Ventricular tachycardia, premature ventricular contractions, and mortality in unselected patients with lung, colon, or pancreatic cancer: a prospective study
Abstract
Aims
Many cancer patients die due to cardiovascular disease and sudden death, but data on ventricular arrhythmia prevalence and prognostic importance are not known.
Methods and results
Between 2005 and 2010, we prospectively enrolled 120 unselected patients with lung, colon, or pancreatic cancer due to one of three diagnoses: colorectal (n = 33), pancreatic (n = 54), or non-small cell lung cancer (n = 33). All were free of manifest cardiovascular disease. They were compared to 43 healthy controls similar in age and sex distribution. Each participant underwent 24 h electrocardiogram recording and cancer patients were followed for up to 12.5 years for survival (median 21 months). Ninety-six cancer patients (80%) died during follow-up [5-year survival: 27% (95% confidence interval 19–35%)]. Non-sustained ventricular tachycardia (NSVT) was more frequent in cancer patients vs. controls (8% vs. 0%, P = 0.021). The number of premature ventricular contractions (PVCs) over 24 h was not increased in cancer patients vs. controls (median 4 vs. 9, P = 0.2). In multivariable analysis, NSVT [hazard ratio (HR) 2.44, P = 0.047] and PVCs (per 100, HR 1.021, P = 0.047) were both significant predictors of mortality, independent of other univariable mortality predictors including tumour stage, cancer type, potassium concentration, prior surgery, prior cardiotoxic chemotherapy, and haemoglobin. In patients with colorectal and pancreatic cancer, ≥50 PVCs/24 h predicted mortality (HR 2.30, P = 0.0024), and was identified in 18% and 26% of patients, respectively.
Conclusions
Non-sustained ventricular tachycardia is more frequent in unselected patients with colorectal, pancreatic, and non-small cell lung cancer and together with PVCs predict long-term mortality. This raises the prospect of cardiovascular mortality being a target for future treatment interventions in selected cancers.
Graphical Abstract
Introduction
It is well known that advanced cancer patients often present with signs and symptoms resembling those of heart failure patients like shortness of breath, congestion, and sudden death.1 Retrospective cohort studies have suggested that 20–30% of cancer patients die from cardiovascular causes,2, 3 independently of the severity of the cancer and the survival time after the cancer diagnosis.4 However, prospective studies are rare.5 Newer analysis suggests that cardiovascular illness is the second most common cause of death in cancer.6 Cardiotoxicity, depending on cancer type and related anti-tumour therapy, occurs in up to 48% of cancer patients and can lead to heart failure and fulminant myocarditis.7 Identifying patients at risk of cardiotoxicity before, during, and after treatment is vital for implementing precautionary measures. Cardiovascular biomarkers such as troponin8 can help to identify patients at risk of, or who have developed cardiotoxicity. Other markers such as an elevated heart rate have been shown to be associated with worse survival in cancer patients with advanced disease, independent of chemotherapy regimens and other confounding factors.9 Oncometabolites, such as D-2-hydroxyglutarate, can induce contractile dysfunction in the heart.10
We hypothesized that metabolic changes in patients with cancer, caused by the combined effects of a catabolic state and anti-cancer therapies, may result in significant cardiac arrhythmias. There have been no studies prospectively examining the prevalence and prognostic significance of ventricular arrhythmias during 24 h electrocardiograms (ECGs) in cancer patients. In this study, we sought to prospectively explore the presence and prognostic impact of non-sustained ventricular tachycardia (NSVT) and premature ventricular contractions (PVCs) in unselected patients with cancer free of manifest cardiovascular disease.
Methods
Patient recruitment
From 2005–2010, we recruited 169 cancer patients with histologically confirmed diagnoses of pancreatic (PaC, n = 91), colorectal (CRC, n = 37), or non-small cell lung cancer (NSCLC, n = 41) at the Charité Medical School.8 Of these, a total of 120 patients agreed to undergo 24 h ECG and were included in this analysis. Also 43 healthy subjects of similar age and sex – recruited concomitantly – were included in this study.
