (BNP) levels (hazard ratio [HR] 2.41, 95% confidence interval [CI] 1.34–4.31, p = 0.003), CMR left ventricular ejection fraction (LVEF) (HR 0.96, 95% CI 0.94–0.98, p < 0.0001) and maximum standardized uptake value of FDG-PET (HR 1.11, 95% CI 1.04–1.19, p = 0.001) to be independent predictors of outcome. These findings remained robust for different patient subgroups.
Conclusion
Cardiac sarcoidosis is associated with significant morbidity and mortality, particularly in those with cardiac involvement as the first manifestation. Higher BNP levels, lower LVEF and more active myocardial inflammation were independent predictors of outcomes.
Graphical Abstract
BNP, CMR-LVEF and SUVmax in FDG-PET: independent predictors of outcome in a contemporary cardiac sarcoidosis population remaining robust in subgroup subanalysis. AV, atrioventricular; BNP, brain natriuretic peptide; CMR, cardiac magnetic resonance; FDG-PET, fluorodeoxyglucose positron emission tomography; HF, heart failure; LVEF, left ventricular ejection fraction; SCD, sudden cardiac death; SUVmax, maximum standardized uptake value; VT, ventricular tachycardia.
Introduction
Sarcoidosis is a multi-system inflammatory disorder, characterized by the formation of non-caseating granulomas in the affected organs.1 Cardiac involvement may occur in 5–10% of patients with systemic sarcoidosis leading to arrhythmias, heart failure (HF) and premature death.2 However, necropsy studies report a higher prevalence of cardiac involvement of 20–30%.3 In recent years, the incorporation of cardiac magnetic resonance (CMR) imaging and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) has played a central role in improving the detection and management of CS.4-9 To secure a diagnosis of CS, the Heart Rhythm Society (HRS) recommends either histological evidence of CS from endomyocardial biopsy, or histological confirmation of extra-cardiac sarcoidosis (ECS) with at least one typical manifestation of cardiac disease.10 Using HRS criteria, the prevalence of CS has increased to 25–30% of sarcoidosis population.4, 5
The potential clinical pathways leading to a diagnosis of CS include the following: (i) detection of new cardiac involvement in those with known histologically confirmed ECS (group A), (ii) cardiac manifestations as the first presentation of previously undetected and subsequently confirmed ECS (group B), or (iii) isolated CMR and/or FDG-PET findings in a compatible clinical context leading to a diagnosis of isolated CS. Left ventricular ejection fraction (LVEF) at presentation has been identified as an independent predictor of outcome described in many case series.11-15 However, there are no studies to assess whether the different clinical presentations may influence subsequent morbidity and mortality and to identify the independent predictors in CS patients with preserved LVEF failing to meet the criteria for implantation of an implantable cardioverter defibrillator (ICD) as prophylaxis (group C) or those with left ventricular (LV) impairment (group D) representing the more advanced CS group of patients.
The aim of our study was to assess clinical characteristics, imaging findings, morbidity and mortality rates, and predictors of outcome among a contemporary cohort of CS patients, and to perform a comparative analysis between different subgroups.
Methods
Study population
This was a retrospective study of patients referred to the Royal Brompton Hospital between 2008 and 2018. This cohort included patients with known ECS who developed cardiac-sounding symptoms ± abnormal screening investigations, or those initially presenting with myocardial disease suspicious of CS in the absence of previously diagnosed systemic sarcoidosis.
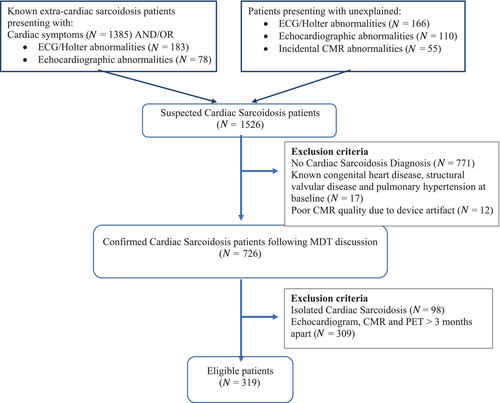
All patients underwent echocardiography and CMR imaging, and from 2012 onwards FDG-PET imaging was also performed. For each individual, imaging was completed within a 3-month timeframe. Serum biomarkers including brain natriuretic peptide (BNP), C-reactive protein (CRP), high-sensitivity troponin (hs-troponin), angiotensin-converting enzyme (ACE) and white blood cells (WBC) were collected at baseline presentation. Neutrophil/lymphocyte ratio (NLR) was calculated. Each case was discussed in our CS multidisciplinary team meeting consisting of respiratory physicians and cardiologists with specific expertise in HF, pacing and cardiac imaging, respectively. A high confidence diagnosis of CS was made in accordance with HRS recommendations.10 Patients with presumed isolated CS were excluded in the absence of histological confirmation.10 Other exclusion criteria included known congenital heart disease, at least moderate primary valvular disease, and pulmonary hypertension. Figure 1 summarizes the patient selection. After diagnosis, immunosuppressive, HF and anti-arrhythmic treatment strategies were applied according to guidelines.16, 17
Transthoracic echocardiography
All echocardiographic studies were performed using a commercially available system equipped with a 3.5 MHz transducer. Echocardiography was performed with the patient lying in a semi-supine, left lateral position. Images were acquired in standard parasternal, apical and sub-costal views, and sector size, depth and gain settings were adjusted to achieve optimal visualization. The minimum dataset of two-dimensional, (2D) colour flow and Doppler images was acquired storing three cardiac cycles per image and quantitative echocardiographic measurements were made as per guidelines.18 LVEF was estimated using the biplane Simpson method. All 2D measurements were indexed to body surface area.
