Effect of allogeneic adipose tissue-derived mesenchymal stromal cell treatment in chronic ischaemic heart failure with reduced ejection fraction – the SCIENCE trial
Abstract
Aims
The aim of the SCIENCE trial was to investigate whether a single treatment with direct intramyocardial injections of adipose tissue-derived mesenchymal stromal cells (CSCC_ASCs) was safe and improved cardiac function in patients with chronic ischaemic heart failure with reduced ejection fraction (HFrEF).
Methods and results
The study was a European multicentre, double-blind, placebo-controlled phase II trial using allogeneic CSCC_ASCs from healthy donors or placebo (2:1 randomization). Main inclusion criteria were New York Heart Association (NYHA) class II–III, left ventricular ejection fraction (LVEF) <45%, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels >300 pg/ml. CSCC_ASCs or placebo (isotonic saline) were injected directly into viable myocardium. The primary endpoint was change in left ventricular end-systolic volume (LVESV) at 6-month follow-up measured by echocardiography. A total of 133 symptomatic HFrEF patients were included. The treatment was safe without any drug-related severe adverse events or difference in cardiac-related adverse events during a 3-year follow-up period. There were no significant differences between groups during follow-up in LVESV (0.3 ± 5.0 ml, p = 0.945), nor in secondary endpoints of left ventricular end-diastolic volume (−2.0 ± 6.0 ml, p = 0.736) and LVEF (−1.6 ± 1.0%, p = 0.119). The NYHA class improved slightly within the first year in both groups without any difference between groups. There were no changes in 6-min walk test, NT-proBNP, C-reactive protein or quality of life the first year in any groups.
Conclusion
The SCIENCE trial demonstrated safety of intramyocardial allogeneic CSCC_ASC therapy in patients with chronic HFrEF. However, it was not possible to improve the pre-defined endpoints and induce restoration of cardiac function or clinical symptoms.
Graphical Abstract
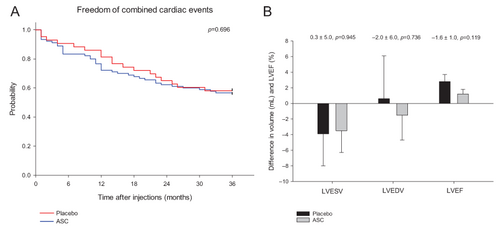
Treatment of chronic ischaemic heart failure with reduced ejection fraction (HFrEF) patients with allogeneic adipose tissue-derived stromal cells (ASC) was safe but without any demonstratable restoration of cardiac function or clinical symptoms. (A) Kaplan–Meier plot of freedom of cumulative combined cardiac-related adverse events during a 3-year follow-up period. (B) Differences in baseline to 6-month follow-up in left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDV) and left ventricular ejection fraction (LVEF) in ASC treated compared to placebo patients.
Introduction
Ischaemic heart failure with reduced ejection fraction (HFrEF) is a serious clinical condition with a poor prognosis despite improvements in medical therapies, cardiac rehabilitation and device-based therapies.1, 2 Cell therapies can potentially be an adjunct approach for improvement of cardiac function and heart failure (HF) symptoms. Several smaller randomized clinical trials have been conducted with different cell populations in both patients with ischaemic HF and refractory angina.3-13 They have all demonstrated safety but so far, the clinical efficacy data have been conflicting.
Many research groups and companies are now focusing on mesenchymal stromal cell (MSC) as the cell of choice for regeneration in many disease indications.6-10 MSCs can be isolated from several different tissues (adipose tissue, bone marrow, umbilical cord, etc.) and culture expanded in large quantities. The MSCs have unique immunomodulatory traits since they evade being recognized by a recipient immune system and can modulate the function of host immune system – most intriguingly by suppressing it.14-20 Allogeneic cell products have several advantages compared to autologous cell products. It is a much more homogeneous cell product, which can be better characterized with potency tests and stored to be used as an off-the-shelf product. Therefore, it is easy to deliver a standardized cell dose to all patients. Moreover, by using healthy donors, the cell product has not been exposed to the risk factors of the patients. Moreover, the MSC regenerative capacity is further related to reduction in fibrosis, anti-apoptosis, and initiation of endogenous tissue regeneration.18
We have established a production of clinical grade allogeneic adipose tissue-derived MSCs (CSCC_ASCs) from healthy donors.21-23 The CSCC_ASCs are stored in sealed vials in nitrogen dry containers as an off-the-shelf product in the hospital ready to be used without any delay. In a phase I study, this cell product demonstrated safety in patients with ischaemic HF and a clinical treatment feasibility concept.24
The aim of the SCIENCE trial was to investigate the safety and clinical efficacy of a single treatment with direct intramyocardial injections of CSCC_ASCs versus placebo in patients with symptomatic chronic ischaemic HFrEF without further treatment options.
Methods
Study design
The SCIENCE trial is an investigator-initiated, randomized, European multicentre, placebo-controlled, double-blind, phase II clinical trial described in detail previously.25 The study was conducted in Denmark, Germany, The Netherlands, Austria, Slovenia, and Poland after a Voluntary Harmonization Procedure approval through the Clinical Trials Facilitation Groups, European Medicines Agency, EudraCT No: 2015–002929-19. Moreover, it was then approved by the National Competent Authorities and Ethics Committees in the participating countries. The study was conducted according to Good Clinical Practice and follows the latest version of the Helsinki Declaration adopted in 2013 at the 64th World Medical Association Assembly, Brazil. The study is registered in ClinicalTrial.gov (NCT02673164).
