Heart failure diagnosis in the general community – Who, how and when? A clinical consensus statement of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC)
Abstract
A significant proportion of patients experience delays in the diagnosis of heart failure due to the non-specific signs and symptoms of the syndrome. Diagnostic tools such as measurement of natriuretic peptide concentrations are fundamentally important when screening for heart failure, yet are frequently under-utilized. This clinical consensus statement provides a diagnostic framework for general practitioners and non-cardiology community-based physicians to recognize, investigate and risk-stratify patients presenting in the community with possible heart failure.
Introduction
Heart failure, a frequent consequence of a wide range of cardiac diseases, results in substantial morbidity and mortality, and a significant socioeconomic burden. An estimated 64 million people worldwide are suffering from heart failure, and an increasing prevalence is attributed to an increasingly elderly population, better survival from myocardial infarction (MI) and increasing prevalence of other comorbidities which predispose to heart failure.1 The last three decades have seen dramatic progress in the management of patients with heart failure, therefore an early diagnosis and implementation of disease-modifying therapy is of paramount importance. Unfortunately, the diagnosis of heart failure is often missed or delayed in the general community, leading to delayed treatment and potentially avoidable hospitalizations and deaths. For example, one-in-six persons aged >65 years presenting to primary care with breathlessness on exertion will have unrecognized heart failure (mainly heart failure with preserved ejection fraction [HFpEF]).2 Furthermore, of patients presenting to hospital with heart failure for the first time, it has been reported that approximately 40% had presented to their primary care physician in the preceding 5 years and reported at least one symptom of heart failure.3 A key reason contributing to the under-diagnosis of heart failure is the non-specific nature of the symptoms and signs, necessitating objective diagnostic tests which primary care practitioners may or may not have access to. These diagnostic challenges are particularly relevant for HFpEF – a condition which primary care practitioners often feel ill-equipped to screen for and diagnose. With the advent of treatment options that significantly lower event rates in patients with heart failure across the entire spectrum of left ventricular ejection fraction (LVEF) from ‘reduced’ to ‘preserved’, there is an urgent need to provide a simple, practical clinical pathway to guide heart failure screening and diagnosis in primary care settings.4
- Who should be screened for heart failure?
- How should the diagnosis of heart failure in the primary care setting be established with emphasis on recognition of the clinical syndrome of heart failure and objective diagnostic tools that are available in primary care (e.g. electrocardiography, chest X-ray, natriuretic peptides, point-of-care ultrasound); as well as important ‘mimickers’ of HFpEF not to miss (e.g. amyloidosis)?
- When should suspected or confirmed heart failure patients be referred to a specialist?
General practitioners, community nurses and pharmacists play a vital role in the prevention, diagnosis and management of heart failure and are often the first healthcare professional a patient will present to with symptoms of heart failure. Given that most patients present with undifferentiated symptoms in primary care settings, general practitioners are most well-positioned to identify patients with possible heart failure, perform the diagnostic work-up, and initiate timely treatment. Following the diagnosis of heart failure, primary care teams are also essential for encouraging adherence to appropriate guideline-directed medical therapy (including its continuous adjustment and monitoring), supporting healthy lifestyle measures, managing comorbidities in conjunction with other specialties, as well as coordinating care and communication with the family and caregivers of the heart failure patient. Under- and misdiagnosis of heart failure can occur at the primary care level for several reasons, including lack of awareness of heart failure as a condition, underreporting of symptoms by patients, the non-specificity of symptoms of heart failure, the presence of comorbidities, or uncertainty in the diagnosis of heart failure as a possible diagnosis in the absence of a frank reduction in LVEF (i.e. the diagnosis of HFpEF).2, 5 There is a longstanding perception of heart failure as a condition causing acute breathlessness with pulmonary and peripheral oedema. There is little appreciation of heart failure as a chronic condition that frequently presents with subtle breathlessness in the absence of signs of fluid overload.
The objective of this paper is to provide primary care teams with guidance for heart failure screening and diagnosis that is clear, feasible, clinically relevant, simple but sound, and with universal applicability globally. Key concepts are summarized and readers are referred to source documents (such as 2021 European Society of Cardiology [ESC] guidelines for heart failure, 2021 universal definition and classification of heart failure, 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America [AHA/ACC/HFSA] guidelines for heart failure and 2019 Heart Failure Association [HFA] consensus statement for the diagnosis of HFpEF) for details and in-depth considerations.6-9
Definition and classification of heart failure
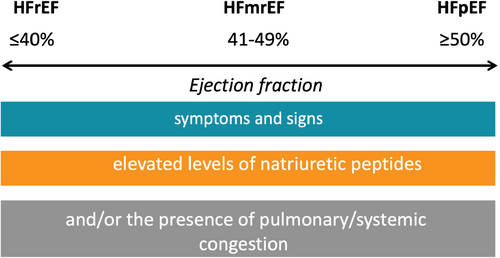
Heart failure is a clinical syndrome characterized by symptoms and signs due to a structural and/or functional abnormality of the heart that results in elevated intracardiac pressures and/or inadequate cardiac output at rest and/or during exercise.6 As with other clinical syndromes its diagnosis therefore begins with recognizing the typical constellation of symptoms and signs (Table 1). Importantly, heart failure is neither a single pathological diagnosis (and therefore a cause should always be sought), nor is it solely defined by reduced LVEF.