A detailed medical history and anamnesis was assessed in every patient. Exclusion criteria included: (i) age <18 years; (ii) clinical signs of ongoing infection, fever, or treatment with antibiotics; (iii) inflammatory disease; (iv) another cancer diagnosis within 5 years; (v) predicted life expectancy <2 months; (vi) chronic obstructive pulmonary disease with forced expiratory volume in the first second <50%; (vii) clinical signs or symptoms of chronic heart failure; (viii) prior myocardial infarction, (ix) prior treatment-related cardiotoxicity; (x) evidence of ischaemic heart disease; (xi) other known significant cardiovascular diseases, except uncomplicated hypertension or type 2 diabetes mellitus; (xii) chemo-, immuno-, radio-, targeted therapy within 21 days before inclusion; (xiii) major surgery within the last 3 months; and (xiv) an Eastern Cooperative Oncology Group (ECOG) performance status of >2. All cancer patients had been on stable medical regimens for at least 4 weeks prior to enrolment. All chemotherapy regimens were unchanged and administered as planned.
Age-similar controls were included with a ratio of patients to controls of three to one. All were generally healthy and free of known significant cardiovascular diseases, except uncomplicated hypertension or type 2 diabetes mellitus. We performed a complete blood count and routine clinical biochemistry in all patients, according to the standard laboratory operating procedures. All cancer patients and control subjects underwent a 24 h ECG and physical examination. Follow-up of the patients was conducted by monitoring the electronic database of the Charité and telephone contact with patients, relatives and home physicians. If the patients died in the Charité and an autopsy was conducted, we recorded the cause of death from the autopsy protocol. If the patients died in the Charité, but no autopsy was conducted, two independent reviewers not involved in the clinical care of the patient (M.S.A., S.v.H.) assessed the specific cause of death for each patient by reviewing the medical records. If they disagreed, a third reviewer (S.D.A.) made the final decision. Controls were allowed to receive anti-hypertensive and anti-diabetic medication as clinically indicated. The study was approved by the Charité ethics committee. Written informed consent was obtained from all subjects, and the study complies with the Declaration of Helsinki.
24 h electrocardiograms
All patients had a standard 24 h ECG. They were analysed with the help of MTM CardioScan software (MTM multitechmed GmbH, Hünfelden-Dauborn, Germany) and verified by two independent medical reviewers. We recorded average 24 h heart rate in beats per minute (bpm), total number of PVCs and premature atrial contractions, presence of NSVT, standard deviation of all normal sinus RR intervals over 24 h in milliseconds (ms), and heart rhythm. NSVT was defined according to the WHO/ISFC and EHRA/HRS/APHRS11, 12 as ≥3 consecutive heart beats originating from the ventricular chamber exceeding a heart rate of 100 bpm.
Statistical analyses
Results are presented as mean ± standard deviation, or median with interquartile range. To assess normal distribution Kolmogrov–Smirnov test was used. Unpaired Student's t-test, Mann–Whitney U test, analysis of variance, and Fisher's post hoc test were used as appropriate. For the analysis of the contingency tables, we preferably used Chi-squared tests. If the contingency tables contained more than two columns or more than two rows and at least one cell assignment was smaller than five, the Fisher's exact test was chosen.13 The Barnard's test was used for 2 × 2 tables with a cell assignment smaller than five.14, 15 Univariable and multivariable Cox-proportional hazard survival analyses (log-rank test) hazard ratios (HRs) are given with 95% confidence intervals (CI) for risk factors. All univariable significant predictors of death, which all represented clinical relevant variables, as well as age, were simultaneously entered into the multivariable Cox survival analysis for constructing the model as recommended by Judd and McClelland.16 For illustrative purposes, Kaplan–Meier survival curves were constructed. Since this study was not designed as a confirmatory study, but to identify patterns, it was not necessary to make an adjustment for tests for multiplicity. The significance tests used therefore have a descriptive character.17, 18 Statistical analyses were conducted using the ‘Stat View 5.0 software’ (SAS Institute Inc., Cary, NC, USA), ‘SAS 9.4’ and ‘R 3.5.1’. In all analyses we considered a P-value <0.05 as statistically significant.
