Long-term outcomes following catheter ablation versus medical therapy in patients with persistent atrial fibrillation and heart failure with reduced ejection fraction
Abstract
Aims
The ARC-HF and CAMTAF trials randomized patients with persistent atrial fibrillation (AF) and heart failure (HF) to early routine catheter ablation (ER-CA) versus pharmacological rate control (RC). After trial completion, delayed selective catheter ablation (DS-CA) was performed where clinically indicated in the RC group. We hypothesized that ER-CA would result in a lower risk of cardiovascular hospitalization and death versus DS-CA in this population.
Methods and results
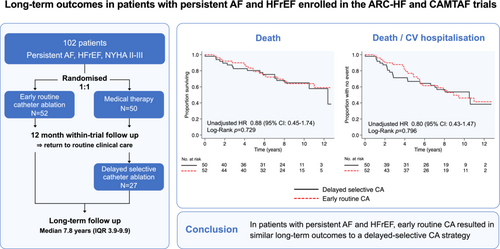
Overall, 102 patients were randomized (age 60 ± 11 years, left ventricular ejection fraction [LVEF] 31 ± 11%): 52 to ER-CA and 50 to RC. After 12 months, patients undergoing ER-CA had improved self-reported symptom scores, lower New York Heart Association class (i.e. better functional capacity), and higher LVEF compared to patients receiving RC alone. During a median follow-up of 7.8 (interquartile range 3.9–9.9) years, 27 (54%) patients in the RC group underwent DS-CA and 34 (33.3%) patients died, including 17 (32.7%) randomized to ER-CA and 17 (34.0%) randomized to RC. Compared with DS-CA, a strategy of ER-CA exhibited similar risk of all-cause mortality (adjusted hazard ratio [aHR] 0.89, 95% confidence interval [CI] 0.44–1.77, p = 0.731) and combined all-cause mortality or cardiovascular hospitalization (aHR 0.80, 95% CI 0.43–1.47, p = 0.467). However, analyses according to treatment received suggested an association between CA and improved outcomes versus RC (all-cause mortality: aHR 0.43, 95% CI 0.20–0.91, p = 0.028; all-cause mortality/cardiovascular hospitalization: aHR 0.48, 95% CI 0.24–0.94, p = 0.031).
Conclusions
In patients with persistent AF and HF, ER-CA produces similar long-term outcomes to a DS-CA strategy. The association between CA as a treatment received and improved outcomes means there is still a lack of clarity regarding the role of early CA in selected patients. Randomized trials are needed to clarify this question.
Graphical Abstract
In intention-to-treat analyses, early routine catheter ablation (CA) resulted in similar long-term outcomes to a delayed selective CA strategy in patients with persistent or long-standing persistent atrial fibrillation (AF) and symptomatic heart failure with reduced ejection fraction (HFrEF) enrolled in the ARC-HF and CAMTAF clinical trials. Medical therapy refers to pharmacological rate control. Kaplan–Meier survival curves shown for all-cause death (left) and a composite outcome of all-cause death or cardiovascular (CV) hospitalization (right). CI, confidence interval; HR, hazard ratio; IQR, interquartile range; NYHA, New York Heart Association.
Introduction
Atrial fibrillation (AF) commonly occurs in patients with heart failure (HF) and is associated with increased rates of morbidity and mortality versus either condition occurring alone.1, 2 The management of AF is challenging in patients with HF due to a higher prevalence of persistent versus paroxysmal AF, increased susceptibility to antiarrhythmic drug (AAD) side effects, and overlapping symptoms related to HF and other comorbidities. To date, several randomized controlled trials have shown that catheter ablation (CA) of AF achieves short-term improvements in quality of life, left ventricular ejection fraction (LVEF), and functional capacity in selected patients with HF with reduced ejection fraction (HFrEF).3-6 However, only one trial (Catheter Ablation vs. Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation [CASTLE-AF]) has shown a reduction in all-cause mortality with CA compared with medical therapy in HF, as part of a composite primary endpoint including HF hospitalization.7 A second trial, the Ablation versus Amiodarone for Treatment of Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted ICD/CRTD (AATAC) trial showed a similar mortality benefit but as a secondary endpoint.8 The median follow-up in CASTLE-AF was 3 years post-ablation and in AATAC, endpoints were assessed at 2 years. Whether a prognostic benefit for CA extends over a longer duration in patients with HF is unclear.
Current AF and HF clinical guidelines recommend the use of CA as second-line therapy, in clinically eligible symptomatic patients with HFrEF and AF, for whom initial attempts at pharmacological rate or rhythm control have failed to control symptoms.9, 10 The Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET 4) trial, reported a lower risk of adverse cardiovascular outcomes with early rhythm control strategies, including but not limited to CA, within 1 year of AF onset, compared with usual care, and included patients with HF (n = 798, 28.6%).11 The median duration of follow-up was 5.1 years and the majority of patients had paroxysmal AF. In theory, earlier CA may have greater efficacy for restoring sinus rhythm when the extent of atrial structural and electrical remodelling is less, and potentially require fewer repeat procedures. Earlier restoration of sinus rhythm in the clinical course of HF may also reduce or prevent adverse AF-mediated myocardial remodelling, contributing to improved HF prognosis.