| Sensitivity | Specificity | |
|---|---|---|
| Dyspnoea | 50% | 73% |
| Dyspnoea on exertion | 66% | 52% |
| Orthopnoea | 66% | 47% |
| Third heart sound | 73% | 42% |
| Bilateral leg oedema | 94% | 10% |
| Weight change | 9% | 97% |
| Jugular venous reflux | 50% | 75% |
| Resting jugular venous distention | 70% | 79% |
| Jugular venous distention >8 cm | 48% | 78% |
| Hepatomegaly | 51% | 62% |
| Chest X-ray findings | ||
| Cardiomegaly | 66% | 96% |
| Redistribution | 60% | 68% |
| Interstitial oedema | 60% | 73% |
| Pleural effusion | 43% | 79% |
- Data adapted from Mullens et al.11
- Symptoms and/or signs caused by a structural and/or functional cardiac abnormality, and
-
Corroboration by at least one of the following:
- Elevated natriuretic peptide levels
- Objective evidence of cardiogenic pulmonary or systemic congestion.
Of note, impaired LVEF can lead to the clinical syndrome of heart failure, but heart failure can also be present with near-normal or normal LVEF. Based on LVEF, heart failure is classified into heart failure with reduced ejection fraction (HFrEF) when LVEF is ≤40%, heart failure with mildly reduced ejection fraction (HFmrEF) if LVEF ranges from 41% to 49%, and HFpEF, if above criteria for heart failure are present, but LVEF is 50% or more (Figure 1). In addition, there is a category referred to as ‘heart failure with improved ejection fraction’, when patients recover from a baseline LVEF ≤40%, with a follow-up LVEF >40% and at least 10% absolute increase from baseline.6
Who to screen for heart failure?
Patients with suggestive symptoms and signs of heart failure
As mentioned above, the diagnosis of heart failure requires the presence of symptoms. Typical symptoms in the majority of patients are caused by congestion as a result of elevated cardiac filling pressures.6, 10-12 Table 1 describes the typical constellation of symptoms and signs of heart failure that should raise suspicion for the diagnosis, although the sensitivity and specificity of individual features may be limited.
While most aspects of the physical examination and radiographic assessment have low accuracy for detecting clinical congestion, the presence of an elevated jugular venous pressure combines both moderately high sensitivity (70%) and specificity (79%) to identify individuals with elevated left ventricular filling pressures.13-18 Changes in body weight have a high specificity for progressive congestion (97% likelihood that a patient with known heart failure who gains 2–4 pounds [∼1–2 kg] in 24–36 h requires hospitalization), but sensitivity is only 9%.19 Undiagnosed heart failure should also be considered a possibility in patients who are prescribed diuretic therapy for peripheral oedema in the absence of an established diagnosis of heart failure.
Whilst overt symptoms and signs of heart failure (‘clinical’ congestion) may not always be present at rest, such patients may have subclinical elevated cardiac filling pressures (‘haemodynamic’ congestion).20, 21 The diagnosis of heart failure (especially HFpEF) can be challenging in these circumstances and may require specialist input and the use of provocative testing (e.g. exercise stress testing) and/or invasive haemodynamic evaluation to make the diagnosis of heart failure.9
Patients with risk factors for heart failure
Common risk factors for the development of heart failure are coronary artery disease, including a previous MI, hypertension, atrial fibrillation (AF), valvular heart disease, diabetes, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), obesity, a history of high alcohol intake, cardiotoxic drugs (e.g. trastuzumab), or a family history of cardiomyopathy.22, 23 Therefore, a diagnosis of heart failure should be thought of in patients with a history of these diseases (or a family history of cardiomyopathy) who present with one or more of the symptoms detailed previously. Many patients with one or more of these factors will have regular interactions with their primary care team and we strongly advocate that undiagnosed heart failure should be considered and investigated for in patients reporting new symptoms of breathless, fatigue, exercise intolerance and ankle swelling. There are currently no ESC guideline recommendations to support population-level screening for heart failure, however targeted screening is recommended in patients receiving cardiotoxic cancer treatments and there is increasing interest in prospectively testing the value of targeted screening strategies in high-risk populations such as those with diabetes and CKD.24
Coronary heart disease
A previous MI or established coronary artery disease is one of the most common reasons for the development of heart failure. The relationship between the development of heart failure and a history of MI is complex: survival from acute MI has improved dramatically in the last three decades resulting in a larger pool of survivors with ventricular damage who are at risk of the development of heart failure (predominantly HFrEF).25 Alongside this, however, secondary prevention has also improved with therapies that attenuate adverse left ventricular remodelling (e.g. renin–angiotensin–aldosterone system inhibitors and beta-blockers) along with statin therapy and antiplatelets which reduce the risk of reinfarction and promote infarct-related artery patency in the short and long term. Contemporary estimates are that in the era of primary angioplasty and pharmacotherapy the incidence rates of heart failure hospitalization following acute MI in men and women are approximately 31 and 46 per 1000 person-years, respectively, with the highest rates occurring in the 6 months following MI.26 Therefore, despite these advances, the development of heart failure remains a frequent occurrence in the months and years following acute MI and should be considered in all short- and long-term survivors of MI who present with symptoms consistent with heart failure.
