Delayed cardiac consequences unveiled by magnetic resonance imaging in a high-voltage electric shock survivor
Introduction
Electrocution is a significant danger in the construction industry. Despite a decline in the number of accidents, high-voltage power remains a significant cause of occupational fatalities for workers. High-voltage electric shocks (≥1000 V) pose significant risks, including skin burns and severe internal injuries, which can be life-threatening.1 The first predictor of injury is caused by a direct high current passing through the victim's body.2 The skin has a wide range of resistance to electricity and acts as a barrier against the burns of other organs when the body is exposed to electricity. When the chest is in the path of an electrical current, the heart is usually involved.1 The two major cardiac complications of electrical shocks are arrhythmias and myocardial tissue injuries.2
Arrhythmias, such as sinus tachycardia, premature ventricular contractions, atrial fibrillation, and ventricular tachycardia, can appear immediately following the incident or at a later time.3
Myocardial tissue damage, on the other hand, can be caused by direct current effects, coronary spasms, or thrombosis and is detectable by imaging techniques such as cardiac MRI. This case report discusses the progressive dysfunction of the left (LV) and right ventricles (RV) following such an electrical injury.
Case presentation
A 44 year-old construction worker experienced a high-voltage electric shock (20 000 V) for 4 s while on a scaffold. During the incident, the patient remained conscious but experienced heart palpitations and tingling sensations without any signs of shortness of breath or chest pain. He sustained burns on his left hand and buttock, attributed to an electric current while working on a scaffold. The patient had a clean medical history with no prior conditions such as diabetes, hypertension, hyperthyroidism or hyperlipidemic and denied any use of illicit substances. He led a physically active lifestyle, engaging in aerobic exercise three times a week.
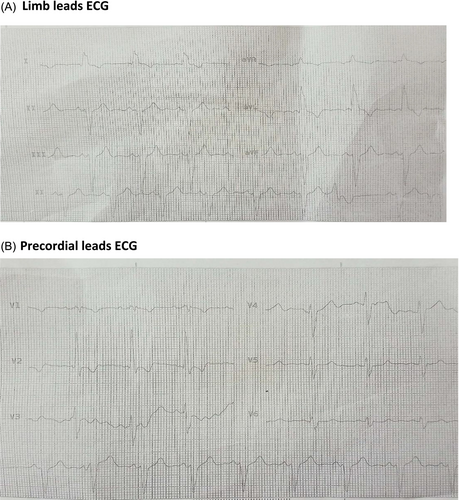
Physical examination demonstrated second-degree burns from the fingertip up to the forearm and his buttock due to electric current. The most likely path was from the left hand to the feet. The initial ECG revealed atrial fibrillation but later returned to a normal sinus rhythm. Subsequent ECG readings in sinus rhythm displayed a narrow QRS with nonspecific ST-T changes. Troponin I level was increased (80 μg/dL, by VIDAS based on the enzyme-linked fluorescent assay).
The two-dimensional echocardiogram indicated mildly reduced left ventricular ejection fraction (LVEF) of 45% with global hypokinesia and normal right ventricular function (Table 1). Coronary artery angiography revealed normal coronary arteries.
| Assessment | Initial (post-injury) | Follow-up (6 months later) |
|---|---|---|
| ECG findings | Atrial fibrillation (converted to sinus rhythm); narrow QRS, nonspecific ST-T changes | IVCD, LAD; wide QRS complexes (Figure 3) |
| LVEF (%) | 45% (echocardiography) | 38% (CMR), 40% (echocardiography) |
| RVEF (%) | Normal | 35% (CMR) |
| RV function | Normal | Reduced |
| Symptoms | Palpitations, tingling sensations | Chronic shortness of breath (NYHA Class II) |
| Imaging modality | Echocardiography | Echocardiography, CMR |
Following the diagnosis of an initial LVEF of 45%, the patient was started on standard therapies for heart failure, which include metoprolol (23.75 mg daily) and captopril (12.5 mg every 12 h), to improve cardiac function and stop further deterioration. The patient had no additional comorbidities, and the coronary angiography revealed no anomalies; therefore, a progression of heart failure was not anticipated.