Cardiac magnetic resonance imaging
All CMR studies were performed on a 1.5 T clinical scanner. Cine images were acquired using a steady-state free-precession sequence in standard two-, three-, and four-chamber long-axis views, with subsequent contiguous short-axis cines from the mitral annulus to the apex. STIR-T2-weighted images were acquired in the same long- and short-axis planes. LV volumes, LVEF and LV mass were quantified from the cine images by standard methods. Fifteen minutes after administration of 0.10 mmol/kg of intravenous gadolinium contrast material, late gadolinium enhancement (LGE) was assessed using an inversion recovery-prepared gradient echo sequence in ventricular short- and long-axis planes at matching cine image slice locations. Patients with pacing devices were scanned using established clinical protocols. Gradient echo sequences were used for cine imaging and wideband LGE imaging was used for late enhancement to mitigate or limit device artefact. Active myocardial inflammation was considered present when STIR positive imaging was reported on CMR.
FDG-PET imaging
Our protocol for FDG-PET patient preparation and imaging has been previously described.19 All studies were performed using a Siemens mCT Flow system. Perfusion and cardiac FDG images were displayed side-by-side for comparing perfusion with metabolism. Half body FDG-PET/computed tomography (CT) images were evaluated for extra-cardiac sites of disease. Only patchy focal or multi-focal FDG uptake was considered compatible with CS. Active myocardial inflammation was considered present when the myocardial maximum standardized uptake value (SUVmax) was ≥2.5.20
Follow-up and clinical endpoints
Baseline clinical data were collected from the hospital electronic health records. Event data were documented following scrutinization of hospital and general physician records or contact with the referring centre. The endpoints in each patient's case were confirmed by VK. The primary endpoint was a composite of events consisting of all-cause mortality, major arrhythmic events, unplanned hospitalization for HF and cardiac transplantation. Major arrhythmic events consisted of (i) aborted sudden cardiac death (SCD) from successful external direct current cardioversion or appropriate shock therapy for ventricular tachycardia (VT) or ventricular fibrillation (VF) by an ICD; (ii) sustained VT of >100 bpm lasting >30 s causing haemodynamic compromise; or (iii) documented symptomatic bradyarrhythmias requiring urgent pacing. Time to event was calculated as the period between CS diagnosis and the first event or death. Those not reaching the endpoint were censored at the time of follow-up (July 2019). Given the retrospective, observational nature of data, the Royal Brompton Research Office approved the study and waived informed patient consent.
Statistical analysis
Statistical analysis was carried out with commercially available software (SPSS). Normality of distribution of each variable was assessed with the one-sample Kolmogorov–Smirnov test. Data were expressed as means ± standard deviation or medians with first (Q1) and third quartiles (Q3). Group comparisons were made using Student's t-test for normally distributed variables, Mann–Whitney test for non-normally distributed variables, Chi-square test for categorical variables and Fisher's exact test were used as appropriate. Univariate Cox proportional hazard models were used to assess the association between covariates and endpoints (presented as hazard ratios [HRs] and 95% confidence intervals [CIs]). Clinically important variables with p < 0.10 on univariate analyses were included in multivariate analysis. Multivariable models were performed in a hierarchical fashion firstly including clinical data, then adding screening investigations, and finally the addition of CMR and FDG-PET data. Biomarkers levels were log-transformed to eliminate data skewness. Kaplan–Meier survival curves were created for selected independent predictors to assess differences in cumulative event-free survival. All statistical tests were two-tailed and a p-value of <0.05 was considered statistically significant.
Results
Baseline data of the whole cohort
The study population consisted of 319 patients with a median age of 55 years (range: 18–85 years), of whom 67% were men, 42% had hypertension and 16% had diabetes (Table 1). BNP was available in 301 (94.4%) patients at baseline with median value of 56 ng/ml (Q1–Q3: 25–142 ng/L). A diagnosis of CS was confirmed in 148 (46%) patients by way of preliminary investigations such that electrocardiogram (ECG) and Holter monitoring detecting ≥2nd degree atrioventricular block (AVB) in 18.5% and sustained VT in 26.3% of patients, while echocardiography revealed LVEF <40% in 15.7% of patients. In the remaining 171 (54%) patients, the diagnosis of CS was confirmed by CMR and FDG-PET imaging. Three patients were diagnosed following endomyocardial biopsy. LGE on CMR was present in 93% of patients. Of the 278 patients who underwent FDG-PET imaging, active myocardial inflammation was present in 64% of patients. CRP was low with a median value of 3 mg/L (Q1–Q3: 1–8 mg/L) and was not associated with myocardial inflammation detected on FDG-PET (r = 0.08, p = 0.13). Figures 1 and 2 show clinical information, ECG, echocardiographic, CMR and FDG-PET appearances as examples of CS diagnosis in selected cases from our cohort.