Study population
The study included patients between 30 and 80 years with chronic ischaemic HFrEF, impaired left ventricular ejection fraction (LVEF <45% measured by echocardiography), symptomatic HF (New York Heart Association [NYHA] class II–III), and plasma N-terminal pro-B-type natriuretic peptide (NT-proBNP) >300 pg/ml (>35 pmol/L). All included patients had to be on maximal tolerated doses of guideline-recommended HF medications without indications for further invasive treatment, such as revascularization and mechanical circulatory support. All inclusion and exclusion criteria are listed in Appendix 1 and in the design publication.25
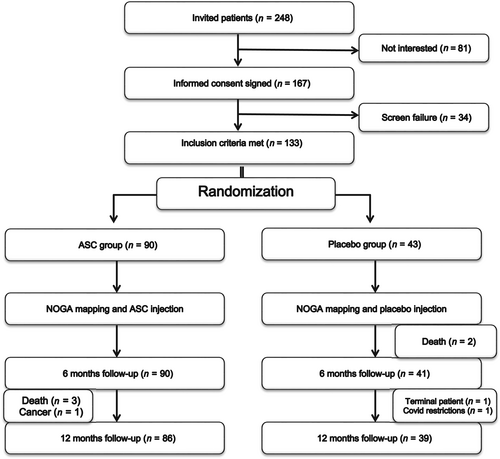
Overview of the study flow
Patients were screened for trial eligibility, included, treated, and followed for 12 months in six European HF centres. Patients were randomized 2:1 to receive intramyocardial injections of CSCC_ASC or placebo (isotonic saline). Treatment randomization was performed by personnel at the Cardiology Stem Cell Centre (CSCC) cell-manufacturing unit before shipment of the treatment vials to the clinical sites. The randomization was for each site performed in blocks of six (four CSCC_ASC and two placebo) to avoid bias in randomization between sites. The vials were stored locally until treatment of patients with direct intramyocardial injections of either 110 × 106 CSCC_ASCs in 5 ml CryoStor or 5 ml saline (placebo). There was no human leucocyte antigen (HLA) tissue type matching between the donor and the patients except for the site in The Netherlands. Here, it was requested by the Medicinal Research Ethics Committee (METC) to perform HLA antibody screening in all patients before randomization and allocate accordingly a donor to which no specific alloantibodies were present, if randomized to CSCC_ASC treatment.
To assure blinding of outcome ascertainment, the clinical teams performing screening and follow-up of the patients were not allowed to participate in the treatment procedure or be in the catheterization laboratory during the treatment. Follow-up visits were conducted 1, 3, 6 and 12 months after treatment for safety and efficacy evaluation. The study algorithm and visit schedule are summarized in Figure 1.
Cell production
The CSCC_ASC and placebo (saline) vials were manufactured at CSCC, Rigshospitalet, University Hospital Copenhagen, Denmark. CSCC holds a manufacturing authorization (no. 23909) and a tissue establishment authorization (no. 32298) issued and inspected every second year by the Danish Medicines Agency and the Danish Patient Safety Authority, respectively. The manufacturing procedure is following the EU Guidelines for Good Manufacturing Practice (GMP) of Medicinal Products for Human Use (certificate of GMP compliance no. DK IMP 92217).
Six healthy volunteer donors, five female (age 26–34 years) and one male (age 30 years), were included in the study. Donors signed an informed consent in compliance with the Declaration of Helsinki. Donors were tested for human immunodeficiency virus 1/2, hepatitis B and C, syphilis, and human T-cell lymphotropic virus I/II serology by serum analysis within 30 days prior to liposuction and by repeated serology and/or nucleic acid testing on the day of donation.
Liposuction was performed under local anaesthesia from abdominal subcutaneous adipose tissue in donors by an experienced plastic surgeon (Printzlau Private Hospital, Denmark) in full compliance with surgical procedures for sterile cosmetic surgery. Approximately 100–150 ml lipoaspirate was obtained from each donor, followed by a xeno-free cell expansion performed with human platelet lysate (Cook Regentec/Sexton Biotechnologies) as a growth supplement in semi-automated and functionally closed bioreactor systems (Quantum Cell Expansion System, Terumo BCT).21-23 The final cell product was on two passages in the bioreactor system. Harvested CSCC_ASCs were cryopreserved in CellSeal vials (COOK Regentec/Sexton Biotechnologies) at doses of 110 × 106 cells in 5 ml CryoStor CS10 (BioLife Solutions) and were stored below −180°C in nitrogen dry storage until clinical use. Release criteria were sterility, endotoxin <70 IU/ml, viability (>80%) and identity (stable positive markers CD90, CD105, and CD73; and negative markers CD45 and HLA-DR) (Appendix 2). Mycoplasma testing was performed on the supernatant from all bioreactor expansions immediately prior to cell harvest. The presence of bacteria, fungi, and endotoxins was tested on the final product, immediately prior to cryopreservation.21-23 A total of 24-month stability has been documented by analyses for sterility, viability, recovery, CSCC_ASC identity, and proliferation potential after thawing of the final product.
Each final CSCC_ASC product vial contained cells from a single donor. Patients randomized to placebo received injections of isotonic saline from a vial stored identical to the CSCC_ASC vials.
World courier shipped the CSCC_ASC and placebo vials to each site in Europe in a qualified portable nitrogen dry shipper, in accordance with the European rules for Good Distribution Practices. The randomization code for each delivered vial was available in a sealed envelope at each site in case there was an acute need to break the code in situations with a serious unexpected serious adverse event (SAE).