We sought to define long-term outcomes following CA in patients with AF and HF who were enrolled in two similar randomized trials (A Randomized Trial to Assess Catheter Ablation Versus Rate Control in the Management of Persistent Atrial Fibrillation in Chronic Heart Failure [ARC-HF]3 and A Randomized Controlled Trial of Catheter Ablation Versus Medical Treatment of Atrial Fibrillation in Heart Failure [CAMTAF]4) comparing CA versus pharmacological rate control (RC). Both trials included patients with persistent AF and HFrEF and completed 12-month follow-up, after which participants returned to routine clinical care. After trial completion, some patients initially randomized to RC underwent CA on clinical grounds. We hypothesized that patients randomized to a strategy of early routine CA (ER-CA) would have a lower risk of long-term mortality and cardiovascular hospitalization, as compared to the usual practice of delayed selective (DS-CA). We further assessed the relationship between AF treatment strategy, cardiac rhythm and survival by evaluating outcomes according to treatment received at any point during follow-up.
Methods
Study population and design
We conducted a retrospective observational study of patients enrolled in the ARC-HF3 and CAMTAF4 clinical trials. Both trials enrolled adults (aged >18 years) with symptomatic HFrEF (New York Heart Association [NYHA] class II–IV) with a LVEF ≤35% in ARC-HF or <50% in CAMTAF, and persistent (>7 days) or long-standing persistent AF (LSPAF). Participants were enrolled between April 2009–June 2012 (ARC) or June 2005–July 2011 (CAMTAF) and randomized in a 1:1 ratio to undergo CA or continue pharmacological RC. To be enrolled, patients needed to be eligible for both treatment strategies, i.e. patients with contraindications to CA or a previous ablation procedure were excluded. Previous failure of pharmacological rhythm control was not a pre-requisite for either study. Full eligibility criteria are provided in online supplementary Table S1 and have been reported previously.3, 4 All participants provided written informed consent and both trials conformed to the ethical guidelines of the 1975 Declaration of Helsinki with prior approval by independent ethics committees at participating sites.
In the present analysis, patients were followed through to September 2020 for late outcomes, including all-cause mortality and hospitalization due to a cardiovascular cause. Additional CA procedures (or cross-over to CA for those initially randomized to RC) were performed, as clinically indicated, for persistent symptoms or poor RC,10 at the discretion of treating physicians.
Catheter ablation
Ablation strategy and peri-procedural management have been described previously.2, 3 Both centres employed a stepwise approach to CA, comprising pulmonary vein isolation in all patients with additional ablation of complex fractionated atrial electrograms and/or linear ablation as directed by the operator. The CA strategy was the same for patients randomized to CA during the trial period (ER-CA) and those undergoing DS-CA after trial completion. The assigned blanking period was 2 months in ARC-HF and 3 months in CAMTAF, during which any recurrence of atrial arrhythmia was managed using AADs and/or electrical cardioversion and was not considered to reflect procedural outcome. Use of AADs or documentation of atrial arrhythmia lasting greater 30 s was regarded as procedural failure as per clinical guidelines.10, 12 Patients with atrial arrhythmia greater than 3 months following CA were offered a repeat procedure.
Pharmacological therapy
Pharmacological therapy for HFrEF was optimized for 1–3 months before randomization including angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB), β-blockers and, as appropriate, a mineralocorticoid receptor antagonist (MRA), in line with contemporary HF recommendations.9 All patients were prescribed guideline-directed oral anticoagulation.9, 10 Pharmacological RC therapy for AF comprised β-blockers or digoxin, with a target heart rate ≤ 80 bpm at rest on 24-h ambulatory monitoring and ≤110 bpm after a 6-min walk (ARC-HF) or ≤110 bpm after moderate exertion on exercise testing (CAMTAF). Rate control criteria were mandated before enrolment in CAMTAF but could be achieved during the study in ARC-HF, if not present at baseline.