After 6 months, the patient returned with persistent shortness of breath and symptoms indicating of New York Heart Association Class II heart failure. A follow-up echocardiogram revealed a further decrease in LVEF to 40% and additional signs of right ventricular dysfunction (Table 1). A cardiac MRI was conducted because of ongoing shortness of breath and NYHA Class II heart failure, utilizing this technique to offer more comprehensive insights into the underlying pathology and the patient's condition. With a left ventricular end-diastolic volume (EDV) of 169 mL/m2 and a right ventricular EDV of 143 mL/m2, the magnetic resonance imaging (MRI) showed elevated indexed volumes of both ventricles. Considering LVEF = 38% and RVEF = 35%, there was a significant decrease in both EFs.
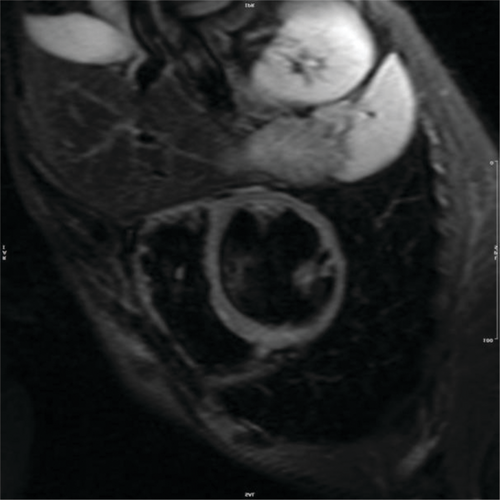
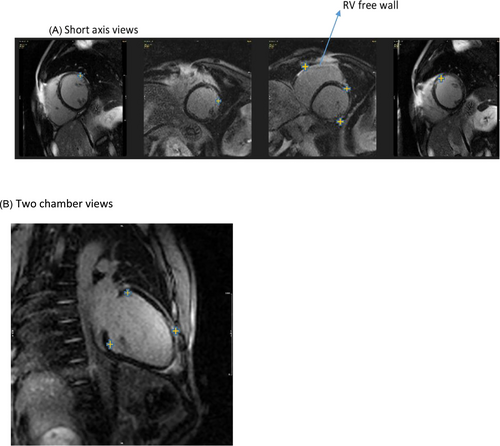
There was a noticeable reduction in thickness in the basal to mid anterior and lateral walls, along with a lack of movement and the development of small aneurysms in the right ventricular outflow tract (RVOT). No signs of myocardial oedema were observed on short tau inversion recovery (STIR) sequences (Figure 1). In the late-phase gadolinium study, there was a notable presence of mixed subendocardial and subepicardial enhancement observed in the basal to mid anterior, anterolateral, and inferior walls, along with the RV-free wall and anterior RVOT (Figure 2A,B, and Data S1–S4). There was moderate regurgitation of the mitral valve and mild regurgitation of the tricuspid valve, yet no thrombus was detected in the left or right ventricular cavities. Currently, the ECG revealed wide QRS complexes accompanied by intraventricular conduction delay and left axis deviation (Figure 3A,B).
Discussion
The patient's CMR demonstrated dilation of both ventricles and delayed subepicardial/subendocardial enhancement, a pattern previously observed in chronic myocarditis with heart failure.3 Although viral myocarditis, particularly due to COVID-19, cannot be definitively ruled out, the initial ECG changes, lack of coronary artery disease risk factors and diffuse late gadolinium enhancement (LGE) pattern strongly support electrical injury as the cause of progressive heart failure in this case.