Cardiac echocardiographic scans and analyses
The investigation is described in detail in the design publication.25 The echocardiographic (ECHO) scans were performed at each site following the same ECHO site instruction manual.
Patients had an ECHO scan at baseline and after 6 months for inclusion and evaluation of the primary endpoint.
All ECHO imaging data were stored in a central server at the Netherlands Heart Institute & Lygature/TraIT, Durrer Center, Amsterdam, The Netherlands.
The baseline and 6-month ECHO investigations were analysed blinded by two independent groups at the Stem Cell Imaging Core Lab, University Medical Center Utrecht, Utrecht, The Netherlands, and at the Advanced Heart Failure and Transplantation Centre, University Medical Centre, Ljubljana, Slovenia. The two groups did not have any access to each other's analyses.
If a difference between the two observers of more than ± 2 standard deviations (SD) in left ventricular end-systolic volume (LVESV) and left ventricular end-diastolic volume (LVEDV) existed, then data were re-analysed by a third observer from another group. The mean values between the two/three observers of the measurements were used for further statistical analysis.
NOGA-guided injection
A three-dimensional (3D) left ventricular mapping was created with the NOGA® system (Biologics Delivery Systems). Point by point measurement generates an electromechanical 3D left ventricular map. The system distinguishes between viable (unipolar voltage >12 mV, bipolar voltage >2.5 mV, local linear shortening [LLS] >6%), non-viable-myocardium (unipolar voltage <6 mV, bipolar voltage <1.5 mV, LLS <4%) and border zone (unipolar voltage 6–12 mV, bipolar voltage 1.5–2.5 mV, LLS 4–6%) around myocardial scar tissue. To ensure appropriate injection into the ventricle wall, the injection catheter tip was located perpendicular to the ventricle wall and a ventricular extrasystole was elicited when extending the needle into the wall before any injection. Approximately 15 injections of 0.3 ml CSCC_ASC CryoStor solution (total cell dose 100 × 106) or placebo were performed into the viable myocardium judged by the 3D map in the border zone of infarcted tissue using the NOGA Myostar® catheter (Biological Delivery System, Cordis, Johnson & Johnson).8-10, 24
Quality of life
Quality of life was evaluated by the Kansas City Cardiomyopathy Questionnaire (KCCQ) and EQ-5D-3L Questionnaire. The KCCQ is a self-administered questionnaire with 23 items addressing specific HF health domains: physical limitation, symptoms, quality of life, social limitation, symptom stability, and self-efficacy. The first four domains combine into a clinical summary score. Scores range from 0 to 100, and higher scores point to lower symptom burden and better quality of life. The EQ-5D-3L Questionnaire focuses on mobility, self-care, usual activities, discomfort, and anxiety. These five elements can be ranged from no, some, and extreme problems. Each element results in a score and all scores can be combined into one, which express the health status of the patient.
Endpoints
The primary endpoint was change in LVESV at 6-month follow-up measured by ECHO.25
Safety of CSCC_ASC treatment was registered as incidence and severity of SAEs and suspected unrelated SAEs at 12-month follow-up.
Secondary endpoints were changes in LVEF and LVEDV, NYHA class, KCCQ, EQ-5D-3L Questionnaire, 6-min walk test (6MWT) and NT-proBNP-levels at 6-month follow-up.
In addition, we analysed a composite endpoint of death, HF hospitalization, cardiac resynchronization therapy device implantation, ventricular tachycardia, or aborted sudden cardiac death 1, 2, and 3 years after treatment.
Power calculation and statistics
Power calculation has been described previously.25 In short, it was based on the MSC-HF trial, a placebo-controlled comparison of intramyocardial injections of autologous bone marrow-derived MSCs (randomized in a 2:1 pattern) in patients with ischaemic HFrEF.9 It was estimated that with a maximum dropout rate of 15% before the 6-month follow-up, then it would be possible to detect an absolute difference between the two groups in LVESV of 9.5 ml (estimated SD 15 ml) and 12.7 ml (estimated higher SD of 20 ml) by including 138 patients, a difference in LVEF of 3.2% (estimated SD 5%) and of 5.1% (estimated higher SD of 8%) with a statistical power of >90%. An alpha value of 5% was used in all calculations.24
Statistical analyses were performed using SPSS 25 (SPSS Inc., Chicago, IL, USA). For baseline characteristic comparison and follow-up data between and within groups, an independent sample t-test and paired t-test for normally distributed continuous data was used, respectively. Mann–Whitney U test was used for between-group comparisons of continuous non-normally distributed data. For baseline categorical data, we used Pearson's chi-square or Fisher's exact test, as appropriate. For follow-up data with more than two time-points, we used repeated measures analysis with autoregressive covariance structure. For nominal repeated data, we used generalized estimating equations. Person's chi-square or Fisher's exact test was used for comparison of occurrences of SAEs between groups. Kaplan–Meier curves using log-rank test was used to analyse the incidence of combined cardiac endpoint. A two-sided p-value <0.05 was considered statistically significant.