Hypertension
Hypertension is another common cause of heart failure (both HFrEF and HFpEF) with systolic and diastolic dysfunction resulting from chronically high afterload and compensatory hypertrophy.27, 28 In addition, hypertension may indirectly contribute towards heart failure as it is a risk factor for other conditions causally linked to heart failure, such as coronary artery disease. In the Framingham Heart Study men and women with hypertension had a two-fold and three-fold higher risk of developing heart failure, respectively, as compared with normotensive individuals.29
Atrial fibrillation
Atrial fibrillation and heart failure are inextricably linked, with each being a risk factor for the other.30 All patients with AF should be assessed for echocardiographic signs of heart failure due to the high prevalence of systolic dysfunction in patients with AF. It is important to assess for the presence of systolic dysfunction using echocardiography as this has important consequences for the management of AF including consideration of early rhythm control therapy in the setting of tachycardiomyopathy (systolic dysfunction secondary to uncontrolled ventricular rate) and the contraindication to the use of rate-limiting calcium channel blockers.6
Valvular heart disease
Valvular heart disease, both significant stenotic and regurgitant lesions, can cause heart failure. One of the values of routine echocardiography in patients with suspected heart failure is to assess for the presence of valvular pathology which can be addressed surgically or percutaneously, as in many cases this can result in an improvement or resolution of heart failure secondary to valve disease.
Diabetes
Heart failure is a common complication of diabetes, and its presence is frequently underappreciated, often due to the poor specificity of heart failure symptoms and overlap with symptoms of diabetes and its other complications. In the Framingham cohort, the presence of diabetes was associated with a two-fold increase in the risk of heart failure for men and five-fold for women.31 Poor glycaemic control is associated with a greater risk of the development of heart failure.32 In patients with diabetes, the incidence of heart failure has been reported, along with peripheral arterial disease, to be the most common presentation of cardiovascular disease highlighting the importance of considering heart failure in patients with diabetes who present with symptoms.33 Indeed, the prevalence of undiagnosed heart failure in patients with diabetes has been reported to be as high as 28%, with approximately 80% of these diagnosed as having HFpEF.5
Chronic obstructive pulmonary disease
In clinical practice, the coexistence of COPD and heart failure is common. In contemporary HFrEF trials, the prevalence of COPD is approximately 12–15%.34, 35 The risk of developing heart failure is 4.5 times higher among patients with COPD as compared to individuals without COPD.36 As a consequence of common risk factors (cigarette smoking, advanced age and systemic inflammation) and symptoms, the diagnosis of heart failure in patients with COPD may be challenging and frequently underappreciated. Furthermore, each is an independent predictor of poor outcomes.37 The presence of COPD is an independent predictor of adverse outcomes in patients with heart failure and the same applies for the presence of heart failure in patients with COPD.38, 39
Chronic kidney disease
A frequent comorbidity in patients with coronary artery disease, hypertension and diabetes is CKD, which is also an independent risk factor for the development of heart failure.40 Heart failure doubles the rate of decline in estimated glomerular filtration rate over time, as compared to patients without heart failure, and the combination of heart failure and diabetes is associated with a four-fold increase in the rate of decline. Beyond the diagnostic challenge of attributing fluid overload to heart failure versus CKD, the presence of advanced CKD, including the use of renal replacement therapy, poses therapeutic challenges for the management of coexistent heart failure, therefore we would encourage early specialist input in this patient population.41
Obesity
Obesity increases the risk for heart failure, particularly HFpEF. Observational data demonstrate that the risk of developing heart failure increases by 5% in men and 7% in women for every 1 unit of body mass index above the normal range.42 As detailed in the following section, the diagnosis of heart failure in patients with obesity has its unique challenges due to falsely low natriuretic peptide levels in this population and the presence of obesity-related dyspnoea.
Cardiotoxins
Patients who abuse cardiotoxins such as alcohol or have had exposure to cardiotoxic drugs are at high risk of developing heart failure.23 There is increasing awareness and understanding of the long-term cardiac complications of cancer treatment, many of which are known to induce left ventricular dysfunction, including a range of chemotherapy agents including anthracyclines, trastuzumab, and tyrosine kinase inhibitors.24 Heart failure can be a late complication of cancer treatment and so should be considered in any patients with a history of cancer and exposure to relevant anticancer treatments.24
Risk scores
As discussed previously, the symptoms of heart failure lack specificity and signs can be difficult to detect clinically. Therefore, the availability of easy-to-use clinical risk scores to aid identification of patients with a high likelihood of heart failure, or even to identify patients at high risk of developing heart failure in the future, may be desirable to help guide further targeted testing. Numerous such scores have been developed from large population cohorts such as the Framingham Heart Study, the Multi-Ethnic Study of Atherosclerosis (MESA), the Atherosclerosis Risk in Communities (ARIC) study or the Health Aging and Body Composition (Health ABC) study (Table 2).43-46 A specific score – WATCH-DM (weight [body mass index], age, hypertension, creatinine, high-density lipoprotein cholesterol, diabetes control [fasting plasma glucose], electrocardiogram [ECG] QRS duration, MI, and coronary artery bypass grafting) – has been developed for patients with type 2 diabetes and has been shown to perform well in predicting incident heart failure in external validation cohorts and shown to have improved predictive capacity when used in combination with natriuretic peptide concentrations.47, 48