High-voltage or direct current can induce ventricular asystole, sinus bradycardia, bundle branch blocks and various degrees of atrioventricular blocks, potentially necessitating a permanent pacemaker.3-5 Ventricular fibrillation or conduction disorders are the leading causes of death following electrocution.4 The extent of myocardial involvement is influenced by factors such as pre-existing myocardial injury, the amount of electrical energy passing through the heart, the current (direct vs. alternating), the duration of electrocution and the current pathway.4, 6
Guidelines suggest that patients who are admitted after high-voltage electrical injuries and present with normal ECG results, normal troponin levels and no signs of arrhythmias, loss of consciousness or trauma can typically be discharged after a minimum of 24 h of ECG monitoring.1
The electrical current is suspected to be the cause of the progressive reduction in LV function in this instance. This hypothesis is corroborated by the combined subepicardial and subendocardial LGE pattern observed in cardiac MRI, which is indicative of fibrosis. Myocardial cell membranes can be disrupted by electrical injury, resulting in cellular oedema, altered electrical conductivity and subsequent fibrotic alterations.3, 6
Progressive myocardial remodelling and ventricular dysfunction may also be affected by microvascular injury and impaired coronary microcirculation. The absence of myocardial oedema on CMR suggests that the damage is not the result of ongoing inflammation, but mainly from fibrotic remodelling that is a consequence of the initial electrical injury. Arrhythmias and conduction abnormalities are prevalent conditions that result from electrical injuries. The patient's progression from initial atrial fibrillation to IVCD and LAD on follow-up ECGs is indicative of the ongoing electrical instability within the myocardium as a result of structural alterations. These conduction disturbances have the potential to exacerbate ventricular dysfunction and elevate the risk of sudden cardiac death.
Early diagnosis enables starting the treatment, which is associated with reduced cardiovascular mortality and hospitalizations. For instance, patients diagnosed as outpatients had a 1.83 times lower risk of adverse outcomes compared to those diagnosed in hospitals.7
Echocardiography is an insufficient tool to evaluate these patients due to its inability to consider the multifactorial nature of the condition.8
For example, echocardiography is unable to detect fibrosis. The location and extent of cardiac fibrosis is important for personalizing treatment strategies, as fibrosis is highly heterogeneous and requires tailored therapies. A multi-target approach addressing interconnected pro-fibrotic pathways is likely more effective in this complex and heterogeneous condition.9
The patient's heart failure was confirmed by echocardiography and CT scans, which revealed a decline in ventricular function and worsening symptoms. The lack of an FDG-PET scan limited the ability to assess active inflammation, but the lack of oedema on CMR STIR sequences reduced the likelihood of significant ongoing inflammation. The patient did not start taking steroid immunosuppressive medication and instead continued with their usual heart failure treatment regimen. However, the possibility of chronic low-grade inflammation cannot be excluded entirely.
If fibrosis is the dominant pathology, emerging anti-fibrotic treatments (such as agents targeting the TGF-β pathway) or experimental therapies may be considered.
CMR performs a superior assessment of heart failure phenotypes compared to echocardiography, including early atrial dysfunction, ventricular changes and fibrosis.10 It can also identify specific aetiologies like amyloidosis.11
CMR-based cardiac phenotyping can accurately characterize different forms of heart failure, providing insights into pathophysiological processes and the potential for personalized management. This technique can detect subtle myocardial deformation and fibrosis that echocardiography may miss, particularly in asymptomatic patients at risk for heart failure, enabling early identification and timely interventions.12
Conclusions
Follow-up care for this patient should initially occur at short intervals to closely monitor disease progression and response to therapy, and can be spaced out to longer intervals once stability is achieved. Despite receiving standard heart failure treatment, the patient's symptoms have worsened, indicating the need to target the underlying cause of heart failure. If inflammation is confirmed, for example through biomarkers or advanced imaging like FDG-PET, initiating anti-inflammatory therapy such as corticosteroids may be warranted. However, if fibrosis is the primary driver, tailored therapies addressing fibrotic remodelling should be considered. We recommend that if follow-ups reveal any signs of reduced ejection fraction, traditional heart failure treatments may not suffice, and advanced imaging techniques such as cardiac MRI or FDG-PET should be employed to identify the underlying pathology, enabling more targeted interventions. Additionally, earlier use of cardiac MRI could have provided critical insights into the underlying pathology, such as fibrosis or inflammation, allowing for timely and personalized interventions that might have slowed or mitigated the progression of heart failure.
Ethics statement
Written consent was obtained from the patient.
Conflict of interest
The authors declare that they have no competing interests.
Funding
No funding to declare.