| Characteristics | All patients (n = 319) | Group A (n = 207) | Group B (n = 112) | p-value* |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 55.4 ± 12 | 57.2 ± 11.3 | 52.2 ± 12.4 | <0.0001 |
| Male sex, n (%) | 213 (66.8) | 134 (64.7) | 79 (70.5) | NS |
| White ethnicity, n (%) | 233 (73) | 150 (72.5) | 83 (74.1) | NS |
| Comorbidities | ||||
| Smoking (current-/ex-/non-), n | 8/100/211 | 6/62/139 | 2/38/72 | NS |
| Hypertension, n (%) | 135 (42.3) | 91 (44) | 44 (39.3) | NS |
| Diabetes, n (%) | 51 (16) | 39 (18.8) | 12 (10.7) | NS |
| Ischaemic heart disease, n (%) | 33 (10.3) | 26 (12.6) | 7 (6.3) | NS |
| Treatment for systemic sarcoidosis, n (%) | 113 (35.4) | 113 (54.6) | 0 (0) | <0.0001 |
| Sarcoidosis disease duration, years | 5.1 [0–5.1] | 7.8 [2–12] | 0 | <0.0001 |
| Organ involvement, n (%) | ||||
| Pulmonary | 202 (63.3) | 144 (69.6) | 58 (51.8) | 0.0001 |
| Intra-thoracic LN | 217 (68) | 130 (62.8) | 87 (77.7) | 0.0006 |
| Skin | 76 (23.8) | 66 (31.9) | 10 (8.9) | <0.0001 |
| Other organ | 65 (20.4) | 55 (26.6) | 10 (8.9) | <0.0001 |
| Cardiac symptoms, n (%) | ||||
| Palpitations | 188 (58.9) | 109 (52.7) | 79 (70.5) | 0.002 |
| Chest pain | 70 (21.9) | 44 (21.3) | 26 (23.2) | NS |
| Syncope | 66 (20.7) | 24 (11.6) | 42 (37.5) | <0.0001 |
| Lung function tests | ||||
| FVC, % predicted | 93.6 ± 21.3 | 91.5 ± 22.1 | 98.4 ± 18.8 | 0.011 |
| DLCO, % predicted | 68.2 ± 20.8 | 65.5 ± 20.4 | 74.1 ± 20.6 | 0.002 |
| Laboratory tests | ||||
| BNP, ng/L | 132 [25–142] | 102 [21–102] | 187 [38–178] | 0.03 |
| Hs-troponin, μg/L | 1 [1–6] | 1 [1–3] | 1 [1–29] | 0.004 |
| CRP, mg/L | 3 [1–8] | 4 [2–8] | 3 [1–8.8] | 0.013 |
| ACE, IU/L | 30 [13.5–39] | 37 [17–65] | 26 [9.3–37.5] | 0.04 |
| WBC, n × 109/L | 7.4 [5.8–9.4] | 7.2 [5.6–9.2] | 7.6 [5.8–9.9] | NS |
| NLR, % | 4.5 [2.9–7.6] | 4.5 [2.8–7.7] | 4.4 [2.9–7.6] | NS |
| ECG/Holter, n (%) | ||||
| AVB (≥2nd degree) | 59 (18.5) | 24 (11.6) | 35 (31.3) | <0.0001 |
| SVT | 39 (12.2) | 25 (12.1) | 14 (12.5) | NS |
| VT | 84 (26.3) | 33 (15.9) | 51 (45.5) | <0.0001 |
| Echocardiography | ||||
| LVIDd, mm | 5.1 ± 0.9 | 5.0 ± 0.8 | 5.3 ± 0.9 | 0.003 |
| LVIDs, mm | 3.6 ± 1.1 | 3.5 ± 1 | 3.8 ± 1.1 | 0.004 |
| IVSd, mm | 1.0 ± 0.3 | 1.1 ± 0.3 | 1 ± 0.3 | NS |
| LV mass index, g/m2 | 87.2 ± 34.2 | 87.5 ± 31.2 | 86.9 ± 38.9 | NS |
| LVEDV index, ml/m2 | 56.9 ± 24.6 | 54 ± 24.3 | 62.1 ± 24.5 | 0.008 |
| LVESV index, ml/m2 | 28.3 ± 21.8 | 25.8 ± 21.3 | 32.6 ± 22.1 | 0.01 |
| LVEF, % | 54.4 ± 12.5 | 56 ± 12 | 51.4 ± 12.8 | 0.002 |
| RWMA, n (%) | 104 (32.6) | 23 (11.1) | 27 (24.1) | 0.003 |
| LA volume index, ml/m2 | 29.3 ± 12.5 | 28.1 ± 12.1 | 31.4 ± 12.8 | 0.04 |
| RV base, mm | 3.9 ± 0.7 | 3.8 ± 0.6 | 4 ± 0.8 | 0.04 |
| PASP, mmHg | 30.7 ± 10.6 | 32 ± 11.4 | 28.8 ± 8.9 | 0.02 |
| TAPSE, mm | 2.1 ± 0.5 | 2.1 ± 0.5 | 2.1 ± 0.5 | 0.96 |
| GLS, % | −16.4 ± 3.2 | −16.4 ± 4.4 | −16.4 ± 5.2 | 0.99 |
| CMR | ||||
| LGE, n (%) | 298 (93.4) | 188 (90.8) | 110 (98.2) | 0.009 |
| STIR, n (%) | 50 (15.7) | 25 (12.1) | 25 (22.3) | 0.002 |
| RWMA, n (%) | 117 (36.7) | 56 (27.1) | 61 (54.4) | <0.0001 |
| RV-LGE, n (%) | 25 (7.8) | 5 (2.4) | 20 (17.8) | <0.0001 |
| LVEDV index, ml/m2 | 78.7 ± 25.6 | 76.2 ± 24.3 | 84.4 ± 27.5 | 0.02 |
| LVESV index, ml/m2 | 34.5 ± 23.2 | 32 ± 22.8 | 40.2 ± 23.4 | 0.01 |
| LVEF, % | 59.1 ± 13.2 | 60.9 ± 12.6 | 55 ± 13.6 | 0.001 |
| LV mass index, mg/m2 | 74.4 ± 24.4 | 69.8 ± 20.2 | 85 ± 29.4 | <0.0001 |
| RVEDV index, ml/m2 | 76.7 ± 22.4 | 75.7 ± 20.6 | 78.9 ± 26 | NS |
| RVESV index, ml/m2 | 34.4 ± 15.1 | 33 ± 13.3 | 37.6 ± 18.2 | 0.05 |
| RVEF, % | 56.3 ± 9.9 | 57.4 ± 9.2 | 53.7 ± 11 | 0.012 |
| FDG-PET | (n = 278) | (n = 170) | (n = 108) | |
| SUVmax | 3.1 [0–5.4] | 3 ± 2.8 | 5 ± 5.3 | <0.0001 |
| RV-FDG, n (%) | 30 (10.8) | 10 (5.9) | 20 (18.5) | <0.0001 |
| PET positive, n (%) | 177 (63.7) | 106 (62.9) | 71 (65.7) | 0.02 |
- ACE, angiotensin-converting enzyme; AVB, atrioventricular block; BNP, brain natriuretic peptide; CMR, cardiac magnetic resonance; CRP, C-reactive protein; DLCO, diffusion factor for carbon monoxide; ECG, electrocardiogram; FDG, 18F-fludeoxyglucose; FVC, forced vital capacity; Hs, high-sensitivity; IVSd, interventricular septal diameter in diastole; LA, left atrial; LGE, late gadolinium enhancement; LN, lymph node; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; NLR, neutrophil/lymphocyte ratio; PASP, pulmonary artery systolic pressure; PET, positron emission tomography; RV, right ventricular; RVEDV, right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end-systolic volume; RWMA, regional wall motion abnormality; SUVmax, maximum standardized uptake value; STIR, short-tau inversion recovery; SVT, supraventricular tachycardia; TAPSE, tricuspid annular plane systolic excursion; VT, ventricular tachycardia; WBC, white blood cell.