| Risk score | Population | Cohort and follow-up | Endpoint | Variables |
|---|---|---|---|---|
| Prediction of incident heart failure | ||||
| MESA study44 | General population aged 45–84 years | 6814 (median 4.7 years) | Risk to develop heart failure events | Age, male gender, smoking, BMI, SBP, HR, T2DM, NT-proBNP, LVMI |
| ARIC study45 | General population aged 45–64 years old | 13 555 (15.5 years) | Risk to develop heart failure events | Age, African American, male, HR, SBP, BP-lowering medication, T2DM, smoking (current/former), BMI, NT-proBNP |
| Health ABC study46 | Elderly population aged 70–79 years | 2935 (65) | Risk of hospitalization for new-onset heart failure | Age, CAD, smoking, SBP, HR, glucose, creatinine, albumin, LV hypertrophy |
| WATCH-DM47 | ACCORD trial (patients with T2DM) | 8756 (median 4.9 years) | Risk to develop heart failure events | Weight (body mass index), age, hypertension, creatinine, high-density lipoprotein cholesterol, diabetes control (fasting plasma glucose), ECG QRS duration, myocardial infarction, and coronary artery bypass grafting |
| Diagnosis of heart failure | ||||
| Framingham Heart Study43 | General population aged 30–62 years | 5192 (16 years) | Presence of heart failure |
Major criteria: nocturnal dyspnoea or orthopnoea, neck-vein distention, rales, cardiomegaly, acute pulmonary oedema, S3 gallop, venous pressure >16 cm of water, circulation time ≥25 s, hepatojugular reflux Minor criteria: ankle oedema, night cough, dyspnoea on exertion, hepatomegaly, pleural effusion, vital capacity less than two-thirds of maximum, tachycardia (≥120/min) Major or minor criterion: weight loss ≥4.5 kg in 5 days in response to treatment Two major or one major and two minor criteria had to be present concurrently |
| H2FPEF49 | Single-centre population (Mayo Clinic) referred for invasive exercise testing for unexplained dyspnoea | 414 (NA) | Presence of heart failure with preserved ejection fraction | BMI, treatment with two or more antihypertensives, AF, age, E/e' ratio, echo-derived PASP |
| HFA-PEFF9 | Consensus recommendation | NA | Presence of heart failure with preserved ejection fraction | e′ velocity, E/e′ ratio, TR velocity, longitudinal strain, LA volume, LV wall thickness, LV mass, natriuretic peptide concentrations |
- AF, atrial fibrillation; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; ECG, electrocardiogram; HR, heart rate; LA, left atrial; LV, left ventricular; LVMI, left ventricular mass index; NA, not available; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PASP, pulmonary artery systolic pressure; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TR, tricuspid regurgitation.
Scores have also been developed to aid the diagnosis of heart failure (Framingham) and specifically for the identification of patients with HFpEF – a diagnosis which is frequently challenging (Table 2).43 These include the H2FPEF score and the HFA-PEFF score, both of which require echocardiographic data.9, 49 It is interesting to note that most risk models to identify heart failure share a set of common variables (age, sex, New York Heart Association [NYHA] class, blood pressure, diabetes, body mass and indices of renal function). However, none of the scoring systems have found widespread use in clinical practice and current European guidelines do not recommend the use of score-based approaches, with the exception of HFpEF, in which the use of scoring systems may facilitate diagnosis.6 However, the guidelines acknowledge that general physicians often do not have access to all tests and parameters required by the specific diagnostic algorithms in HFpEF and are largely limited to the realms of specialist cardiology clinics. Future research should focus on the potential benefit of identifying high-risk patients by routine implementation of these risk scores (e.g. WATCH-DM in patients with diabetes).
The future of screening for heart failure
It is foreseeable that in the future, artificial intelligence and machine learning assisted algorithms for the detection of unrecognized heart failure will become increasingly available. They will aid the identification of patients at risk for heart failure in whom targeted screening may be appropriate and potentially patients with undiagnosed heart failure.50, 51 Numerous such algorithms have already been developed. They follow two basic strategies: one is to evaluate multiple documented parameters, e.g. in electronic patient records, to identify patients at risk for developing clinical heart failure or heart failure events.47, 52 Another strategy with promising results is the use of machine learning techniques to interpret single diagnostic modalities such as the conventional ECG or echocardiogram in order to identify patients with unrecognized heart failure or to aid in the interpretation of results.53-57
So far, none of these algorithms have found their way into routine clinical practice. Reasons include the ‘black box’ nature of machine learning algorithms, the fact that some of them require the inclusion of a multitude of variables limiting their usability, as well as potential ‘overfitting’ of the models to the derivation cohorts, which can limit generalizability to external data sets.50 As long as artificial intelligence algorithms use the same parameters as the more traditional scores listed above, no significant improvement of their predictive power can be expected. However, the possibility of interpreting standard ECG recordings by machine learning algorithms in order to identify patients with a likelihood of heart failure as the cause of non-specific symptoms, or at high risk of developing heart failure in the future, may be a potentially valuable asset in community-based screening strategies. Future research therefore needs to determine whether such algorithms retain their accuracy when applied to patients with broader demographics, and to compare their diagnostic value to current standards, e.g. to the point-of-care measurement of natriuretic peptides and routine cart-based echocardiography.