Outcomes
The primary outcome for this study was all-cause mortality. Secondary outcomes included: (i) a composite of all-cause mortality and cardiovascular hospitalization and (ii) rhythm status at last follow-up. Recurrent arrhythmia was defined as documented AF or atrial tachycardia lasting >30 s regardless of symptoms and including AAD use, following the blanking period of the last CA procedure.10 Rhythm at final follow-up is reported as sinus rhythm, paroxysmal atrial arrhythmia, or persistent atrial arrhythmia (AF or atrial tachycardia). Mortality and hospitalization outcomes were ascertained from the time of the index ablation procedure (ER-CA group) or randomization date (RC group) until the time of death or last clinic follow-up. All outcomes, including cardiovascular (unplanned/emergency) hospitalization were clinically adjudicated (NA, AT). In case of disagreement, a third adjudicator arbitrated (RZ). Planned hospitalizations, including admission for routine device interrogation in patients with a cardiac implanted electronic device (CIED), were not included. Outcome data were obtained by a combination of phone calls and examination of electronic health records (EHR). In ARC-HF, telephone follow-up was obtained for 49 of 52 patients and last clinical contact (EHR follow-up) for the remaining three patients. In the CAMTAF trial, recent clinical contact recorded in the EHR was used as the first-line follow-up approach (n = 25 of 50). For patients with no recent face-to-face clinical contact, telephone follow-up was obtained (n = 25 of 50). Among survivors, all patients were either contactable by telephone or had a recent entry in the EHR.
Statistical analysis
Patient characteristics at baseline were compared using the Student's t-test for normally distributed continuous variables, Wilcoxon rank sum test for non-normally distributed continuous variables, and chi-squared or Fisher's exact test for categorical variables.
Initial analyses were based on an intention-to-treat comparison of the effects of ER-CA (all patients initially randomized to CA) versus a DS-CA strategy, the latter comprising all patients initially randomized to pharmacological RC, some of whom underwent delayed CA. A secondary analysis was performed according to the treatment received, comparing the effect of CA performed at any time during follow-up versus continued RC alone, with CA included as a time-dependent variable. Kaplan–Meier survival curves were calculated for each group, with event times measured from the index ablation (or randomization date for the DS-CA or RC group).
Survival time to each outcome was summarized using Kaplan–Meier product limit estimates and the non-parametric log-rank tests were used for comparison of survival curves between treatment groups. Cox proportional hazards models were used to assess the risk of all-cause mortality (primary outcome) and the secondary composite outcome of all-cause mortality or first cardiovascular hospitalization between groups. Multivariable models were adjusted for age, sex, LVEF at baseline. The proportional hazard assumption was examined graphically and using formal tests, as described by Grambsch13; no major deviations from this assumption were observed. Atrial arrhythmia recurrence after CA was assessed from the post-blanking date of the last CA procedure until the last available date of clinical follow-up, with event times measured from the index ablation. Cox proportional hazards regression was performed to estimate the risk of recurrence and Kaplan–Meier survival curves were used to display freedom from arrhythmia recurrence by treatment strategy.
Analyses were performed using STATA/IC 16.1 (StataCorp, College Station, TX, USA). Analyses were two-tailed and p-values <0.05 were considered statistically significant.
Results
Study population
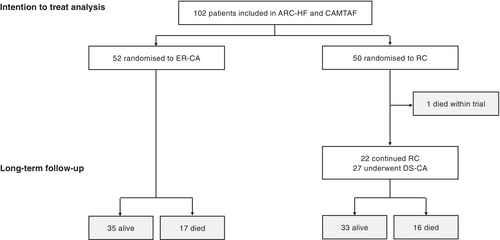
Overall, 102 patients underwent randomization: 52 patients were assigned to ER-CA and 50 patients to pharmacological RC (Figure 1). The median (interquartile range [IQR]) age at randomization was 61 (53–68) years, 91.2% participants were men. All patients were in NYHA class II or III at randomization (51.0% NYHA class III). HF aetiology was ischaemic in 38 (37.3%) patients and 6 (5.9%) patients had evidence of secondary valve disease (mild or moderate severity). The median duration of AF was 36 months in ARC-HF (69% LSPAF) and 24 months in CAMTAF (100% LSPAF) (Table 1). All patients received guideline-recommended oral anticoagulation, 99% of patients were prescribed an ACEI or ARB, 96% were prescribed a β-blocker, and 33% were taking MRAs. Thirty-six percent of patients had a history of previous (failed) AAD use before randomization and 14% patients had a CIED. Comorbidities and baseline characteristics were similar between randomized groups, with the exception of a marginally greater prescription rate for MRAs among patients randomized to CA (Table 1).