- * p > 0.05 deemed non-significant.
Baseline data of patient subgroups
A total of 207 (64.9%) patients had previously known ECS (Group A) at the time of CS diagnosis with a median disease duration of 3 years (Q1–Q3: 1–9 years). Approximately a half of those patients (113/207, 54.6%) were already on a degree of immunosuppression for known ECS. The remaining 112 (35.1%) patients originally presented with a cardiac manifestation of CS without previously known ECS (Group B). Prior to CS diagnosis, no Group B patients were on immunosuppressive therapy (Table 1).
Similarly, 202 of 319 patients (63.3%) patients had preserved LVEF (>55%) (Group C) and 117 (36.7%) had LV systolic dysfunction at the time of CS diagnosis (Group D). A total of 41 (20.3%) of Group C and 63 (53.8%) of Group D patients had a device in situ at the time of CS diagnosis (Table 2).
| Characteristics | All patients (n = 319) | Group C (n = 202) | Group D (n = 117) | p-value* |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 55.4 ± 12 | 55.1 ± 11.4 | 56.1 ± 12.9 | NS |
| Male sex, n (%) | 213 (66.8) | 130 (64.4) | 83 (70.9) | NS |
| White ethnicity, n (%) | 233 (73) | 152 (75.2) | 81 (69.2) | NS |
| Comorbidities | ||||
| Smoking (current-/ex-/non-), n | 8/100/211 | 5/64/133 | 3/36/78 | NS |
| Hypertension, n (%) | 135 (42.3) | 84 (41.6) | 51 (43.6) | NS |
| Diabetes, n (%) | 51 (16) | 32 (15.8) | 19 (16.2) | NS |
| Ischaemic heart disease, n (%) | 33 (10.3) | 19 (9.4) | 14 (12.0) | NS |
| Treatment for systemic sarcoidosis, n (%) | 113 (35.4) | 60 (29.7) | 53 (45.3) | <0.0001 |
| Sarcoidosis disease duration, years | 5.1 [0–5.1] | 5.7 ± 6.9 | 6.2 ± 8.2 | NS |
| Organ involvement, n (%) | ||||
| Pulmonary | 202 (63.3) | 123 (60.9) | 79 (67.5) | NS |
| Intra-thoracic LN | 217 (68) | 118 (58.4) | 99 (84.6) | <0.0001 |
| Skin | 76 (23.8) | 57 (28.2) | 19 (16.2) | 0.01 |
| Other organ | 65 (20.4) | 60 (29.7) | 5 (4.3) | <0.0001 |
| Cardiac symptoms, n (%) | ||||
| Palpitations | 188 (58.9) | 118 (58.4) | 70 (59.8) | NS |
| Chest pain | 70 (21.9) | 49 (24.3) | 21 (17.9) | NS |
| Syncope | 66 (20.7) | 35 (17.3) | 31 (26.5) | NS |
| Lung function tests | ||||
| FVC, % predicted | 93.6 ± 21.3 | 95.8 ± 22.1 | 89.5 ± 19.2 | 0.02 |
| DLCO, % predicted | 68.2 ± 20.8 | 69.2 ± 21.7 | 66.1 ± 18.9 | NS |
| Laboratory tests | ||||
| BNP, ng/L | 132 [25–142] | 39 [19–82] | 97 [52–213] | <0.0001 |
| Hs-troponin, μg/L | 1 [1–6] | 1 [1–4.3] | 2.6 [1–29] | NS |
| CRP, mg/L | 3 [1–8] | 3 [1–8] | 4 [1–9] | <0.001 |
| ACE, IU/L | 30 [13.5–39] | 32 [14.5–59.5] | 18 [9–37.3] | 0.05 |
| WBC, n × 109/L | 7.4 [5.8–9.4] | 7.2 [5.9–9.2] | 7.6 [5.8–9.5] | NS |
| NLR, % | 4.5 [2.9–7.6] | 4.3 [2.8–7.2] | 4.6 [2.8–7.5]] | NS |
| ECG/Holter, n (%) | ||||
| AVB (≥2nd degree) | 59 (18.5) | 34 (16.8) | 24 (20.5) | NS |
| SVT | 39 (12.2) | 24 (11.9) | 15 (12.8) | NS |
| VT | 84 (26.3) | 31 (15.3) | 50 (42.7) | <0.0001 |
| Echocardiography | ||||
| LVIDd, mm | 5.1 ± 0.9 | 4.7 ± 0.7 | 5.7 ± 0.9 | <0.0001 |
| LVIDs, mm | 3.6 ± 1.1 | 3.1 ± 0.5 | 4.5 ± 1.2 | <0.0001 |
| IVSd, mm | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | NS |