Results
Study population
Cancer patients and controls (all Caucasians) had similar age and sex [60 ± 10 years vs. 61 ± 11 years; P = 0.67; men: 65 (54%) vs. 24 (56%); P = 0.85]. Baseline characteristics are shown in Table 1 and baseline medication in online supplementary Table S1. In 106 cancer patients, left ventricular ejection fraction (LVEF) was assessed: mean 60 ± 8%, with no one with an LVEF <35%. In eight of the nine patients with a NSVT during 24 h ECG recording, LVEF was assessed by echocardiography and none of these patients had an LVEF <50% (patients with vs. without NSVT, average LVEF 58 ± 5% vs. 60 ± 8%, P = 0.46). Patients were followed until August 2018 for a median of 21 months [for survivors a minimum of 7 years and a maximum >12.5 (i.e. 154 months)]. Ninety-four patients (78%) had advanced cancer stage (III/IV). A total of 96 patients (80%) died during follow-up: 1-year mortality 31% (95% CI 23–39%), 3-year mortality 68% (60–76%), and 5-year mortality 73% (65–81%). Thirty-three patients (34%) died in the Charité. Of these, five patients had an autopsy in which the exact cause of death was determined by a pathologist. In the other 28 patients a cause of death could be adjudicated. The cause of death of these 33 patients were: 15 (45%) cancer progression, 11 (33%) sepsis/pneumonia/infection, 4 (12%) gastrointestinal bleeding, 3 (9%) cardiovascular (1x pulseless electrical activity due to cardiac decompensation, 2× sudden cardiac death – one of the patients that died due to sudden cardiac death had a NSVT during our 24 h ECG analysis). At time of death, 24 patients (73%) had diagnosed cachexia. With regard to all 96 deceased patients, 63 (66%) died at home or in a hospice and therefore adjudicating a cause of death was not possible. Information about all-cause mortality was available for all patients and hence used for further analyses.
| Variable | Controls (n = 43) | Cancer patients (n = 120) | P-value | Cancer patient survivors at 5 years (n = 32) | Cancer patients with fatal event at 5 years (n = 88) | P-value |
|---|---|---|---|---|---|---|
| Clinical characteristics | ||||||
| Age, years | 61 ± 11 | 60 ± 10 | 0.67 | 61 ± 10 | 59 ± 10 | 0.40 |
| Male sex, n (%) | 24 (56) | 65 (54) | 0.85 | 14 (44) | 51 (58) | 0.17 |
| BMI, kg/m2 | 26.1 ± 3.8 | 24.5 ± 4.7 | 0.041 | 26.0 ± 5.1 | 23.9 ± 4.3 | 0.032 |
| Systolic BP, mmHg | 133 ± 19 | 124 ± 19 | 0.0089 | 125 ± 17 | 124 ± 19 | 0.93 |
| Diastolic BP, mmHg | 80 ± 11 | 74 ± 10 | 0.0014 | 74 ± 10 | 75 ± 10 | 0.95 |
| LVEF, % | 63 ± 6 | 60 ± 8 | 0.059 | 59 ± 7 | 60 ± 8 | 0.70 |
| UICC 3 or 4, n (%) | – | 94 (78) | – | 14 (44) | 51 (58) | 0.17 |
| Laboratory parameters | ||||||