How to diagnose heart failure
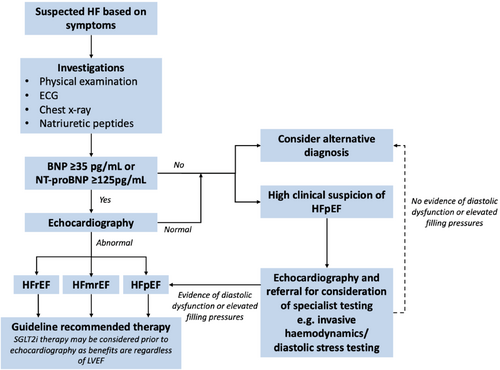
The presence of symptoms and signs suggestive of heart failure lack the specificity to be solely sufficient to make a diagnosis of heart failure and further confirmatory objective evidence of cardiac dysfunction and elevated filling pressures is required to make this diagnosis (Figure 2).58-61 In addition to history taking and clinical examination to detect suggestive symptoms and signs, the ESC guidelines for the management of heart failure advocate for a standard set of investigations to be performed in all patients suspected of having heart failure (Table 3).6 It is important to highlight that in many patients being investigated for potential heart failure, other diagnoses with overlapping symptoms may be present or made concurrently (e.g. COPD or coronary artery disease) and the presence of these diseases should not dissuade clinicians from also investigating for heart failure.
| Diagnostic tool | Positive findings suggestive of heart failure in the presence of symptoms | Requires specialist interpretation | Comments |
|---|---|---|---|
| Physical examination |
Elevated jugular venous pressure Hepatojugular reflux Lung rales Peripheral oedema Third heart sound |
No | Physical examination may be normal in patients with normal filling pressures at rest. |
| ECG |
AF Bundle branch block Left axis deviation Left ventricular hypertrophy Signs of previous myocardial infarction |
No | A normal ECG virtually excludes a diagnosis of HFrEF but may be present in 35–45% of patients with HFpEF. |
| Chest X-ray |
Cardiomegaly Pulmonary venous congestion Interstitial oedema Pleural effusion |
No |
A normal chest X-ray does not rule out a diagnosis of heart failure. Chest X-ray is valuable in screening for alternative causes of symptoms (e.g. lung cancer or pulmonary fibrosis). |
| Natriuretic peptides |
NT-proBNP ≥125 pg/ml BNP ≥35 pg/ml |
No |
Natriuretic peptide concentrations are higher in patients with AF, chronic kidney disease and the elderly. Concentrations are lower in patients who are obese. Up to 25% of patients with invasively proven HFpEF may have NT-proBNP <125 pg/ml. |
| Echocardiography |
LVEF <50% (HFrEF [LVEF ≤40%] and HFmrEF [LVEF 41–49%]) HFpEF: signs of LV diastolic dysfunction/elevated LV filling pressures:
|
Yes | These thresholds are based on the 2021 ESC heart failure guidelines.6 |
| Right heart catheterization | Pulmonary capillary wedge pressure ≥15 mmHg at rest | Yes |
Only available in specialist centres. Diagnostic of elevated left ventricular filling pressures at rest. |
| Diastolic stress testing |
Invasive: pulmonary capillary wedge pressure ≥25 mmHg at peak exercise. Stress echocardiography:
|
Yes |
Only available in specialist centres. TR velocity may only be measurable in 50% of patients with HFpEF. |
- AF, atrial fibrillation; BNP, B-type natriuretic peptide; ECG, electrocardiogram; ESC, European Society of Cardiology; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PASP, pulmonary artery systolic pressure; TR, tricuspid regurgitation.
Electrocardiography
The 12-lead ECG is an important part of the screening process in all patients with suspected heart failure as it is not only a sensitive screening tool but also provides additional information regarding potential personalized treatment options in patients confirmed to have heart failure. A normal 12-lead ECG is rare in patients with a diagnosis of HFrEF and virtually excludes a diagnosis of HFrEF.62-65 In a cohort of 534 patients, 96 had left ventricular systolic impairment and all these patients had an abnormal ECG with 90 (94%) classed as having ‘major abnormalities’ (AF, previous MI, left ventricular hypertrophy, bundle branch block and/or left axis deviation).62 However, in the same cohort, major ECG abnormalities were present in 39% of those without heart failure. This highlights that the finding of ECG abnormalities is a highly sensitive marker for a diagnosis of HFrEF but lacks specificity. On the other hand, the sensitivity of the ECG in HFpEF is lower than in HFrEF, and a normal ECG may be present in 35–45% of patients with HFpEF.66
In patients with signs and symptoms of heart failure, particular ECG abnormalities may provide clues as to the aetiology of heart failure and lead to specific investigations and treatment strategies. These include the presence of changes consistent with a previous MI, AF with an uncontrolled ventricular rate, high percentage of right ventricular pacing, and left bundle branch block. Specific treatments including anticoagulation in the context of AF, and cardiac resynchronization therapy in patients with a LVEF ≤35% and a left bundle branch block are recommended in patients with these specific ECG abnormalities.6
Chest X-ray
A normal chest X-ray does not rule out a diagnosis of heart failure. Approximately a third of patients with a depressed LVEF have a normal cardiothoracic ratio (<0.5) on chest X-ray.67 Conversely, the presence of an enlarged cardiac silhouette on a chest X-ray does not necessarily signify the presence of left ventricular dilatation and systolic impairment. Alternative causes of radiographic cardiomegaly include pericardial effusion, pericardial fat, left ventricular hypertrophy and valvular heart disease. Several features on a chest X-ray including pulmonary venous congestion, interstitial oedema and bilateral effusions may support a diagnosis of heart failure, however these findings along with the presence of an increased cardiothoracic ratio offer little additive diagnostic value to that of an abnormal ECG.68 In patients who are suspected to have heart failure, in addition to detecting signs which may support a diagnosis of heart failure such as cardiomegaly, there is value in a chest X-ray as a screening tool for alternative lung pathologies which may cause breathlessness (e.g. lung cancer or pulmonary fibrosis).