| LV mass index, g/m2 | 87.2 ± 34.2 | 79.5 ± 24.6 | 101.5 ± 40.6 | <0.0001 |
| LVEDV index, ml/m2 | 56.9 ± 24.6 | 47.3 ± 12.5 | 73.3 ± 30.9 | <0.0001 |
| LVESV index, ml/m2 | 28.3 ± 21.8 | 18.4 ± 6.5 | 45.4 ± 27.7 | <0.0001 |
| LVEF, % | 54.4 ± 12.5 | 62.1 ± 4.5 | 41.3 ± 10.5 | <0.0001 |
| RWMA, n (%) | 104 (32.6) | 40 (19.8) | 62 (53.0) | <0.0001 |
| LA volume index, ml/m2 | 29.3 ± 12.5 | 29.0 ± 10.8 | 33.7 ± 12.0 | <0.0001 |
| RV base, mm | 3.9 ± 0.7 | 3.8 ± 0.6 | 4.1 ± 0.8 | 0.01 |
| PASP, mmHg | 30.7 ± 10.6 | 31.2 ± 10.6 | 30.0 ± 10.7 | NS |
| TAPSE, mm | 2.1 ± 0.5 | 2.1 ± 0.4 | 2.0 ± 0.5 | NS |
| GLS, % | −16.4 ± 3.2 | −18.1 ± 3.5 | −13.1 ± 3.6 | <0.001 |
| CMR | ||||
| LGE, n (%) | 298 (93.4) | 187 (92.6) | 111 (94.9) | NS |
| STIR, n (%) | 50 (15.7) | 36 (17.8) | 14 (12.0) | NS |
| RWMA, n (%) | 117 (36.7) | 23 (11.4) | 81 (69.2) | <0.0001 |
| RV-LGE, n (%) | 25 (7.8) | 12 (5.9) | 13 (11.1) | NS |
| LVEDV index, ml/m2 | 78.7 ± 25.6 | 70.2 ± 17.6 | 98.2 ± 30.1 | <0.0001 |
| LVESV index, ml/m2 | 34.5 ± 23.2 | 25.4 ± 11.1 | 55.4 ± 29.6 | <0.0001 |
| LVEF, % | 59.1 ± 13.2 | 64.7 ± 8.6 | 46.2 ± 12.9 | <0.0001 |
| LV mass index, mg/m2 | 74.4 ± 24.4 | 67.9 ± 19 | 89.6 ± 28.6 | <0.0001 |
| RVEDV index, ml/m2 | 76.7 ± 22.4 | 75.4 ± 19.3 | 79.6 ± 28.3 | NS |
| RVESV index, ml/m2 | 34.4 ± 15.1 | 31.6 ± 11.6 | 40.8 ± 19.6 | <0.0001 |
| RVEF, % | 56.3 ± 9.9 | 58.8 ± 8.1 | 50.4 ± 11.3 | <0.0001 |
| FDG-PET | (n = 278) | (n = 174) | (n = 104) | |
| SUVmax | 3.1 [0–5.4] | 3.2 [0–5.0] | 3.0 [0–6.0] | NS |
| RV-FDG, n (%) | 30 (10.8) | 14 (6.9) | 16 (13.7) | NS |
| PET positive, n (%) | 177 (63.7) | 112 (64.4) | 67 (64.4) | NS |
- ACE, angiotensin-converting enzyme; AVB, atrioventricular block; BNP, brain natriuretic peptide; CMR, cardiac magnetic resonance; CRP, C-reactive protein; DLCO, diffusion factor for carbon monoxide.; ECG, electrocardiogram; FDG, 18F-fludeoxyglucose; FVC, forced vital capacity; GLS, global longitudinal strain; Hs, high-sensitivity; IVSd, interventricular septal diameter in diastole; LA, left atrial; LGE, late gadolinium enhancement; LN, lymph node; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole; NLR, neutrophil/lymphocyte ratio; PASP, pulmonary artery systolic pressure; PET, positron emission tomography; RV, right ventricular; RVEDV, right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end-systolic volume; RWMA, regional wall motion abnormality; SUVmax, maximum standardized uptake value; STIR, short-tau inversion recovery; SVT, supraventricular tachycardia; TAPSE, tricuspid annular plane systolic excursion; VT, ventricular tachycardia; WBC, white blood cell.
- * p > 0.05 deemed non-significant.
Baseline cardiac implantable electronic devices data
At the time of baseline assessment, 104 (32.6%) patients had a cardiac device in situ. This included 39 permanent pacemakers (PPM), 42 dual-chamber ICDs and 23 cardiac resynchronization therapy-defibrillators; 13 of these defibrillators were implanted for primary prevention. Of the 52 secondary prevention defibrillator devices, the indication was sustained VT in 29 (56%), advanced AVB in 17 (33%) and LVEF <35% despite optimal HF therapy in 6 (12%).