Results
Patients
A total of 133 patients (122 men and 11 women) with stable symptomatic chronic ischaemic HFrEF were included in the study. Demographic data are presented in Table 1. The ASC and placebo groups were comparable at baseline regarding cardiovascular risk factors, medication, and medical history except for a higher systolic blood pressure and a more frequent use of diuretics in the ASC group. Approximately two-thirds of the patients were in NYHA class II and one-third in NYHA class III in both groups. There was no significant difference between groups in LVESV, LVEDV, or LVEF. In the 6MWT, the ASC patients walked 402 ± 104 m (mean ± SD) and the placebo group 393 ± 107 m. There was no difference in blood NT-proBNP, C-reactive protein (CRP), or renal function between the groups.
| Parameter | ASC group (n = 90) | Placebo group (n = 43) | p-value |
|---|---|---|---|
| Patient profile | |||
| Age (years) | 66.4 ± 8.1 | 64.0 ± 8.8 | 0.122 |
| Male sex | 84 (93.3) | 38 (88.4) | 0.333 |
| Smoking | |||
| Current | 15 (16.7) | 5 (11.6) | 0.447 |
| Former | 60 (66.7) | 29 (67.4) | 0.930 |
| Diabetes mellitus | |||
| Type I | 1 (1.1) | 1 (2.3) | 0.544 |
| Type II | 37 (41.1) | 16 (37.2) | 0.667 |
| Stroke | 7 (7.8) | 5 (11.6) | 0.472 |
| TIA | 8 (8.9) | 5 (11.6) | 0.622 |
| PAD | 14 (15.6) | 7 (16.3) | 0.915 |
| Pulmonary disease | 18 (20.0) | 10 (23.3) | 0.667 |
| BMI (kg/m2) | 28.5 ± 4.6 | 29.9 ± 3.8 | 0.084 |
| Blood pressure | |||
| Systolic (mmHg) | 125 ± 17 | 118 ± 18 | 0.033 |
| Diastolic (mmHg) | 77 ± 11 | 74 ± 11 | 0.190 |
| Heart rate (bpm) | 68 ± 10 | 71 ± 12 | 0.089 |
| 6-min walk test (m) | 402 ± 104 | 393 ± 107 | 0.656 |
| Cardiac history | |||
| Previous MI | 69 (76.7) | 39 (90.7) | 0.053 |
| Previous PCI | 68 (75.6) | 34 (79.1) | 0.654 |
| Previous CABG | 44 (48.9) | 15 (34.9) | 0.128 |
| Hypertension | 72 (80.0) | 29 (67.4) | 0.115 |
| Hypercholesterolaemia | 81 (90.0) | 39 (90.7) | 0.113 |
| Family history of premature IHD | 36 (40.0) | 15 (34.9) | 0.538 |
| Cardiac device implant | 53 (58.9) | 27 (62.8) | 0.667 |
| Cardiac valve operation | 3 (3.3) | 4 (9.3) | 0.212 |
| Baseline endpoints | |||
| LVESV (ml) | 154.5 ± 54.8 | 150.0 ± 57.7 | 0.567 |
| LVEDV (ml) | 221.8 ± 63.0 | 214.1 ± 65.8 | 0.460 |
| LVEF (%) | 31.6 ± 7.2 | 32.0 ± 8.9 | 0.712 |
| NYHA class | 2.3 ± 0.5 | 2.3 ± 0.5 | 0.919 |
| II | 62 (68.9) | 30 (69.8) | 0.918 |
| III | 28 (31.1) | 13 (30.2) | |
| CCS class | 1.7 ± 0.6 | 1.3 ± 0.5 | 0.258 |
| I | 6 (6.7) | 4 (9.3) | 0.497 |
| II | 8 (8.9) | 2 (4.7) | |
| III | 1 (1.1) | 0 (0.0) | |
| Medication | |||
| Acetylsalicylic acid | 48 (53.3) | 29 (67.4) | 0.123 |
| Clopidogrel | 9 (10.0) | 6 (14.0) | 0.562 |
| Prasugrel | 3 (3.3) | 2 (4.7) | 0.658 |
| Ticagrelor | 7 (7.8) | 5 (11.6) | 0.523 |
| Warfarin | 35 (38.9) | 11 (25.6) | 0.131 |
| Angiotensin-converting enzyme inhibitor | 41 (45.6) | 18 (41.9) | 0.688 |
| Angiotensin II receptor blocker | 40 (44.4) | 24 (55.8) | 0.220 |
| β-blocker | 87 (96.7) | 41 (95.3) | 0.685 |
| Calcium channel blocker | 11 (12.2) | 2 (4.7) | 0.222 |
| Diuretic agent | 68 (75.6) | 25 (58.1) | 0.041 |
| Aldosterone | 53 (58.9) | 30 (69.8) | 0.226 |
| Statins | 81 (90.0) | 41 (95.3) | 0.502 |
| Non-statin cholesterol-lowering drug | 10 (11.1) | 8 (18.6) | 0.237 |
| Nitrate | 15 (16.7) | 9 (20.9) | 0.550 |
| Insulin | 12 (13.3) | 5 (11.6) | 0.783 |
| Liraglutide | 3 (3.3) | 1 (2.3) | 1.000 |
| Oral anti-diabetic | 27 (30.0) | 10 (23.3) | 0.417 |
| Biochemical profile | |||
| NT-proBNP (pg/ml) | 1495 ± 2242 | 1828 ± 2376 | 0.503 |
| Total cholesterol (mg/dl) | 133 ± 52 | 136 ± 68 | 0.833 |
| HDL-cholesterol (mg/dl) | 39 ± 20 | 47 ± 17 | 0.195 |
| Creatinine (μmol/L) | 111.3 ± 39.0 | 106.3 ± 32.9 | 0.585 |
| CRP (mg/L) | 8.7 ± 13.3 | 7.4 ± 8.5 | 0.690 |
| KCCQ | |||
| Total symptom score | 70 ± 22 | 74 ± 20 | 0.241 |
| Clinical summary score | 68 ± 19 | 65 ± 21 | 0.585 |
| Overall summary score | 64 ± 19 | 65 ± 21 | 0.671 |
| Physical limitation | 67 ± 20 | 66 ± 21 | 0.820 |
| Symptom stability | 51 ± 14 | 49 ± 8 | 0.549 |
| Symptom frequency | 67 ± 21 | 71 ± 20 | 0.292 |
| Symptom burden | 72 ± 26 | 78 ± 24 | 0.243 |
| Self-efficacy | 78 ± 20 | 78 ± 16 | 0.840 |
| Quality of life | 60 ± 23 | 60 ± 25 | 0.898 |
| Social limitation | 58 ± 24 | 59 ± 27 | 0.790 |
| EQ-5D-3L score | 59 ± 17 | 63 ± 19 | 0.221 |
- Values are mean ± standard deviation, or n (%).