| Sodium, mmol/L | 141 ± 2 | 140 ± 4 | 0.062 | 139 ± 3 | 140 ± 4 | 0.53 |
| Potassium, mmol/L | 4.3 ± 0.4 | 4.2 ± 0.5 | 0.21 | 4.3 ± 0.3 | 4.1 ± 0.5 | 0.083 |
| Creatinine, mg/dL | 0.90 ± 0.18 | 0.82 ± 0.25 | 0.075 | 0.88 ± 0.31 | 0.80 ± 0.23 | 0.12 |
| GGT, U/L, median (IQR) | 23 (15–30) | 57 (31–113) | <0.0001 | 36 (27–74) | 62 (36–143) | 0.0078 |
| Haemoglobin, g/dL | 14.0 ± 1.6 | 11.6 ± 1.6 | <0.0001 | 12.2 ± 1.7 | 11.4 ± 1.5 | 0.016 |
| Leucocytes, G/L | 6.5 ± 1.5 | 5.8 ± 2.6 | 0.080 | 6.8 ± 2.6 | 5.4 ± 2.5 | 0.0090 |
| Platelets, G/L | 236 ± 65 | 261 ± 138 | 0.26 | 284 ± 179 | 252 ± 120 | 0.26 |
| 24 h ECG | ||||||
| Sinus rhythm, n (%) | 43 (100) | 119 (99) | 0.59 | 32 (100) | 87 (99) | 0.59 |
| Average 24 h heart rate, bpm | 71 ± 9 | 80 ± 12 | <0.0001 | 76 ± 13 | 82 ± 11 | 0.022 |
| Premature atrial contractions, n, median (IQR) | 62 (16–256) | 21 (5–75) | 0.0019 | 24 (8–96) | 19 (5–71) | 0.60 |
| PVCs, n, median (IQR) | 9 (2–49) | 4 (1–37) | 0.22 | 3 (1–31) | 4 (1–39) | 0.88 |
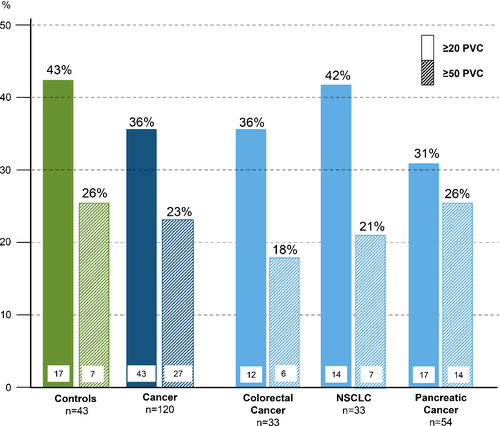
| ≥1 PVCs/24 h, n (%) | 36 (84) | 96 (80) | 0.59 | 26 (81) | 70 (80) | 0.84 |
| ≥50 PVCs/24 h, n (%) | 11 (26) | 27 (23) | 0.68 | 6 (19) | 21 (24) | 0.55 |
| ≥1000 PVCs/24 h, n (%) | 4 (9) | 5 (4) | 0.39 | 1 (3) | 4 (5) | 0.76 |
| NSVT, n (%) | 0 | 9 (8) | 0.021 | 0 | 9 (10) | 0.019 |
| SDNN 24 h, ms | 134 ± 35 | 105 ± 34 | <0.0001 | 114 ± 31 | 102 ± 35 | 0.11 |
- Data are counts (%), mean ± standard deviation, or median (IQR).
- BMI, body mass index; BP, blood pressure; ECG, electrocardiogram; GGT, gamma-glutamyl transferase; IQR, interquartile range; LVEF, left ventricular ejection fraction; NSVT, non-sustained ventricular tachycardia; PVC, premature ventricular contraction; SDNN 24 h, standard deviation of all normal sinus RR intervals over 24 h; UICC, Union Internationale Contre le Cancer tumour stage.
Seventy-nine patients (66%) had an operation due to the underlying cancer disease >3 months prior to enrolment, 89 patients (74%) had received chemotherapy prior to enrolment, and 38 patients (32%) had previously received potentially cardiotoxic chemotherapies. No significant age and sex distribution differences were noted between groups (online supplementary Table S2). Body mass index, haemoglobin and blood pressure were lower in cancer patients. There was no difference in the frequency of use of anti-diabetic medication, beta-blockers, or angiotensin-converting enzyme inhibitor/angiotensin II receptor antagonist (all P > 0.1) between controls and the overall cancer cohort. Within the cancer cohort, subgroup analysis showed that NSCLC patients were more often treated with beta-blockers [39% vs. 9% in CRC (P = 0.0043) and vs. 11% in PaC (P = 0.0068); online supplementary Table S3).