Natriuretic peptides
The natriuretic peptides are a group of circulating peptides which are released in response to increased cardiac wall stress with cardioprotective vasodilatory, natriuretic, antifibrotic and sympatholytic properties.69 The most well-known and studied of the natriuretic peptides is B-type natriuretic peptide (BNP) and its biologically inactive precursor fragment N-terminal prohormone of BNP (NT-proBNP) which is released in equimolar concentrations to bioactive BNP. Elevated concentrations of BNP and NT-proBNP reflect increased left ventricular wall stress. As well as being a powerful independent predictor of adverse outcomes in patients with established heart failure, elevated concentrations of the natriuretic peptides are a sensitive marker when investigating patients with suspected heart failure in both the acute hospital and non-acute community settings.70-72 For this purpose of this article, we will focus on the utility of natriuretic peptide measurement in the non-acute setting.
In a study by Cowie et al.,71 atrial natriuretic peptide (ANP) and BNP were measured in 122 consecutive patients referred by their general practitioner with suspected heart failure. Mean ANP and BNP were significantly higher in those patients adjudicated by a panel of cardiologists to have a new diagnosis of heart failure based on clinical assessment, chest X-ray and transthoracic echocardiography. A BNP cut-off of ≥22.2 pmol/L (76 pg/ml) had a negative predicative value for heart failure of 98% and provided a greater degree of sensitivity (97%), specificity (84%) and positive predictive value (70%) than ANP or N-terminal ANP. The high sensitivity of natriuretic peptides for the community-based diagnosis of heart failure was further confirmed in a series of studies in a range of populations and countries.61, 73-76 In a randomized controlled trial in patients with suspected heart failure based on symptoms, a diagnostic strategy including NT-proBNP had a greater diagnostic accuracy than a strategy without NT-proBNP.77 NT-proBNP may also have a role as a screening tool for undiagnosed left ventricular systolic dysfunction and/or heart failure in high-risk populations to guide preventative treatments.78, 79
Based on these data, the measurement of BNP or NT-proBNP has been recommended as a key test in the assessment of patients with suspected signs and symptoms of heart failure. Current international guidelines use BNP and NT-proBNP cut-off values of ≥35 pg/ml and ≥125 pg/ml, respectively.6, 8 Concentrations below these thresholds have a negative predictive value of 95–99% indicating that a diagnosis of heart failure is unlikely, therefore eliminating the subsequent need for echocardiography.
There are some factors and comorbidities which influence circulating natriuretic peptide concentrations and should be considered when interpreting results. Age, AF, diabetes, and CKD are all associated with higher natriuretic peptide concentrations.80, 81 It has been suggested that specific cut-offs for age and AF status be incorporated into diagnostic guidelines, however such a strategy requires further testing in prospective clinical trials.82 Conversely, obesity, a frequent comorbidity in patients with HFpEF, is associated with lower circulating levels of natriuretic peptides.80 It has been suggested that a substantial proportion of ambulatory patients with early/minimally symptomatic HFpEF may have normal natriuretic peptide levels despite increased filling pressures as measured by invasive exercise haemodynamic testing.83, 84 Indeed, the dynamic nature of elevated filling pressures (and therefore natriuretic peptides) in HFpEF warrants consideration, with many patients having normal filling pressures at rest with elevations occurring only during exercise. Subsequently, specific HFpEF diagnostic guidelines have been suggested which permit the diagnosis of HFpEF in the absence of elevated natriuretic peptide levels, however we would encourage such cases to be discussed with specialists in this area.9, 49
Future use of NT-proBNP in the diagnosis of suspected heart failure may involve the use of diagnostic support tools incorporating artificial intelligence to utilize electronic healthcare records recording clinical characteristics such as age, sex, kidney function and AF status along with NT-proBNP concentrations assessed as continuous variables (rather than dichotomous cut-off values). Such an approach has been shown to be a more accurate diagnostic tool for the diagnosis of heart failure in patients presenting with acute dyspnoea rather than an individual cut-off value.85 The utility of these instruments in patients presenting to their general practitioner in the community with symptoms of heart failure has yet to be reported.
Echocardiography
In patients with elevated natriuretic peptides suspected of having heart failure, several cardiac imaging modalities may be used to assess the presence of cardiac systolic and/or diastolic dysfunction along with indirect metrics of elevated filling pressures. Imaging also plays an important role in ruling out conditions (and directing appropriate treatments) which may also elevate natriuretic peptides and cause symptoms suggestive of heart failure including hypertrophic obstructive cardiomyopathy, valvular heart disease, and cardiac amyloidosis. The most widely used imaging modality is transthoracic echocardiography, but others include cardiac magnetic resonance imaging, radionuclide ventriculography and cardiac computed tomography.