Major cardiovascular event data
Eighty patients met the composite endpoint during a median follow-up period of 2.2 years (Q1–Q3: 1.2–4.3 years) (Table 3). Twenty-six (8.2%) patients died and first events in survivors consisted of 26 aborted SCD, 19 VT without haemodynamic compromise, 4 AVB requiring pacing and 5 HF hospitalizations. A further 26 subsequent events were recorded during follow-up giving a cumulative total of 106 events. The annualized mortality rate was 2.7% and the annualized composite event rate was 8.4%. The 5- and 10-year mortality rates were 6.2% and 7.5%, respectively. During the follow-up period, 28 new defibrillators were implanted of which 7 were upgrades to pre-existing PPMs.
| Events | All patients (n = 319) | Group A (n = 207) | Group B (n = 112) | p-value | Group C (n = 202) | Group D (n = 117) | p-value |
|---|---|---|---|---|---|---|---|
| Composite primary events, n (%) | 80 (25.1) | 40 (19.3) | 40 (35.7) | 0.002 | 35 (17.3) | 45 (38.5) | <0.001 |
| Total cumulative events, n (%) | 106 (33.2) | 53 (25.6) | 53 (47.3) | <0.0001 | 39 (19.3) | 67 (57.3) | <0.0001 |
| All-cause mortality | 26 (8.2) | 19 (9.2) | 7 (6.3) | NS | 13 (6.4) | 13 (11.1) | NS |
| Aborted sudden cardiac death | 34 (14.4) | 8 (3.9) | 26 (23.2) | <0.0001 | 9 (4.5) | 25 (21.4) | <0.0001 |
| VT/VF episode | 23 (7.2) | 10 (4.8) | 13 (11.6) | <0.0001 | 10 (5) | 13 (11.1) | NS |
| Complete heart block | 6 (1.9) | 5 (1.6) | 1 (0.9) | NS | 3 (1.5) | 3 (2.6) | NS |
| Heart failure hospitalization | 14 (4.4) | 10 (4.8) | 4 (3.6) | NS | 4 (2) | 10 (8.5) | 0.01 |
| Cardiac transplantation | 3 (0.9) | 1 (0.5) | 2 (1.8) | NS | 0 (0) | 3 (2.6) | <0.0001 |
| Follow-up period, months | 35.9 ± 28.8 | 38.7 ± 29.6 | 30.8 ± 26.5 | NS | 38.3 ± 30.2 | 32.4 ± 26.3 | NS |
| Annualized mortality rate, % | 2.7 | 2.9 | 2.4 | NS | 2 | 4.1 | NS |
| Annualized composite event rate, % | 8.4 | 6 | 13.9 | 0.001 | 5.4 | 14.3 | <0.001 |
| Annualized total cumulative event rate, % | 11.1 | 7.9 | 18.5 | <0.0001 | 6 | 21.2 | <0.0001 |
- VF, ventricular fibrillation; VT, ventricular tachycardia.
Predictors of outcome
A summary of the univariate and multivariate Cox regression analyses for the prediction of the composite endpoint is provided in Table 4. The multivariate model containing significant univariate parameters from the clinical, ECG and echocardiography domains, showed that BNP was the only independent predictor of adverse events (HR 5.12 [2.64–9.96], p < 0.0001). Following the addition of CMR and FDG-PET parameters to the model, BNP (HR 2.41 [1.34–4.31], p = 0.003) remained a predictor of the composite endpoint, and CMR-LVEF (HR 0.96 [0.94–0.98], p < 0.0001) and SUVmax (HR 1.11 [1.04–1.19], p = 0.001) were also independent predictors.
| Covariates | Univariate analysis (HR; 95% CI) | p-value | Step 1 (HR; 95% CI) | p-value | Step 2 (HR; 95% CI) | p-value |
|---|---|---|---|---|---|---|
| Clinical domain | ||||||
| Age | 1.02 (1.00–1.04) | 0.02 | 0.99 (0.96–1.02) | NS | – | – |
| Sex | 0.91 (0.57–1.47) | NS | – | – | ||
| Ethnicity | 1.17 (0.75–1.85) | NS | – | – | ||
| Hypertension | 1.06 (0.68–1.66) | NS | – | – | ||
| Diabetes mellitus | 1.00 (0.57–1.76) | NS | – | – | ||
| Group A/B | 2.04 (1.31–3.18) | 0.002 | 1.62 (0.73–3.58) | NS | – | – |
| Group C/D | 0.37 (0.24–0.57) | <0.001 | ||||
| Palpitations | 1.10 (0.70–1.73) | NS | – | – | ||
| Chest pain | 1.22 (0.72–2.08) | NS | – | – | ||
| Syncope | 1.24 (0.74–2.07) | NS | – | – | ||
| ECG domain | ||||||
| Atrioventricular block | 2.02 (1.22–3.34) | 0.006 | 0.52 (0.28–1.92) | NS | – | – |
| Ventricular tachycardia | 1.43 (0.91–2.26) | NS | – | – | ||
| Biomarker domain | ||||||
| BNP | 3.68 (2.40–5.63) | <0.0001 | 5.12 (2.64–9.96) | <0.0001 | 2.50 (1.39–4.49) | 0.002 |
| Hs-troponin, μg/L | 1.40 (0.97–2.02) | NS | ||||
| CRP, mg/L | 1.62 (1.03–2.55) | 0.04 | 1.05 (0.49–2.26) | |||
| ACE, IU/L | 0.99 (0.98–1.00) | NS | NS | |||
| WBC, n × 109/L | 0.44 (0.11–1.72) | NS | ||||
| NLR, % | 1.02 (0.99–1.06) | NS | ||||
| Echo domain | ||||||
| LVEF | 0.97 (0.96–0.99) | <0.0001 | 0.99 (0.96–1.03) | NS | – | – |
| RWMA | 1.77 (1.12–2.79) | 0.01 | 0.80 (0.27–2.39) | NS | – | – |
| RV diameter | 1.68 (1.08–2.63) | 0.02 | 1.25 (0.69–2.28) | NS | – | – |
| GLS | 1.09 (1.01–1.17) | 0.02 | 0.88 (0.72–1.07) | NS | – | – |
| CMR domain | ||||||
| LGE | 6.01 (0.84–43.21) | 0.08 | 3.22 (0.43–24.25) | NS | ||
| RV-LGE | 1.2 (0.57–2.51) | NS | ||||
| STIR | 1.31 (0.69–2.48) | NS | – | – | ||
| RWMA | 3.03 (1.72–5.37) | <0.0001 | 1.22 (0.52–2.85) | NS | ||
| LVEF | 0.97 (0.95–0.98) | <0.0001 | 0.96 (0.94–0.98) | 0.01 | ||
| RVEF | 0.95 (0.93–0.97) | <0.0001 | 0.99 (0.98–1.01) | NS | ||
| PET domain | ||||||
| SUVmax | 1.06 (1.01–1.12) | 0.02 | 1.11 (1.04–1.18) | 0.001 | ||
| RV-FDG | 2.36 (1.25–4.43) | 0.008 | 1.29 (0.47–3.51) | NS | ||
| PET positivity | 1.41 (0.83–2.41) | NS | – | – | ||
- ACE, angiotensin-converting enzyme; BNP, brain natriuretic peptide; CI, confidence interval; CMR, cardiac magnetic resonance; CRP, C-reactive protein; ECG, electrocardiogram; FDG, 18F-fludeoxyglucose; GLS, global longitudinal strain; HR, hazard ratio; Hs, high-sensitivity; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; NLR, neutrophil/lymphocyte ratio; PET, positron emission tomography; RV, right ventricular; RVEF, right ventricular ejection fraction; RWMA, regional wall motion abnormality; SUVmax, maximum standardized uptake value; STIR, short-tau inversion recovery; WBC, white blood cell.