- ASC, adipose tissue-derived stromal cell; BMI, body mass index; CABG, coronary artery bypass graft; CCS, Canadian Cardiovascular Society; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; IHD, ischaemic heart disease; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; MI, myocardial infarction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack.
Safety
A small pericardial effusion was detected by ECHO after the NOGA procedure in one patient, which was treated with diuretics. During the movement of the catheter in the left ventricle, one patient developed ventricular tachycardia, which was converted to sinus rhythm with direct current cardioversion and the treatment was continued. Beside this event, no procedure-related complications were seen to the direct intra-myocardial injections.
The SAEs reported within the first year are outlined in Table 2. There was no significant difference in occurrence of events between the two groups. There were three deaths due to progression of HF in the ASC group and two in the placebo group. One patient in the ASC group was diagnosed with B-cell lymphoma with large pelvic tumor 10 months after CSCC_ASC treatment, which was judged not to be related to the treatment.
| ASC group (n = 90) | Placebo group (n = 43) | p-value | |
|---|---|---|---|
| Death | 3 (3.3) | 2 (4.7) | 1.000 |
| Hospitalizations for: | |||
| Cancer | 1 (1.1) | 0 | 1.000 |
| Heart failure worsening | 14 (15.5) | 7 (16.3) | 1.000 |
| VT/VF | 6 (6.6) | 0 | 0.177 |
| MI | 4 (4.4) | 1 (2.3) | 1.000 |
| PCI or CABG | 2 (2.2) | 0 | 1.000 |
| Stroke or TIA | 1 (1.1) | 1 (2.3) | 1.000 |
| Angina worsening | 1 (1.1) | 1 (2.3) | 1.000 |
| Pneumonia | 4 (4.4) | 2 (4.7) | 1.000 |
| Syncope non-cardiac | 1 (1.1) | 0 | 1.000 |
| Cutaneous abscess | 2 (2.2) | 1 (2.3) | 1.000 |
| Pulmonary embolism | 1 (1.1) | 0 | 1.000 |
| Epididymitis | 2 (2.2) | 0 | 1.000 |
| Cholecystitis | 1 (1.1) | 0 | 1.000 |
| Colon perforation | 1 (1.1) | 0 | 1.000 |
| Incarcerated abdominal hernia | 1 (1.1) | 0 | 1.000 |
| Diabetic foot amputation | 1 (1.1) | 0 | 1.000 |
| Acute ischaemic leg | 0 | 1 (2.3) | 0.331 |
| Aortic valve operation | 1 (1.1) | 0 | 1.000 |
| Ulcus ventriculi | 0 | 1 (2.3) | 0.331 |
| Gout | 1 (1.1) | 0 | 1.000 |
| Severe headache | 0 | 1 (2.3) | 0.331 |
| Struma multinodular | 1 (1.1) | 0 | 1.000 |
| Costa fracture - bicycle accident | 1 (1.1) | 0 | 1.000 |
| Depression | 1 (1.1) | 0 | 1.000 |
| Diabetic derailment | 1 (1.1) | 0 | 1.000 |
- Values are1 n (%).
- ASC, adipose tissue-derived stromal cell; CABG, coronary artery bypass graft; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack; VF, ventricular fibrillation; VT, ventricular tachycardia.
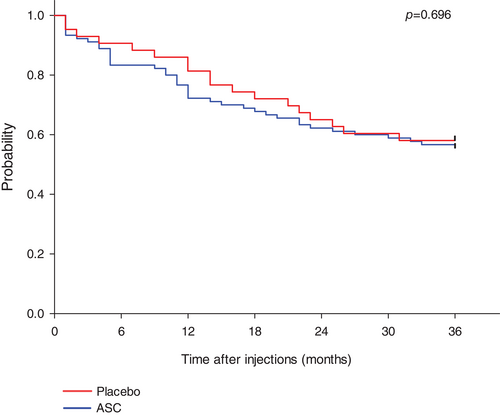
The overall mean time to occurrence of combined cardiac adverse event during the 3-year follow-up period was 26 ± 1 months in the ASC group and 27 ± 2 months in the placebo group (p = 0.696) (Figure 2).