24 h electrocardiograms
In 24 h ECG analyses, we found that all but one cancer patient were in sinus rhythm. One NSCLC patient had normo-frequent atrial fibrillation. Cancer patients vs. controls more frequently showed NSVT [9 (8%) vs. 0, P = 0.021; Figure 1], had a higher mean 24 h heart rate and reduced heart rate variability (Table 1), whereas controls showed more premature atrial contractions. In eight of the nine cancer patients with detection of NSVTs, an echocardiogram was performed (all LVEF >50%). Some patients had more than one episode of NSVT. The total number of NSVT episodes in these patients were the following: PaC 43, 35, 7, 6, 1, and 1; NSCLC 1 and 1; CRC 1. There was no association between occurrence of NSVT and the Union Internationale Contre le Cancer (UICC) tumour stage [UICC stage 1: 0% (0/14 patients) NSVT, UICC stage 2: 8% (1/12 patients) NSVT, stage 3: 6% (2/36 patients) NSVT, UICC stage 4: 10% (6/58 patients) NSVT; P = 0.76]. The number of PVCs was not significantly different between cancer patients and controls (Figure 2).
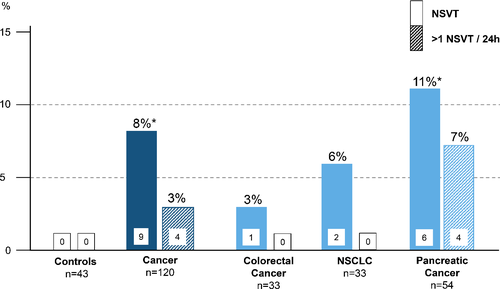
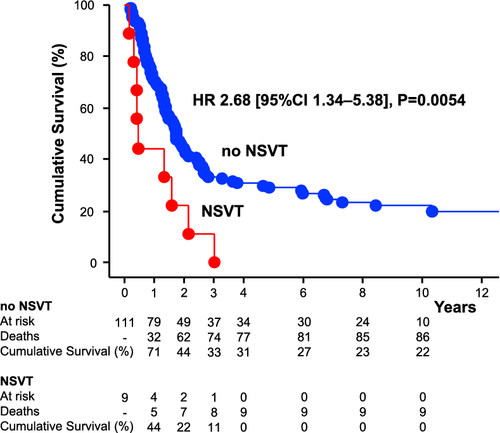
Survival analyses
With respect to baseline characteristics, we compared patients with a fatal event during the first 5 years with cancer patient survivors (Table 1). Patients with fatal events had lower baseline body mass index, haemoglobin, and leucocytes, and higher gamma-glutamyl transferase. Patients with fatal events demonstrated higher average 24 h heart rates and more frequently had NSVTs [9 (10%) vs. 0, P = 0.019).
Subgroup analyses showed that patients with NSVTs in their 24 h ECG were older, had higher creatinine, more atrial premature beats, and PVCs (online supplementary Table S4).
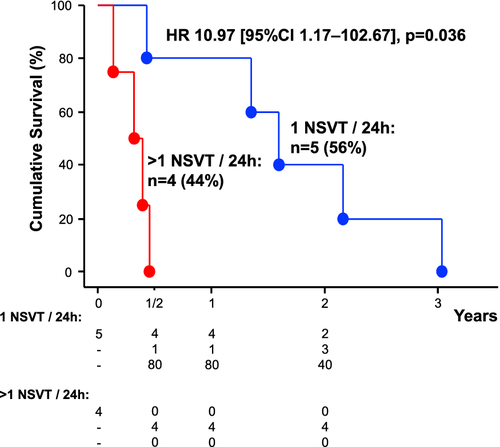
All patients with NSVTs in 24 h ECG had died within 37 months. In univariable and multivariable Cox-proportional hazard analyses, including all univariable and clinical relevant predictors of mortality as well as age, the presence of NSVT and the number of PVCs/24 h were associated with higher mortality (Table 2). When we included LVEF (n = 106), NSVT and PVCs/24 h remained significant predictors of mortality. The number of premature atrial contractions/24 h and LVEF (in %) in cancer patients were no significant predictors of mortality (P = 0.8 and P = 0.9, respectively). The Kaplan-Maier curve emphasizes the underlying survival advantage of patients without presence of NSVT (Graphical Abstract). The subgroup of cancer patients with >1 NSVT in 24 h had worse prognosis than patients with only one NSVT episode [HR 10.97 (95% CI 1.17–102.67), P = 0.036; Figure 3].
| Variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age (per year) | 0.99 | 0.97–1.01 | 0.32 | 0.99 | 0.97–1.02 | 0.56 |
| Sex (male vs. female) | 1.61 | 1.07–2.42 | 0.022 | 1.28 | 0.82–2.00 | 0.28 |
| Average 24 h heart rate (per 1 bpm) | 1.023 | 1.007–1.039 | 0.0049 | 1.027 | 1.005–1.049 | 0.016 |
| PVCs/24 h (per 100) | 1.036 | 1.016–1.056 | 0.0005 | 1.021 | 1.0003–1.042 | 0.047 |
| NSVT (yes vs. no) | 2.68 | 1.34–5.38 | 0.0054 | 2.44 | 1.01–5.87 | 0.047 |
| SDNN 24 h (per 10 ms) | 0.93 | 0.87–0.99 | 0.021 | 1.005 | 0.997–1.013 | 0.20 |
| BMI (per 1 kg/m2) | 0.94 | 0.90–0.99 | 0.013 | 1.00 | 0.95–1.05 | 0.92 |
| Diagnosis (CRC vs. NSCLC vs. PaC) | –a | –a | <0.0001 | –b | –b | 0.0074 |
| Tumour stage (UICC 4 vs. 1–3) | 2.06 | 1.37–3.10 | 0.0005 | 1.22 | 0.73–2.04 | 0.45 |
| Surgery (no vs. yes) | 1.75 | 1.16–2.65 | 0.0080 | 1.44 | 0.88–2.37 | 0.15 |
| Haemoglobin (per 1 g/dL) | 0.81 | 0.71–0.93 | 0.0020 | 0.89 | 0.76–1.04 | 0.15 |
| Potassium (per 1 mmol/L) | 0.55 | 0.32–0.94 | 0.029 | 0.58 | 0.33–1.05 | 0.072 |
| Prior cardiotoxic chemotherapy (yes vs. no) | 0.62 | 0.39–0.97 | 0.038 | 0.72 | 0.43–1.20 | 0.20 |
| Anticoagulation (yes vs. no) | 2.03 | 1.18–3.51 | 0.011 | 1.34 | 0.72–2.50 | 0.36 |
- BMI, body mass index; CI, confidence interval; CRC, colorectal cancer; HR, hazard ratio; NSCLC, non-small cell lung cancer; NSVT, non-sustained ventricular tachycardia; PaC, pancreatic cancer; PVC, premature ventricular contraction; SDNN 24 h, standard deviation of all normal sinus RR intervals over 24 h; UICC, Union Internationale Contre le Cancer.
- a Univariable subgroup differences: PaC vs. CRC: HR 3.29 (95% CI 1.91–5.69), P = <0.0001; PaC vs. NSCLC: HR 1.71 (95% CI 1.07–2.75), P = 0.025; CRC vs. NSCLC: HR 0.52 (95% CI 0.29–0.95), P = 0.032.
- b Multivariable adjusted subgroup differences: PaC vs. CRC: HR 2.63 (95% CI 1.33–5.18), P = 0.0052; PaC vs. NSCLC: HR 1.83 (95% CI 1.06–3.15), P = 0.030; CRC vs. NSCLC: HR 0.69 (95% CI 0.33–1.45), P = 0.33.