| Total (n = 102) | Early routine catheter ablation (n = 52) | Rate control (n = 50) | p-value | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, years | 61 (53,68) | 60 (51,71) | 63 (55,67) | 0.359 |
| Male sex | 93 (91.2) | 46 (88.5) | 47 (94.0) | 0.324 |
| Coronary artery disease | 38 (37.3) | 17 (32.7) | 21 (42.0) | 0.331 |
| Valvular heart diseasea | 6 (5.9) | 4 (7.7) | 2 (4.0) | 0.428 |
| Hypertension | 33 (32.4) | 16 (30.8) | 17 (34.0) | 0.727 |
| Duration of AF (months since AF onset) | ||||
| ARC (n = 52) | 36 (12,66) | 48 (18, 72) | 21 (9,60) | 0.216 |
| CAMTAF (n = 50) | 24 (15,47) | 24 (17, 33) | 24 (12, 38) | 0.991 |
| Previous DCCV | 59 (57.8) | 30 (57.7) | 29 (58.0) | 0.975 |
| Medication | ||||
| ACEI/ARB | 101 (99.0) | 51 (98.1) | 50 (100.0) | 0.324 |
| β-blocker | 98 (96.1) | 50 (96.2) | 48 (96.0) | 0.968 |
| MRA | 34 (33.3) | 21 (40.4) | 13 (26.0) | 0.123 |
| Failed AAD therapy | ||||
| ARC | 18 (34.6) | 9 (34.6) | 9 (34.6) | 1.000 |
| CAMTAF | 24 (48.0) | 14 (53.8) | 10 (41.7) | 0.392 |
| Overall | 42 (41.2) | 23 (44.2) | 19 (38.0) | 0.524 |
| CIED | 14 (13.7) | 10 (19.2) | 4 (8.0) | 0.099 |
| NYHA class | 0.846 | |||
| II | 50 (49.0) | 25 (48.1) | 25 (50.0) | |
| III | 52 (51.0) | 27 (51.9) | 25 (50.0) | |
| Baseline | ||||
| Heart rate, bpm | 80 (72, 88) | 78 (70, 88) | 80 (75, 87) | 0.212 |
| Holter mean heart rate, bpm | 83 (75,91) | 83 (75, 90) | 84 (74, 91) | 0.961 |
| Left atrial diameter, mm | 50 (44,53) | 51 (44, 54) | 47 (42,53) | 0.227 |
| Left atrial area, mm2 | 28.3 (24.0,33.0) | 27.9 (25.0,31.5) | 29.0 (23.2, 33.0) | 0.656 |
| LV ejection fraction, % | 30.0 (24.0, 37.7) | 29.9 (23.5, 33.5) | 31.9 (24.0,43.1) | 0.116 |
| MLWHFQ score, /105 | 44 (29,64) | 35 (22,58) | 49 (32, 64) | 0.142 |
| BNP, pg/ml | 233 (152,489) | 283 (175,558) | 195 (123, 444) | 0.323 |
| Peak VO2, ml/kg/min | 17.3 (13.8, 22.7) | 16.2 (12.8, 22.7) | 18.3 (14.9,22.4) | 0.269 |
| 12-month follow-up (survivors, n = 101) | ||||
| BNP, pg/ml | 135 (87, 286) | 139 (47, 243) | 135 (95, 350) | 0.084 |
| Δ BNPb, pg/ml | −59 (−223, 24) | −110 (−291, −28) | −22 (−100, 53) | 0.456 |
| LV ejection fraction, % | 34.0 (28.0,44.0) | 34.1 (30.0, 44.0) | 34.0 (27.1, 41.5) | 0.308 |
| Δ LV ejection fractionb, % | 4.0 (−4.0, +12.0) | 8.4 (0, +15) | −2.0 (−10.0, +6.0) | 0.0003 |
| MLWHFQ score, /105 | 31 (10, 53) | 16 (4,38) | 46 (27,59) | <0.001 |
| Δ MLWHFQ scoreb,/105 | −9 (−21, +3) | −12 (−28, −5) | −1 (−15, +10) | 0.0009 |
| NYHA class (12 months), n (%) | <0.001 | |||
| I | 28 (28.3) | 25 (50.0) | 3 (6.1) | |
| II | 47 (47.5) | 18 (36.0) | 29 (59.2) | |
| III | 22 (22.2) | 6 (12.0) | 16 (32.7) | |
| IV | 2 (2.0) | 1 (2.0) | 1 (2.0) | |
| Last follow-up | ||||
| AAD use | 12 (11.8) | 6 (11.5) | 6 (12.0) | 0.942 |
- Data are presented as median (interquartile range) or n (%) as appropriate.
- AAD, antiarrhythmic drug; ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; CCB, calcium channel blocker; CIED, cardiac implanted electronic device; DCCV, direct current cardioversion; LV, left ventricular; MLWHFQ, Minnesota Living With Heart Failure Questionnaire score (higher score = worse symptoms); MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
- a Secondary valve disease with mild or moderate severity (patients with primary or severe valve disease at baseline were excluded).
- b Change from baseline to 12-month follow-up.