Cardiac function
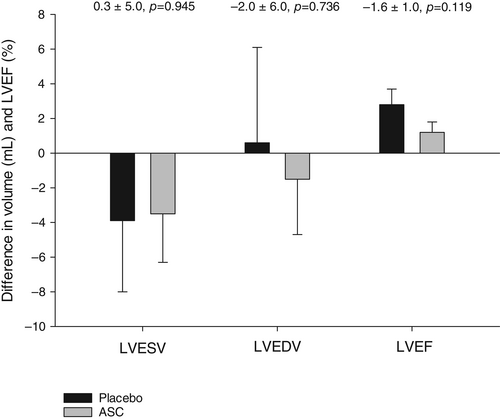
The LVESV, LVEDV and the LVEF did not change from baseline to 6-month follow-up between the two groups (Table 3). Change in the primary endpoint of LVESV from baseline to follow-up was −3.5 ± 2.8 ml (p = 0.216) in the ASC group and −3.9 ± 4.1 ml (p = 0.358) in the placebo group (Table 3). The change in LVEDV was −1.5 ± 3.2 ml (p = 0.646) in the ASC group and 0.6 ± 5.5 ml (p = 0.919) in the placebo group. There was a significant increase in LVEF from baseline to 6-month follow-up in the ASC (1.2 ± 0.6%, p = 0.044) and placebo (2.8 ± 0.9%, p = 0.003) group, respectively. There were no significant differences between groups during follow-up in LVESV (0.3 ± 5.0 ml, p = 0.945), LVEDV (−2.0 ± 6.0 ml, p = 0.736), or LVEF (−1.6 ± 1.0%, p = 0.119) (Figure 3).
| ASC group | Placebo group | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6-month FU | Change from baseline to 6-month FU | p-value | Baseline | 6-month FU | Change from baseline to 6-month FU | p-value | |
| LVESV (ml) | 154.5 ± 54.8 | 151.0 ± 55.2 | −3.5 ± 2.8 | 0.216 | 150.0 ± 57.7 | 146.2 ± 70.5 | −3.9 ± 4.1 | 0.358 |
| LVEDV (ml) | 221.8 ± 63.0 | 220.3 ± 63.6 | −1.5 ± 3.2 | 0.646 | 214.1 ± 65.8 | 214.6 ± 83.5 | 0.6 ± 5.4 | 0.919 |
| LVEF (%) | 31.6 ± 7.2 | 32.8 ± 7.5 | 1.2 ± 0.6 | 0.044 | 32.0 ± 8.9 | 34.7 ± 9.7 | 2.8 ± 0.9 | 0.003 |
- Values are mean ± standard deviation.
- FU, follow-up; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume.
Functional status and quality of life
There were no changes in 6MWT, NT-proBNP, or the inflammatory marker CRP during the first year in any of the two groups (Table 4). The NT-proBNP level was significantly higher in the placebo group, compared to the ASC group at 12-month follow-up (p = 0.034, online supplementary Figure S2). However, no differences in response between the two groups were detected for 6MWT and CRP at follow-up (online supplementary Figure S1).
| n | ASC group | p-value baseline to FU | p-value overall | n | Placebo group | p-value baseline to FU | p-value overall | p-value for between-group differences baseline to FU | |
|---|---|---|---|---|---|---|---|---|---|
| 6MWT | |||||||||
| Baseline | 90 | 419 ± 12 | 43 | 423 ± 18 | |||||
| 6 months | 90 | 422 ± 13 | 0.676 | 41 | 450 ± 20 | 0.040 | 0.089 | ||
| 12 months | 86 | 432 ± 13 | 0.092 | 0.372 | 39 | 451 ± 19 | 0.051 | 0.183 | 0.097 |
| NT-proBNP | |||||||||
| Baseline | 90 | 1907 ± 381 | 43 | 1329 ± 419 | |||||
| 6 months | 90 | 1687 ± 281 | 0.485 | 41 | 1875 ± 486 | 0.227 | 0.403 | ||
| 12 months | 86 | 1607 ± 274 | 0.759 | 0.429 | 39 | 1652 ± 595 | 0.495 | 0.079 | 0.033 |
| CRP | |||||||||
| Baseline | 90 | 5.5 ± 0.8 | 43 | 5.8 ± 1.5 | |||||
| 6 months | 90 | 5.1 ± 0.7 | 0.428 | 41 | 5.7 ± 1.4 | 0.341 | 0.952 | ||
| 12 months | 86 | 5.5 ± 0.5 | 0.438 | 0.483 | 39 | 5.0 ± 0.5 | 0.377 | 0.489 | 0.711 |
- Values are mean ± standard deviation.
- 6MWT, 6-min walk test; CRP, C-reactive protein; FU, follow-up; NT-proBNP, N-terminal pro B-type natriuretic peptide.
The NYHA class did not change in the two groups or between groups (Table 5).
| NYHA class | ASC group | Placebo | p-value for between-group differences | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6-month FU | 12-month FU | Baseline | 6-month FU | 12-month FU | Baseline to 6-month FU | Baseline to 12-month FU | |
|
I |
11 (12.2) | 11 (12.2) | 7 (16.3) | 9 (20.9) | ||||
| II | 62 (68.9) | 62 (68.9) | 59 (65.6) | 30 (69.8) | 26 (60.5) | 23 (53.5) | ||
| III | 28 (31.1) | 15 (16.7) | 16 (17.8) | 13 (30.2) | 8 (18.6) | 6 (14.0) | ||
| IV | 1 (1.1) | 1 (2.3) | 0.632 | 0.341 | ||||
- ASC, adipose tissue-derived stromal cell; FU, follow-up; NYHA, New York Heart Association.
The KCCQ quality of life evaluation demonstrated significant improvements in total symptoms, clinical summary and total summary scores in both the ASC and placebo groups within the 1-year follow-up. However, there were no differences between groups for any parameter (online supplementary Figure S2). All KCCQ parameters are outlined in online supplementary Table S1.