Further analyses were performed dividing plasma BNP and LVEF into sub-categories. In the LVEF subgroups, LVEF <35% (HR 0.15 [0.07–0.31], p < 0.0001), 35–45% (HR 0.27 [0.13–0.57], p = 0.001) and 45–55% (HR 0.31 [0.16–0.6], p = 0.001) were each associated with the composite endpoint, when compared to patients with LVEF >55%.
Brain natriuretic peptide levels of 20–100 ng/L (HR 3.07 [1.09–8.67], p = 0.03), 100–200 ng/L (HR 3.38 [1.09–10.55], p = 0.04) and >200 ng/L (HR 8.78 [3.06–25.16], p < 0.0001) were each associated with the composite endpoint, when compared to patients with BNP levels <20 ng/L. Kaplan–Meier survival curve analysis showed significantly worse event-free survival as LV function decreased and BNP increased (Figure 2).
Subgroup analyses
We performed subgroup analyses depending on patients' original presentation and degree of LV impairment. As shown in Table 1, Group B patients were younger than Group A patients with similar comorbidities. Although less frequently reported than palpitations, syncope was three times more common in Group B patients. Similarly, presentation with VT and AVB had an approximate three-fold higher prevalence in Group B patients resulting in a greater number of devices implanted at baseline (n = 65, 58.7%). Myocardial inflammation was more commonly detected in Group B patients on both CMR (22% vs. 12%, p = 0.002) and FDG-PET scans (65% vs. 62%, p = 0.02). CRP was higher in Group A patients (p = 0.01) but was not associated with myocardial inflammation detected on FDG-PET in either of the groups.
On Kaplan–Meier survival curve analysis, patients in Group B had worse event-free survival with regard to the composite endpoint and life-threatening arrhythmia (Figure 2). However all-cause mortality rates remained similar across both cohorts. Multivariate analysis within Group A showed BNP (HR 4.01 [1.98–8.15], p < 0.0001) and male gender (HR 3.62 [1.25–10.46], p = 0.02) to be predictive of the composite endpoint. Within Group B, CMR-LVEF (HR 0.94 [0.91–0.98], p = 0.001) and SUVmax (HR 1.10 [1.02–1.19], p = 0.01) were the independent predictors.
Group C and Group D patients did not differ in either demographics, comorbidities or symptoms at baseline (Table 2). As expected, Group D patients had worse LV functional parameters in both echocardiography and CMR resulting in a three-fold higher rate of VT at presentation. The rate of AVB at presentation did not differ between the two groups. There was no difference in the presence or extent of myocardial inflammation on FDG-PET scans despite a higher CRP level in Group D patients (p < 0.001). CRP was not associated with myocardial inflammation detected on FDG-PET in either group.
On Kaplan–Meier survival curve analysis, patients in Group D had worse event-free survival with regard to the composite endpoint and life-threatening arrhythmias (Figure 2). However, all-cause mortality rates again remained similar across both cohorts. Multivariate analysis within Group C showed BNP (HR 1.49 [1.04–2.14], p = 0.03) and AVB (HR 3.2 [1.20–8.6], p = 0.02) to be predictive of the composite primary endpoint. Within Group D, CMR-LVEF (HR 0.94 [0.91–0.98], p = 0.002) and SUVmax (HR 1.14 [1.06–1.24], p < 0.0001) were the independent predictors.
Discussion
We evaluated the medium-term outcomes in a large, contemporary real-life population of CS patients, diagnosed using the HRS criteria in an multidisciplinary team setting based on comprehensive phenotyping of clinical, laboratory and imaging findings. To our knowledge, this study represents the largest single centre CS cohort offering real-life experience of the condition and its natural history over a 3-year period, with one-third experiencing the composite endpoint and an 8% mortality. Furthermore, we provide a clinically simple and highly useful triad of risk predictors of future events such as (i) serum levels of baseline BNP, (ii) the degree of LV dysfunction, and (iii) the intensity of myocardial inflammation (Graphical Abstract). Although both LV systolic dysfunction and myocardial inflammation have previously been identified as independent predictors of outcomes in CS,8, 11-15 serum BNP levels emerged as a very potent and novel risk stratification tool that remained robust in subgroup analysis. Of note, BNP was the only independent predictor of outcome among other biomarkers examined in this study. Detection of myocardial inflammation using FDG-PET increases the risk of future events even when the LV function is impaired. On the other hand, prior use of immunosuppressive treatment in patients with known ECS was protective in the subgroup analysis suggesting that immunosuppressive treatment in CS indeed favourably affects the disease's natural history. Finally, comparison of outcomes between different subgroups demonstrated that (i) those with CS as first clinical presentation had significantly worse outcomes than those with known ECS who were screened for cardiac involvement, largely driven by ventricular arrhythmic events; (ii) the independent predictors of outcome in the whole cohort remained statistically significant in the various subgroups; (iii) while preserved LV systolic function confers a good prognosis, a raised BNP and/or the presence of myocardial inflammation may result in adverse outcomes during follow-up.