Treatment received
One patient randomized to ER-CA declined the procedure and continued with RC. All other patients randomized to ER-CA underwent one or more procedures (online supplementary Figure S1). From 50 patients randomized to pharmacological RC, one patient crossed-over to ER-CA by request and 27 (54%) patients underwent clinically indicated DS-CA at some point during follow-up (5 with adjuvant AAD use). The median time from randomization to CA for those selected for delayed CA was 1.2 (IQR 0.9–1.5) years (Figure 1 and online supplementary Figure S1). Patients selected to undergo DS-CA after 12 months were, on average, younger (62 vs. 66 years) and had a higher LVEF (36% vs. 27%) than patients who continued with medical therapy alone (online supplementary Table S2). When all patients undergoing CA at any time were combined (ER-CA and DS-CA), baseline characteristics were similar to patients who continued medical therapy, with the exception of a statistical trend toward younger age (median age [± standard deviation, SD] 59 ± 11 vs. 64 ± 9, p = 0.053; online supplementary Table S3). In the medical therapy group, only one patient received pharmacological rhythm control after the trial period, therefore medical therapy as a treatment received in this study largely comprised RC alone.
Repeat CA procedures were performed in 47 (59.5%) patients. The mean (± SD) number of procedures performed per patient was 1.7 ± 0.7 and was similar between patients who underwent ER-CA (1.8 ± 0.7) versus DS-CA (1.6 ± 0.6, p = 0.323). Procedural complication rates, including delayed and recurrent procedures were also low. Major complications included two instances of pericardial tamponade and one peri-procedural stroke (i.e. 3/79 patients, 3.8%), minor complications included two treated haematomas, and one patient treated for post-procedure pulmonary oedema.
Twelve-month outcomes
At 12 months, patients undergoing ER-CA had, on average, lower Minnesota Living With Heart Failure Questionnaire scores (i.e. fewer self-reported symptoms and better quality of life) and a greater proportion were NYHA class I or II, suggesting better functional capacity, compared to patients receiving RC (Table 1). ER-CA was associated with an improvement in LVEF at 12 months whereas minimal change or even a subtle decline in LVEF was observed in the RC group. Median brain natriuretic peptide values were unchanged after 12 months for either group.
Long-term outcomes
Intention-to-treat analysis: early routine versus delayed selective ablation strategy
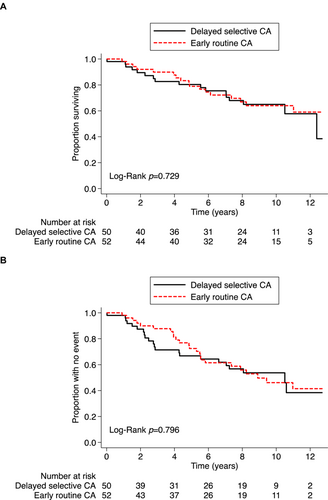
During a median (IQR) follow-up of 7.8 (3.9–9.9) years, 34 (33.3%) patients died, including 17 (32.7%) patients randomized to ER-CA and 17 (34.0%) patients randomized to RC, with or without subsequent delayed CA (p = 0.899). Kaplan–Meier survival curves were similar between ER-CA and DS-CA strategies (Figure 2A, Graphical Abstract). In an intention-to-treat analysis, there was no statistically significant association between an ER-CA versus DS-CA treatment strategy with respect to long-term all-cause mortality (Table 2).
| Outcomes | Treatment strategy | Unadjusted HR (95% CI) | p-value | Multivariatea HR (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| DS-CA (n = 50) | ER-CA (n = 52) | |||||
| Death | 17 (34.0%) | 17 (32.7%) | 0.88 (0.45–1.74) | 0.722 | 0.89 (0.44–1.77) | 0.731 |
| Death/CV hospitalization | 22 (44.0%) | 24 (46.2%) | 0.92 (0.51–1.64) | 0.768 | 0.80 (0.43–1.47) | 0.467 |
- CI, confidence interval; CV, cardiovascular; DS-CA, delayed selective catheter ablation; ER-CA, early routine catheter ablation; HR, hazard ratio.
- a Adjusted for age, sex, baseline left ventricular ejection fraction.
For the secondary combined endpoint of all-cause death or cardiovascular hospitalization, a total of 46 (45.1%) patients had an event, including 24 (46.2%) patients in the ER-CA group and 22 (44.0%) patients in the DS-CA group (Figure 2B, Graphical Abstract). HF hospitalizations comprised 53% of all cardiovascular hospitalizations. In the intention-to-treat analysis, there was no significant association between an ER-CA versus DS-CA strategy with respect to the combined endpoint, even after adjusting for age, sex, and baseline LVEF (Table 2).
One patient (1.0%) experienced a stroke during follow-up. This patient had been randomized to and underwent ER-CA.