The EQ-5D-3L quality of life evaluation demonstrated improvement in the ASC group from 60 ± 2 to 66 ± 2 (p < 0.001) and no change in the placebo group (64 ± 3 to 66 ± 4; p < 0.09) (online supplementary Table S2). However, there were no significant differences between groups.
Discussion
We have previously demonstrated that direct intramyocardial injections of autologous bone marrow-derived MSCs in patients with ischaemic HFrEF were safe and improved cardiac function.8-10 However, to use a more standardized and quality-controlled cell product, we have established production of an allogeneic CSCC_ASC product obtained from healthy donors stored in nitrogen dry container as an off-the-shelf product ready to be used without any time delay. It has proven safety and indications of efficacy in phase I clinical trials in patients with ischaemic HFrEF, dry eyes caused by Sjogren's syndrome and radiation-induced xerostomia with reduced salvia production.24, 26, 27
The SCIENCE trial is the first European multicentre randomized phase II trial testing the safety and efficacy of direct intramyocardial injections of allogeneic CSCC_ASC product or saline in 133 patients with symptomatic chronic ischaemic HFrEF without any further treatment options. Our hypothesis was that the cell therapy would lead to improvement of left ventricular volumes and function.
The study demonstrated that it was safe to inject CSCC_ASC intramyocardially in patients with chronic ischaemic HFrEF. However, it was not possible to detect any improvement in the primary endpoint of LVESV. Moreover, we did not find any significant beneficial effect of the treatment on the secondary endpoints evaluating cardiac function, HF symptoms or quality of life (Graphical Abstract). These findings were very surprising based on our previous studies with autologous MSC and allogeneic CSCC_ASC in patients with ischaemic HFrEF.8, 10, 24 However, the present results are in line with the results from a parallel conducted single site national Danish CSCC_ASC phase II study with the same CSCC_ASC product and dose in 81 patients with chronic ischaemic HFrEF (unpublished data).
It was very surprising that the use of a more homogeneous and functional proven allogeneic cell product was without any clinical effect. It can be speculated whether an autologous mesenchymal cell product would be more effective than an allogeneic mesenchymal cell product. When changing the production from autologous to allogeneic production, we performed in vitro comparison studies, which demonstrated that the cell markers and functional tests were identical. However, the allogeneic cell product was much more homogeneous in characteristics and functional tests compared to autologous cell products from patients. Based on the accumulating evidence for the potential immunomodulatory and inflammatory regulation mode of action of ASC in different diseases, our hypothesis was probably wrong.28 The ASCs seem not to be able to restore cardiac function by inducing the creation of new cardiomyocytes by the cell's paracrine secretion.
The discrepancy with the previous study could also be due the use of magnetic resonance/computed tomography scans which were more accurate and with less variability than ECHO. With the implementation of prophylactic implantable cardioverter-defibrillator treatment in patients with HFrEF, magnetic resonance scans were no longer an option. Moreover, we were not allowed to use computed tomography scans with contrast by the competent authorities in all countries. Therefore, we decided to use ECHO and perform the analyses by two independent groups in the Netherlands and Slovenia to minimize the variation in analyses.
Allogeneic MSCs and ASCs have already been used in small clinical trials without any side effects.29, 30 The TRIDENT trial compared two doses of allogeneic MSCs, injected directly into the myocardium in 30 patients with ischaemic cardiomyopathy.30 The treatment was safe. Both cell doses (20 × 106 and 100 × 106 cells) decreased scar size, but only the 100 × 106 dose increased LVEF. In the present trial, we only used one total dose of CSCC_ASCs (100 × 106). Therefore, we can only speculate on whether another dose would have changed the results of the study.
Worldwide, there are still no approved stem cell products on the market for HF. Mesoblast Ltd has an allogeneic bone marrow-derived mesenchymal cell product, rexlemestrocel-L, being used in an event-driven study finalized approximately 2 years ago, the phase III DREAM-HF trial in 537 patients with ischaemic and non-ischaemic HFrEF in the US.31 Our findings are in accordance with some of the results announced by Mesoblast on 15 November 2020 demonstrating that the study did not reach the primary endpoint – time to recurrent non-fatal HF-related major adverse cardiac events.32
We have included chronic ischaemic HF patients with no further treatment options. The CSCC_ASC products regenerative mechanisms of action are through paracrine trophic, antifibrotic, angiogenetic, and immunomodulatory processes.18 In a pre-clinical model of ischaemic cardiomyopathy, we found that immune cell activation was the most prominent effect in the myocardium after cell therapy.33 Some patients with ischaemic HF do also have increased immunological activity in the myocardium.34 In chronic HFrEF patients with no further treatment options, the micromilieu in the myocardium may not be able to benefit from the cells delivered. Early cell treatment after presentation of HFrEF may be more effective, which the preliminary data from the DREAM-HF trial also indicate.
It can be discussed whether the cell dose in this study was high enough and whether the delivered cell product remains in the myocardium for enough time to initiate clinically meaningful improvement.35-38 Injection of combined cell and hydrogel solutions may improve efficacy.39 More than one treatment session could potentially also increase efficacy. Many of the cells will pass through the capillaries in the myocardium by time and migrate to the lungs and spleen. Therefore, the cell retention may be too short to initiate the paracrine effects. Also, the number of resident primitive cells in the chronic myocardium may be too low to be stimulated sufficiently by the delivered cells.