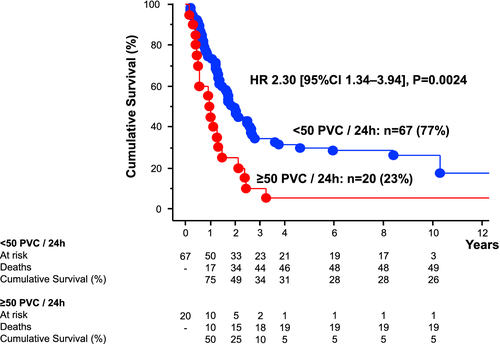
Subgroup analyses revealed that PVCs/24 h were a significant predictor of mortality in patients with CRC [HR per 100 PVCs: 1.13 (1.02–1.25), P = 0.024], and PaC [HR per 100 PVCs: 1.03 (1.01–1.05), P = 0.0036], but not in patients with NSCLC (P > 0.1). Therefore, we analysed the best predictive cut-off value for PVCs to predict survival only in patients with PaC and CRC and found it to be at ≥50 PVCs/24 h [HR 2.30 (1.34–3.94), P = 0.0024; Figure 4] – 26% in PaC and 18% in CRC had ≥50 PVCs/24 h.
Discussion
This is the first study to assess the frequency of ventricular arrhythmias during 24 h ECGs in cancer patients and examine their long-term prognostic value. In unselected patients with cancer but without significant cardiovascular disease, the frequency of NSVT on 24 h ECG recordings compared to healthy controls was increased, while PVCs were not more frequent. However, both the number of NSVT and PVCs carried significant prognostic value in these cancer patients, independent of other univariable predictors of mortality such as tumour stage, diagnosis, potassium, prior surgery, and haemoglobin. In 106 cancer patients where LVEF assessment was available, the prognostic value of NSVT and PVCs was also independent of cardiac function of patients. The subgroup of patients with >1 NSVT had an even higher risk of mortality, but due to the small size of this group (n = 4) we consider this analysis as only hypothesis generating.
We focused on investigating unselected patients with cancer due to one of three histologically confirmed diagnoses, i.e. CRC and PaC as well as NSCLC. All these cancers are associated with very high mortality burden. We cannot know what the frequency of NSVT will be in other cancers in general, and what it is in patients exposed to significant cardiotoxic chemo- and radiation therapy. The fact that we only found one case of atrial fibrillation in our study lends credence to the unselected nature of this cancer population. In contrast, in studies focusing on cancer patients with risk factors of atrial fibrillation including use of cardiotoxic anti-cancer therapy, frequencies of atrial fibrillation prevalence of 4–5%19-21 are reported. In a retrospective investigation, focusing on 261 cancer patients who underwent 24 h ECGs based on recommendations of local cardiologist, the frequency of NSVT with ≥3 beats was found to be 17% (own unpublished observation). For this reason, we believe that the research into the general burden of arrhythmia in cancer requires a prospective and unbiased approach regarding patient selection for inclusion. Pre-selection of patients with higher cardiovascular risk for such analyses – as would be the case for instance in retrospective analyses of 24 h ECGs that were performed in cancer patients for clinical reasons – cannot yield reliable prevalence information for the true general burden of arrhythmia in cancer patients.
We consider a frequency of observed NSVT in 24 h ECG of 8% as surprisingly high. No prior report exists on such data, so comparisons are not possible. Only longer assessments of ECG (over 1–2 weeks or longer) can show whether the frequency of NSVT in cancer patients is even higher than that. Such investigations are ongoing.
The mortality burden represented by ventricular arrhythmias in these cancer patients was also unexpectedly high. The results were independent of administered cardiotoxic chemotherapy. In subgroup analysis, we found PVCs to be predictive of survival only in CRC and PaC and not in NSCLC, but noted that NSCLC patients were more frequently treated with beta-blockers. This treatment is well known to inhibit PVCs22 and therefore could have reduced the number of PVCs in NSCLC patients. The results require independent and prospective validation in larger cohorts and with longer duration of ECG recordings.