The annualized composite event and mortality rates over a 3-year period were 8.4% and 2.7%, respectively. Previously reported morbidity and mortality rates varied considerably,5, 7, 21-25 due to different patient characteristics and smaller sized cohorts. Earlier studies prior to the era of optimal HF therapy tended to quote higher mortality rates. Diagnosis often hinged on a positive endomyocardial biopsy,22 or on the finding of myocardial granulomas at autopsy.23, 24 Mortality rates published more recently in smaller series have been more comparable to our patient population. The better survival rates are likely driven by higher rates of detection of cardiac involvement at an earlier stage,11-14 optimal and greater use of immunosuppressive and HF therapies, in particular ICD implantation.
The most favourable outcomes have been reported by a group from Finland11, 12, 15 showing an annualized death rate of 1.3% over a 10-year period.15 Unlike the Finnish cohort, 20% of our patients had ICDs implanted at the time of diagnosis, increasing to 29% by the end of follow-up. This was necessitated by the high prevalence of VTs in our study cohort, much higher than the 14% reported in the Finnish registry.15 The importance of achieving a prompt diagnosis of CS followed by a proactive approach towards ICD implantation are key components in reducing the mortality rate. A total of 34/104 (32.7%) patients with device in situ experienced appropriate shocks during follow-up resulting in aborted SCD.
Our study further demonstrates an independent prognostic value of BNP in CS patients in line with the results from Nabeta et al.26; albeit the HR was much stronger in our cohort (2.41 vs. 1.28). BNP was the only independent predictor of outcome among several cardiac and non-cardiac sensitive biomarkers such as hs-troponin, CRP, WBC and NLR. Interestingly, neither BNP, a marker of raised intra-cardiac pressures, nor CRP, a marker of systemic inflammation, were associated with the FDG-PET myocardial inflammatory signal. BNP remained an independent predictor of outcome in patients with known ECS as well as those with preserved LV systolic function which represent the wider sarcoidosis population with less overt myocardial involvement. Our findings strongly support the role of BNP, particularly in those with preserved LVEF, in risk stratification of CS at the point of diagnosis.
Our study reaffirms the importance of reduced LVEF as a marker of increased risk in CS. Even a mild reduction in systolic function was associated with a significantly increased risk of events. Current guidelines recommend ICD implantation in HF patients with LVEF <35% (level IA recommendation) for the prevention of SCD.10, 17 However, our data support that CS patients with even mild or moderately reduced LVEF might warrant an ICD. Indeed, the 2017 AHA/ACC/HRS guidelines recommend ICD implantation for patients with preserved LV systolic function and evidence of myocardial scar as detected by CMR or PET, respectively.27 While our study did not assess LGE burden, Ekström et al.28 reported that the extent of scar matters when assessing risk of SCD in CS.
Owing to the variation in local care pathways for CS evaluation, there are limited published data on the prognostic role of FDG-PET in CS. Blankstein et al.8 first reported in a cohort of 112 patients with known or suspected CS that the presence of focal perfusion defects, FDG uptake or right ventricular inflammation on PET conferred a greater risk of major adverse cardiovascular events (MACE). In contrast, Yamamoto et al.29 detected no difference in MACE rates between CS patients with SUVmax values above and below 4. However, 28/73 patients had already received corticosteroid therapy prior to FDG-PET imaging which may have modulated their risk. In our much larger study, we established a greater intensity of inflammation as measured by SUVmax as an indicator of poorer clinical outcomes emphasizing the importance of immunosuppressive therapy. Many of the observed differences in cardiac function and arrhythmic outcomes in our subgroup analysis can be explained by the lack of immunosuppression in Group B at the time of CS diagnosis. Mean SUVmax was not only higher in this cohort but was also an independent predictor of outcome. Correspondingly, nearly a quarter of Group B patients had an LVEF <40% indicating previously covert and therefore untreated inflammation. The right ventricle was also affected with increased echocardiographic and CMR dimensions and worse right ventricular ejection fraction. A lower threshold for ICD implantation may be considered in patients presenting with intense inflammatory signals due to the greater arrhythmic risk.
Limitations
Our sarcoidosis service is delivered in a tertiary referral centre for patients with pulmonary and CS and as a result there may be selection bias towards more advanced disease. In addition, the imaging data were derived from clinical reports from multiple operators and interpreters as part of standard clinical practice rather than from independent imaging adjudicators. As CMR and FDG-PET analyses were non-blinded, the possibility of bias in the interpretation of the images cannot fully excluded. Although the study is comprehensive for incorporating multimodality imaging data, the observational design does not address the full potential of the imaging techniques. Finally, an evaluation of the effects of treatment on serial imaging findings and clinical outcome was beyond the scope of this study.
Conclusion
Cardiac sarcoidosis in a contemporary population is associated with significant morbidity and mortality. Our findings showed that particularly higher BNP serum concentrations, but also lower LVEF and increased myocardial inflammatory signal on FDG-PET were independently associated with a worse outcome. Moreover, CS as the first clinical manifestation of previously undetected systemic sarcoidosis appears to present at a more advanced stage of the disease, with a higher arrhythmic risk.
Conflict of interest: T.F.L. reports research and educational grants from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, Sanofi, Servier and Vifor, outside this study. All other authors have nothing to disclose.