Key echocardiographic measurements required to diagnose heart failure include LVEF, left ventricular volumes and wall thickness, left atrial volume, mitral valve inflow Doppler measurements, tricuspid regurgitation peak velocity, and inferior vena cava diameter among others.6 A full review of these measurements is beyond the scope of this article. Historically the most important of these measures has been LVEF. Based on the inclusion criteria of large, randomized clinical trials in heart failure, LVEF is used to phenotype patients into HFrEF (LVEF ≤40%), HFmrEF (LVEF 41–49%), and HFpEF (LVEF ≥50%). Guidelines provide individualized treatment recommendations for each of these three phenotypes, highlighting the importance of LVEF in guiding treatment decisions.6, 8 Recently, sodium–glucose cotransporter 2 (SGLT2) inhibitors have been shown to reduce the risk of cardiovascular death or heart failure hospitalization across the full spectrum of LVEF, therefore their use may be appropriate in eligible patients with heart failure as indicated by typical symptoms and signs along with elevated natriuretic peptides prior to estimation of LVEF.4
With a growing elderly population along with an increasing burden of risk factors associated with heart failure such as diabetes, obesity and AF, the incidence of heart failure is expected to rise in the coming years, therefore new strategies to facilitate access to diagnostic echocardiography and its time-consuming analysis are urgently required. Point-of-care ultrasound represents an important tool in the move of echocardiography away from large hospital-based cart machines to hand-held portable machines able to be used in the community clinic and in resource-limited settings.86-88 The analysis and interpretation of these scans can be achieved using telehealth models or artificial intelligence assisted automated reporting tools. Such tools have been demonstrated to have similar accuracy to expert sonographer measurements and have the potential to increase access to echocardiography, reduce costs and increase throughput in heart failure screening and diagnosis.55, 89-91
When to refer to the heart failure specialist
A patient-centred partnership between primary care and the heart failure clinical team will ensure the optimal experience of care for the patient. Points in the patient's disease journey where this may be particularly important include: (1) at initial diagnosis in cases of uncertainty; (2) during transitions of care between hospital and community settings (e.g. following discharge from a hospitalization for worsening heart failure); or (3) while monitoring patients for early signs of deterioration/worsening heart failure.
Initial diagnosis
Following implementation of the diagnostic pathway described in Figure 2, patients with confirmed or suspected heart failure should be referred to their local heart failure secondary care team for review and initiation/optimization of guideline-recommended therapy. Where the healthcare system does not provide for universal referral, it is therefore important to identify those patients who stand to benefit from early referral and review by specialist teams. Structured electronic referral pathways for echocardiography and clinical review in patients with elevated natriuretic peptides may help the flow of patients between primary and secondary care.
Importantly, referral to a heart failure specialist should not delay initiation of treatment to address congestion (e.g. with diuretics), potential precipitating factors (e.g. uncontrolled blood pressure, myocardial ischaemia, arrhythmias), and comorbidities (e.g. diabetes, infection, lung disease). Indeed, an improvement of symptoms in response to diuretics may help to confirm the diagnosis of heart failure; and, conversely, a lack of response should raise suspicion for differential diagnoses. Recent data supporting the benefits of SGLT2 inhibitors across the full spectrum of ejection fraction mean that initiation of this treatment may be appropriate prior to echocardiography.4, 92 This strategy may be beneficial for patients in two ways: firstly, the benefits of SGLT2 inhibitors have been shown in clinical trials to be evident within weeks of starting, and secondly, early initiation of SGLT2 inhibition may increase the likelihood of tolerance of other guideline-recommended heart failure treatments including mineralocorticoid receptor antagonists.93-95 In select patients, consideration of advanced therapies including device therapy may be indicated and it is vital to facilitate early review of this group of patients and appropriate specialist input and follow-up.6
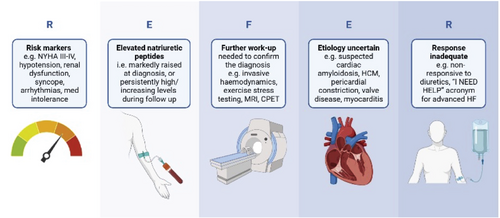
- Risk markers, e.g. NYHA class III–IV, hypotension, renal dysfunction, syncope, arrhythmias, medication intolerance.
- Elevated (persistently high or increasing) natriuretic peptides.
- Further work-up needed to confirm the diagnosis, e.g. invasive haemodynamic evaluation, exercise/diastolic stress testing, magnetic resonance imaging, cardiopulmonary exercise testing, endomyocardial biopsy.
- Etiology (aetiology) uncertain, e.g. suspected cardiac amyloidosis, hypertrophic cardiomyopathy, pericardial constriction, valve disease, myocarditis.
- Response inadequate, e.g. non-responsive to diuretics.
Risk markers
A range of risk scores exist which identify heart failure patients at higher risk of mortality and heart failure hospitalization. These include, but are not limited to, the SHFM (Seattle Heart Failure Model), MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure), BIOSTAT-CHF (A systems Biology Study to Tailored Treatment in Chronic Heart Failure) and PREDICT-HF (http://www.predict-hf.com).96-99 These risk scores may be helpful in identifying high-risk patients, however in the REVeAL-HF (Risk EValuation And its Impact on ClinicAL Decision Making and Outcomes in Heart Failure) trial, disclosure of the estimated 1-year risk of mortality in hospitalized patients, as compared with standard care, was not shown to reduce the composite outcome of 30-day hospital readmission or all-cause mortality at 1 year.100 We suggest that patients who are in NYHA functional class III–IV, have a low ejection fraction (≤35%) or have issues with arrhythmias or hypotension, worsening renal function or syncope which preclude initiation or tolerance of guideline-recommended medications should be referred for expedited specialist assessment.