We have shown before that even chemotherapy-naïve cancer patients with advanced disease demonstrate mildly (but significantly) reduced LVEF and reduced heart rate variability compared to healthy controls.23 This may represent a cancer-associated cardiomyopathy that may predispose both to the Holter abnormalities described and their ability to predict subsequent mortality. Many different factors can act as cardiac stressors. For example, during cardiac contraction in cancer patients, a higher maximal rate of pressure rise and cardiac output has been described, possibly adding additional stress to the myocardium.24 Combined with other cardiac stressors including chemo-, radiation-, immuno-, and targeted therapies,7 the metabolic and immune changes associated with cachexia,25 and other metabolic abnormalities such as hyperkalaemia26 may cause these pathophysiologic changes to become lethal problems. We also hypothesize that the combination of these factors cause significant oxidative stress and tissue inflammation, leading to homeostasis changes in the heart with cardiac wasting, apoptosis, fibrosis and sympathetic nervous system activation, ultimately contributing to arrhythmia development in these patients. This could then lead to an excess mortality.27
In pre-clinical studies focusing on cardiac wasting in cancer, several homeostasis changes have been identified. In cancer mouse models, cardiac wasting was associated with impaired cardiomyocyte ultrastructure, fibrosis, reduced cardiac myocyte size, and fewer sarcomeric proteins.28, 29 Furthermore, autophagy was increased in the cardiac muscle wasting – whereas in skeletal muscle wasting the ubiquitin-proteasome system was increased.27 In a lung cancer mouse model examining the axons of the left ventricle, animals with cardiac wasting had 50% less dense core vesicles and 50% shorter axon.30 In animal models, several biomarkers have been found to be associated with cardiac wasting such as inflammatory cytokines, nuclear factor-κB, and transforming growth factor-β proteins.31 In unison, in a liver cancer rat model, animals with cardiac wasting showed fibrotic remodelling of the heart muscle with increased mortality, whereas bisoprolol and spironolactone both reduced cardiac wasting and improved survival.32
Whether the presence of cardiac wasting is prognostic in cancer patients and whether it can constitute a valid therapeutic target in itself for these patients, we do not know, but believe it could be the case in either case. A study by Barkhudaryan et al.33 analysing autopsy reports of cancer patients found that cancer cachexia in humans was also associated with cardiac wasting. This raises the possibility of cardiac-oriented therapies being effective in reducing the risk of cardiovascular mortality in selected cancer patients. Views on this amongst patients and caregivers may vary greatly. Patients – if asked – might choose longevity over symptoms, or not. Research into this will require an inclusive, careful and open-minded but mindful approach.
Acknowledgements
We thank the patients and staff who were involved in this study. Open access funding enabled and organized by Projekt DEAL.
Conflict of interest: M.S.A. has received personal fees from Servier, outside the submitted work. S.v.H. has been a paid consultant for Bayer, Boehringer Ingelheim, BRAHMS/Thermo Fisher, Chugai, Novartis, Roche, and Vifor Pharma, and research support from Boehringer Ingelheim and BRAHMS/Thermo Fisher. A.J.S.C. has received personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia. J.B. has received research support from the NIH, PCORI, and the European Union; he serves as a consultant for Abbott, Adrenomed, Amgen, Array, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, CVRx, Innolife, Janssen, LinaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sanofi, V-Wave Limited, and Vifor. U.L. reports receiving consulting and/or lecture fees from Amgen, Medicines Company, Bayer, Sanofi, Biotronic, and Abbott. S.D.A. reports grants from Vifor Int and Abbott, and personal fees from Vifor Int, Bayer, Boehringer Ingelheim, Novartis, Servier, Abbott, Actimed, Cardiac Dimensions, Impulse Dynamics. S.D.A. holds a patent on the use of ICDs in cancer patients for the US only (submitted 2006, US8483824B2). This is not associated with any commercial development. All other authors have nothing to disclose.