In conclusion, the SCIENCE trial demonstrated that the direct injection with allogeneic CSCC_ASCs into the myocardium of patients with chronic ischaemic HFrEF was safe during a 3-year follow-up period. However, it was not possible to detect any significant improvement of left ventricular volumes or function, or clinical symptoms 6 months after treatment. Also, in comparison to placebo there was no significant improvement in 6MWT, NYHA class and self-reported indices of quality of life during the follow-up. It has not been tested whether autologous ASCs would be more effective than the present used allogeneic ASCs. However, the clinical effect of autologous ASCs should be highly significant to justice the very expensive production of the inhomogeneous cell product and invasive treatment, which can be difficult to get re-imbursed. Therefore, there is a need for new potential effective treatment strategies and hypotheses to justify clinical trials in this HFrEF patient population.
Acknowledgements
Joanna Ciosek, Sebastian Dworowy, Tomasz Jadczyk, Kai Jaquet, Michal Kozłowski, Aleksandra Michalewska-Włudarczyk, Mira van der Naald, Kasper Westinga, Karin Vlaardingerbroek, Ronja Sagalski, Esther Schlegel, Annette Schmidt, Anna Sikora, Dorota Skiba, Mojdeh Lofti, Kirstine Joo Andresen, Rebekka Harary Søndergaard, Louise Frandsen, and Anne Lavigne were actively involved in the conduction of the SCIENCE trial.
Funding
This work was supported by an EU funding as part of the Horizon 2020 program to conduct this randomized multicentre clinical trial (SCIENCE grant no. 643478). The Innovation Fund Denmark grant (CSCC grant no. 6153-00002A), and Aase and Ejnar Danielsens Foundation.
Conflict of interest: A.E., M.H.S. and J.K. are inventors of a granted patent (‘STEM CELL THERAPY BASED ON ADIPOSE-DERIVED STEM CELLS’) (WO2017068140A1 EP3365432A1) owned by the Capital Region of Denmark and Rigshospitalet, Copenhagen University Hospital, Denmark. The patent is granted in Europe and Australia. Applications are submitted in Canada, China, Hong Kong, Japan, Korea, and USA. A.E., M.H.S. and J.K. are founder of Cell2Cure ApS, which has a license to commercialise the patent. All other authors have nothing to disclose.
Appendix 1: Inclusion and exclusion criteria of the SCIENCE trial
| No. | Inclusion criteria | Exclusion criteria |
|---|---|---|
| 1. | 30 to 80 years of age | Symptomatic heart failure (NYHA class I or IV) |
| 2. | Signed informed consent | Acute coronary syndrome within 6 weeks of inclusion |
| 3. | Chronic stable ischaemic heart disease | Other revascularization treatment within 4 months of treatment |
| 4. | Symptomatic NYHA class II–III | Moderate to severe aortic stenosis (valve area <1.3 cm2) or valvular heart disease with option for surgery or interventional therapy |
| 5. | LVEF ≤45% on echocardiography | Aortic valve replacement with an artificial heart valve. However, a trans-septal treatment approach can be considered in these patients |
| 6. | Plasma NT-proBNP >300 pg/ml (>35 pmol/L) | If the patient is expected to be candidate for MitraClip therapy |
| 7. | Maximal tolerated heart failure medication unchanged 2 months prior to inclusion | Diminished functional capacity for other reasons such as reduced lung function with FEV <1 L/min, moderate to severe claudication or morbid obesity |
| 8. | No option for PCI or CABG | Clinically significant anaemia (Hb <6 mmol/L), leukopenia (leucocytes <2 109/L), leukocytosis (leucocytes >14 109/L) or thrombocytopenia (thrombocytes <50 109/L) |
| 9. | Patients who have had PCI or CABG within 6 months of inclusion must have a new coronary angiography to rule out early restenosis | Reduced kidney function (eGFR <30 ml/min) |
| 10. | Patients cannot be included until 3 months after implantation of a CRT-D and until 1 month after an ICD unit | Left ventricular thrombus |
| 11. | Anticoagulation treatment that cannot be paused during cell injections | |
| 12. | Patients with reduced immune response or known anti-HLA antibodies | |
| 13. | History with malignant disease within 5 years of inclusion or suspected malignity – except treated skin cancer other than melanoma | |
| 14. | Pregnant women | |
| 15. | Other experimental treatment within 4 weeks of baseline tests | |
| 16. | Life expectancy <1 year | |
| 17. | Participation in another intervention trial | |
| 18. | Known hypersensitivity to DMSO, penicillin and streptomycin |
- CABG, coronary artery bypass graft; CRT-D, cardiac resynchronization therapy-defibrillator; DMSO, dimethyl sulfoxide; eGFR, estimated glomerular filtration rate; FVC, forced vital capacity; Hb, haemoglobin; HLA, human leucocyte antigen; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Appendix 2: Release criteria for the clinical stem cell product CSCC_ASC
| Attribute | Acceptance criteria |
|---|---|
| No. of cells | 100–120 × 106 cells |
| CSCC_ASC viability | >80% |
| Donor serology | Negative for anti-HIV 1/2 (antibody + Ag) |
| Negative for anti-HCV | |
| Negative for HBsAg | |
| Negative for anti-HBc | |
|
Negative for syphilis Negative for HTLV I/II |
|
| Sterility | |
| Bacteria/fungi | Negative/negative |
| Endotoxin level | <70 EU/ml |
| Mycoplasma | Negative |
| Characterization (immunophenotype) | CD90 >80% |
| CD105 >80% | |
| CD73 >80% | |
| CD 45 <3% | |
| HLA-DR <5% | |