Treatment-received analysis
During follow-up, 22 (27.9%) patients who underwent CA at any time and 12 patients (52.2%) who received RC alone died (p = 0.029). Kaplan–Meier survival curves showed poorest survival for patients who received RC alone (online supplementary Figure S2A). In a treatment-received analysis, when CA was incorporated as a time-dependent variable, CA was associated with improved survival versus RC on univariate analysis (hazard ratio [HR] 0.43, 95% confidence interval [CI] 0.20–0.89, p = 0.023) and remained significant on multivariate analysis (HR 0.43, 95% CI 0.20–0.91, p = 0.028) (Table 3).
| Outcomes | Treatment strategy | Unadjusted HR (95% CI) | p-value | Multivariatebc HR (95% CI) | p-value | |
|---|---|---|---|---|---|---|
| RC (n = 23) | CA (n = 79) | |||||
| Death | 12 (52.2%) | 22 (27.9%) | 0.43 (0.20–0.89) | 0.023 | 0.43 (0.20–0.91) | 0.028 |
| Death/CV hospitalization | 13 (56.5%) | 33 (41.8%) | 0.53 (0.28–1.02) | 0.056 | 0.48 (0.24–0.94) | 0.031 |
- CI, confidence interval; CV, cardiovascular; CA, catheter ablation (any time); HR, hazard ratio; RC, rate control.
- a It should be noted that assessment by treatment received may be highly biased for many reasons, including patient factors (e.g. those who have died cannot have the procedure) and physician factors (e.g. frail patients with a low chance of procedural success are unlikely to be selected for intervention at a later date).
- b CA considered as a time-dependent variable.
- c Adjusted for age, sex, baseline left ventricular ejection fraction.
The secondary endpoint of death or cardiovascular hospitalization occurred in 33 patients (41.8%) who underwent CA (including 25 [48.1%] randomized to ER-CA and 8 [29.6%] undergoing DS-CA) versus 13 (56.5%) patients who received RC alone (online supplementary Figure S2B). In a treatment-received analysis, incorporating CA as a time-dependent variable, CA was associated with a trend toward lower risk of the secondary endpoint versus RC (HR 0.53, 95% CI 0.28–1.02, p = 0.056), which was statistically significant after adjusting for age, sex and baseline LVEF (HR 0.48, 95% CI 0.24–0.94, p = 0.031) (Table 3).
Rhythm outcomes
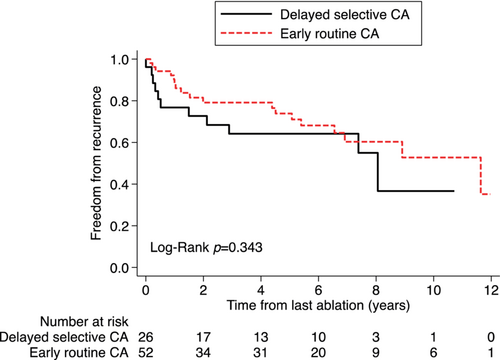
Among 79 patients who underwent CA at any time, rhythm status at long-term follow-up was available in 78 patients. Recurrent atrial arrhythmia occurred in 31 (39.7%) patients, including 19 (37.3%) patients randomized to ER-CA and 12 (44.4%) patients who underwent DS-CA (p = 0.537). Median time to first AF recurrence was numerically longer in patients who underwent ER-CA (median 2.0 years [IQR 1.0–6.6]) versus DS-CA (median 0.5 years [IQR 0.2–2.9], p = 0.149), but did not reach statistical significance. The recurrent arrhythmia was paroxysmal in five patients (all randomized to ER-CA) and persistent in the remaining patients. Six patients with persistent atrial arrhythmia additionally underwent atrioventricular node ablation and were in a paced rhythm at last contact (one randomized to ER-CA and five who underwent DS-CA). Of 47 patients remaining in sinus rhythm at long-term follow-up, 8 (17.0%) were concurrently taking AADs.
Early routine CA was not associated with a significant reduction in the risk of AF recurrence versus DS-CA (univariate HR 0.69, 95% CI 0.32–1.49, p = 0.343; Figure 3). This was also true after adjusting for age, sex, baseline LVEF (adjusted HR 0.75, 95% CI 0.34–1.64, p = 0.463).
Among 50 patients randomized to pharmacological RC, long-term rhythm status was available in 47 patients. Of these, 15 (32%) patients were in sinus rhythm, all of whom had undergone DS-CA and 4 of whom were taking adjuvant AAD therapy at final follow-up. The remaining 32 (68%) patients were in AF, including 12 patients who had undergone (failed) DS-CA and 2 patients on AAD therapy at final follow-up.
Discussion
To our knowledge, this study includes the longest reported follow-up of HFrEF patients undergoing CA for persistent or LSPAF. Over a median duration of 8 years, more than half of all patients randomized to RC eventually underwent DS-CA for clinical indications. Accepting the limitations of an observational analysis, a strategy of ER-CA was not associated with a significant reduction in long-term mortality or cardiovascular hospitalization versus DS-CA (representing usual care). However, analyses based on the treatment received demonstrated a positive association between CA and a reduction in all-cause mortality and cardiovascular hospitalization versus pharmacological RC alone. Success with CA, with respect to AF recurrence rate, was similar between patients undergoing ER-CA or DS-CA, albeit a majority of patients required repeat procedures and a proportion were prescribed AADs. Overall mortality among patients with AF and HF remains unacceptably high, with 21% 5-year mortality observed in this cohort.