Elevated (persistently high or increasing) natriuretic peptides
Elevated natriuretic peptide concentrations are one of the most powerful independent predictors of outcome in heart failure. In patients who have markedly elevated concentrations at diagnosis (e.g. >2000 pg/ml) or persistently high/increasing concentrations during follow-up, accelerated specialist review is advised.101, 102
Further work-up needed to confirm the diagnosis and Etiology uncertain
There may be patients in whom there is high clinical suspicion of HFpEF despite low/normal natriuretic peptide concentrations or equivocal resting echocardiography results. Such patients may benefit from more detailed specialist investigations including diastolic stress testing and/or invasive haemodynamic measurements and should be discussed with the local heart failure team as appropriate.9 In some patients, echocardiography may not provide accurate information regarding cardiac function and in such cases alternative imaging modalities such as cardiac magnetic resonance imaging may be required to confirm a diagnosis. As well as being the gold-standard method of assessing ejection fraction, cardiac magnetic resonance imaging and tissue mapping may also provide information with regard to aetiology with the ability to differentiate between causes of infiltrative cardiomyopathy including Fabry disease, cardiac amyloidosis, haemochromatosis and hypertrophic cardiomyopathy among others.
Response inadequate
- I: intravenous inotropes
- N: NYHA class IIIB/IV or persistently elevated natriuretic peptides
- E: end-organ dysfunction
- E: ejection fraction ≤35%
- D: defibrillator shocks
- H: hospitalizations >1 within last 12 months
- E: edema (oedema) despite escalating diuretics
- L: low systolic blood pressure ≤90 mmHg, high heart rate
- P: prognostic medication; progressive intolerance or down-titration of guideline-directed medical therapy.
Conclusion
Heart failure is a common yet frequently unrecognized syndrome. Earlier recognition of heart failure may improve outcomes for patients and aid the implementation of disease-modifying therapy. Elevated concentrations of natriuretic peptides in patients with signs and symptoms of suspected heart failure should act as a trigger to obtain objective echocardiographic evidence of cardiac dysfunction in order to make a diagnosis of heart failure and its different ejection fraction-based sub-types as this will help direct appropriate treatment. The REFER acronym may be helpful for general practitioners in identifying patients with newly diagnosed or established heart failure who may benefit from expedited specialist review by a heart failure expert.
Conflict of interest: K.F.D. reports that his employer, the University of Glasgow, has been remunerated by AstraZeneca for working on the DAPA-HF and DELIVER trials; he has received speaker's honoraria from AstraZeneca and Radcliffe Cardiology, has served on an advisory board for Us2.ai and Bayer, served on a clinical endpoint committee for Bayer, and has received research grant support from AstraZeneca, Boehringer Ingelheim and Roche outside the submitted work. C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from NovoNordisk and Roche Diagnostics; has served as consultant or on the Advisory Board/Steering Committee/Executive Committee for Alleviant Medical, Allysta Pharma, Amgen, AnaCardio AB, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, CardioRenal, Cytokinetics, Darma Inc., EchoNous Inc, Eli Lilly, Impulse Dynamics, Intellia Therapeutics, Ionis Pharmaceutical, Janssen Research & Development LLC, Medscape/WebMD Global LLC, Merck, Novartis, Novo Nordisk, Prosciento Inc, Radcliffe Group Ltd., Recardio Inc, ReCor Medical, Roche Diagnostics, Sanofi, Siemens Healthcare Diagnostics and Us2.ai; and serves as co-founder and non-executive director of Us2.ai. A.R. reports honoraria from Novartis, Roche Diagnostics and AstraZeneca. A.J.S.C. reports consulting fees from Boehringer Ingelheim, Servier, Actimed, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Respicardia, and honoraria from Astra Zeneca, Bayer, Boehringer Ingelheim, Edwards, Eli Lilly, Menarini, Novartis, Servier, Vifor, Abbott, Impulse Dynamics, Viatris. T.G. reports that her research is funded from the following sources: National Institute for Health Research (BRC-1215-20 008, Remote by Default 2 132 807, LOCOMOTION COV-LT-0016), ESRC (ES/V010069/1), Wellcome Trust (WT104830MA), Health Data Research UK (HDRUK2020.139), NIHR School of Primary Care Research (SPCR594). M.M. received personal fees of minimal amounts in the last 3 years from Amgen, Livanova, and Vifor Pharma as member of Executive or Data Monitoring Committees of sponsored clinical trials; from AstraZeneca, Abbott Vascular, Bayer, Boheringer Ingelheim, Edwards Therapeutics and Roche Diagnositics for participation in advisory boards and/or speeches at sponsored meetings. M.C.P. reports he has received lecture, committee, or advisory board fees from AstraZeneca, Boehringer Ingelheim, Novartis, Novo Nordisk, Abbvie, Bayer, Napp, Takeda, Corvia, Cardiorentis, Pharmacosmos, Siemens, Vifor, and Eli Lilly; and has received grants from Boehringer Ingelheim, Roche, SQ Innovations, AstraZeneca, Novartis, Novo Nordisk, Medtronic, Boston Scientific, and Pharmacosmos. G.M.C.R. was supported by funding of the Italian Ministry of Health (Ricerca Corrente: 20/1819).
Acknowledgement
Open access funding provided by BIBLIOSAN. [Correction added on 2 August 2023, after first online publication: BIBLIOSAN funding statement has been added in this version.]