Therapeutic crossover in catheter ablation trials
In the Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial, which represents the largest outcomes trial of CA versus medical therapy for AF to date, 27.5% of patients randomized to medical therapy (AADs or RC) crossed over to CA over a median of 4.0 years.14 The CABANA population included 317 (14%) patients with HF, though cross-over rates by HF phenotype were not reported. In the CASTLE-AF trial, in a selected HFrEF population, 9.8% of patients in the medical therapy group crossed over to CA within 268 ± 270 days. In the present study, also restricted to patients with HFrEF, the rate of post-randomization cross-over was low during the initial 12-month trial period, but in the post-trial setting was higher than observed in either CABANA or CASTLE-AF, with more than half of the RC arm eventually undergoing clinically indicated ablation. This likely reflects the longer duration of follow-up as well as a high burden of persistent symptoms in patients with AF and HFrEF versus the general AF population in CABANA. Although pharmacological rhythm control was infrequently used as a lone strategy after trial completion in the current analysis (only one from 50 patients initially randomized to RC), the widespread use of CA overall limits the ability to directly compare CA with a RC strategy over the extended duration of this study. It does, however, afford the opportunity to compare a strategy of ER-CA versus a delayed selective approach to CA in patients with HF, using the same intention-to-treat analysis based on initial randomization.
Delayed selective versus earlier catheter ablation strategy in heart failure
The optimal timing of CA in patients with HFrEF remains unclear. Recently, the EAST-AFNET 4 trial reported a reduction in the rate of a primary composite outcome of death, stroke or adverse events related to rhythm-control therapy with an early rhythm-control strategy (either AADs or CA within 1 year of AF onset), as compared with usual care, where rhythm control was second-line therapy.11 Although EAST-AFNET 4 included patients with HF (defined as NYHA class ≥II or LVEF <50%), the majority of patients had paroxysmal AF and preserved or only mildly reduced LVEF. The median follow-up duration was also shorter (5.1 vs. 7.8 years in this study).
In the current analysis, the DS-CA arm is similar to the usual care described in EAST-AFNET 4, while the ER-CA group arguably represents a more aggressive approach to AF management versus EAST-AFNET 4, in which early rhythm control was achieved with AADs for a majority of patients. Interestingly, we found no significant difference between an ER-CA versus DS-CA strategy with respect to rates of all-cause mortality or the composite outcome of all-cause mortality and first cardiovascular hospitalization. Our follow-up duration was longer, hence this may reflect a diminished prognostic effect of CA over time. Additionally, it is unclear whether the findings from EAST-AFNET 4 are generalizable to patients with HF with significantly reduced LVEF and persistent AF as enrolled here.
The number of CA procedures performed was similar between patients undergoing earlier versus delayed CA and AF recurrence rates broadly similar. However, time to first AF recurrence was, on average, longer following earlier versus delayed CA in our study, and although this did not reach statistical significance, may suggest improved CA efficacy when performed earlier. In previous studies, improvements in LVEF and functional capacity have predominantly been seen in patients who remained free of AF recurrence. Survival benefit has also been demonstrated in these patients previously.15 That said, CASTLE AF showed a significant survival benefit despite a moderate burden of persistent AF continuing in the ablation cohort. Therefore, it remains unclear what burden of AF is acceptable from a HF symptomatic and prognostic standpoint.
Our study did not demonstrate a clear survival benefit with ER-CA compared to DS-CA strategy, which may be reassuring to clinicians as the latter arguably best represents current practice. This is despite the marginally older age and lower LVEF of patients selected for delayed CA that may be expected to result in worse outcomes. Hence, while earlier CA may plausibly benefit selected patients with HFrEF, e.g. through its impact on symptoms and left ventricular remodelling,6 further adequately powered trials in HFrEF cohorts are needed to answer this question.
Treatment-received analyses and generalizability
Analyses based on the treatment received did not demonstrate harm from a DS-CA strategy, and conversely showed a potential long-term survival advantage associated with CA versus continued RC. This is consistent with recent clinical trials reporting reduced mortality associated with CA versus medical therapy in patients with AF and HFrEF, either as part of a composite primary (CASTLE-AF,7 CABANA14) or secondary (AATAC8) endpoint. In CASTLE-AF the mortality benefit only emerged after 3 years and in CABANA most of benefits of CA were seen in the second and third year. The current study extends post-CA follow-up over a longer duration. Importantly, however, these data should be interpreted with a high degree of caution. Although all patients were eligible for CA at the time of enrolment, patients selected for DS-CA outside of the clinical trial setting would likely have a favourable clinical risk–benefit assessment that could bias outcomes in favour of CA versus RC. Furthermore, the similarity in outcomes between ER-CA and DS-CA strategies raises questions as to the optimal timing of CA in this population and whether patient selection remains important. For example, previous studies have suggested that absence of ischaemic heart disease and ventricular scarring on cardiac magnetic resonance may predict HF response with restoration of sinus rhythm.6, 16 Randomized studies will be needed to clarify these issues.
Like CASTLE-AF, the ARC-HF and CAMTAF trials exclusively enrolled patients with HFrEF, whereas the majority of patients in CABANA had a LVEF >40%. Therefore the CABANA trial estimates of CA treatment effect largely pertain to a different part of the HF spectrum. Although the current study enrolled patients that were relatively young for a HFrEF cohort (mean age 60 years), the average LVEF and peak oxygen consumption values indicated significant HF and patients enrolled had potentially more advanced HF symptoms compared with CASTLE-AF (51% vs. 28% NYHA class III).17 In a post hoc analysis of CASTLE-AF,18 the benefit of CA varied significantly by NYHA class and was almost exclusively seen among patients in NYHA class I/II. It is important to note that CASTLE-AF enrolled a highly selected population (363 of 3013 patients screened with a CIED) and over one third of patients had paroxysmal AF, which was an exclusion criteria in ARC-HF and CAMTAF. A larger proportion of patients in the current study also had LSPAF (69% in ARC-HF and 100% in CAMTAF vs. <30% in CASTLE-AF), thus our findings may be considered more generalizable to a high-risk HFrEF cohort. Of note, the rate of stroke was extremely low in this population, presumably related to a high rate of oral anticoagulation use.
Limitations
The small cohort size has resulted in wide CIs around the estimated treatment effect for CA, as per similarly sized CA trials.6, 19 This may also reflect interindividual variation in the treatment effect of CA, and therefore our findings would benefit from validation in a larger cohort with similar long-term follow-up. Nevertheless, these long-term data of initially randomized HFrEF patients are unique in the literature, particularly in view of the early termination of the Rhythm Control-Catheter Ablation With or Without Anti-arrhythmic Drug Control of Maintaining Sinus Rhythm Versus Rate Control With Medical Therapy and/or Atrio-ventricular Junction Ablation and Pacemaker Treatment for Atrial Fibrillation (RAFT-AF) trial,20 and provide insights into late outcomes in this population. There were minor differences in study protocol between ARC-HF and CAMTAF (outlined in online supplementary Table S1); however, the main eligibility criteria and ablation techniques were consistent between recruiting sites. We cannot exclude minor variations in CA technique that may have occurred due to patient and operator factors, similar to other multicenter CA trials. We also cannot exclude the possibility of more aggressive up-titration of HF pharmacological therapy in patients who underwent CA, as may occur due to improved haemodynamic parameters and tolerance in sinus rhythm versus AF, that may have influenced outcomes. Optimal dose titration and ‘best’ medical therapy for HF was stipulated as the reason for no difference in LVEF change between CA and medical therapy in the early-terminated Atrial Fibrillation Management in Congestive Heart Failure With Ablation (AMICA) trial.19 As highlighted in the discussion, patients selected for delayed CA were likely to have a favourable clinical profile and prognosis, as compared to those continuing RC, which may have confounded outcomes in favour of CA. In the treatment-received analyses, we utilized a time-dependent modelling approach to examine the prognostic effect of CA. Updated information regarding patient characteristics at the time of the procedure was unavailable and may have influenced patient selection as well as outcomes. Finally, the lack of systematic monitoring of arrhythmia recurrence by CIEDs is an important limitation; asymptomatic AF or short durations of AF recurrence may have been missed.
Conclusions
Mortality for patients with AF and HFrEF remains high. Long-term outcomes for CA of persistent AF in this cohort remain relatively effective, accepting the need for repeat ablation and AADs in a majority of patients. A strategy of ER-CA produced similar long-term outcomes to a DS-CA strategy with respect to all-cause mortality and cardiovascular hospitalization. Notably, as with CABANA and compatible with CASTLE-AF, an as-treated analysis raised the possibility of a survival benefit among patients undergoing ablation. Randomized studies are needed to validate current selection criteria and identify which patients with HFrEF are most likely to benefit from an earlier CA approach.
Acknowledgements
We sincerely thank all of the patients and healthcare professionals who participated in both trials.
Funding
R.Z. is funded by the King's College London British Heart Foundation Center for Cardiovascular Research Excellence and a King's Prize Fellowship. The ARC-HF trial was supported by the National Institute for Health Research cardiovascular Biomedical Research Unit at the Royal Brompton & Harefield NHS Foundation Trust. The CAMTAF trial was supported by a British Heart Foundation project grant (PG/08/130): Catheter Ablation versus Medical Treatment of AF in Heart Failure (CAMTAF) trial. January 2009.
Conflict of interest: R.J.S. declares research grants and speaker fees for Medtronic, Boston Scientific, Abbott medical and is a shareholder in Rhythm AI. All other authors have nothing to disclose.