Cardiac magnetic resonance left atrioventricular coupling index as a prognostic tool in hypertrophic cardiomyopathy
Abstract
Aims
A novel marker left atrioventricular coupling index (LACI) has been proved to be associated with cardiovascular events in patients without history of cardiovascular disease. However, the studies on cardiac magnetic resonance-derived LACI in hypertrophic cardiomyopathy (HCM) patients are limited, and the prognostic value of LACI has still not been studied thoroughly, so we aimed to explore the association between LACI and adverse clinical outcomes in HCM patients.
Methods
A total of 206 HCM patients underwent cardiac magnetic resonance examination were retrospectively enrolled. LACI is defined by the ratio between the left atrial (LA) volume and the left ventricular (LV) volume in LV end-diastolic phase. The composite endpoint was categorized into death-related, heart failure-related, and arrhythmia-related events, reflecting mortality risk, heart failure progression, and arrhythmia burden, respectively. Receiver operating characteristics curve analysis was used to determine the optimal cut-off value for LACI to distinguish HCM patients at high risk of adverse clinical outcome. Multivariable Cox regression models were built including significant clinical variables, LA ejection fraction (LAEF), LA volume index (LAVI), late gadolinium enhancement (LGE) extent and LACI. The improvement of discrimination by adding LACI to a clinical model was assessed using C-statistic, net reclassification improvement (NRI) and integrated discrimination improvement (IDI).
Results
Thirty-four HCM patients reached the endpoint during a median follow-up time of 60 [interquartile range (50–68)] months. In the multivariate Cox regression analysis, LACI [hazard ratio 1.054, 95% confidence interval (CI): 1.037, 1.071; P < 0.001] was an independent predictor of the composite events after adjustment for age and atrial fibrillation. Then 40.09% was identified as an optimal cut-off for LACI in the risk stratification. Integrating LACI to the clinical model yielded higher C-statistic 0.892 with 95% CI (0.861, 0.922) compared with LA diameter, LAEF, LAVI and LGE extent, providing an improvement in prediction of high-risk patients (NRI = 0.627, 95% CI: 0.112–0.934; IDI = 0.295, 95% CI: 0.016–0.709).
Conclusions
LACI is an independent risk factor for clinical adverse outcome and is superior to conventional LA parameters and LGE extent for the identification of high-risk HCM patients.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common inherited heart disease, characterized by an increase in left ventricular (LV) wall thickness or mass that is not be solely explained by abnormal loading conditions.1 The prevalence of HCM continues to rise and largely accompanied with adverse clinical outcomes.2 A large cohort study involving 52 091 HCM adults reported that 12 676 patients (24.0%) died during a median follow-up of 3 years, with all-cause mortality was 8.10%/year and cardiovascular death mortality was 2.76%/year.3 Sudden cardiac death (SCD), heart failure and stroke caused by atrial fibrillation are the three leading causes of death in HCM patients, and SCD is the most severe outcome.4 Identifying individuals at high risk for HCM has important clinical significance for following treatment and decision making. Two SCD risk model algorithms were proposed in international guidelines in 2014 and 2020,5, 6 a global consensus on the risk stratification of SCD in HCM remains unmet. The latest 2023 European Society of Cardiology (ESC) guidelines recommended combing the risk factors from the two algorithms to provide primary prevention of SCD in HCM patients.1 The prevention of adverse complications such as heart failure and atrial fibrillation warrants further attention. Therefore, risk assessment for HCM patients is still tough and complicated in clinical practice.
HCM encompasses multiple pathophysiological changes, including LV out-flow tract obstruction, mitral regurgitation, LV diastolic dysfunction and myocardial ischaemia.5-7 LV diastolic dysfunction is an important factor in the process of the left atrial (LA) function impairment, ultimately leading to LA enlargement.8, 9 Recent studies highlight the utility of cardiac magnetic resonance (CMR)-derived pulmonary capillary wedge pressure (PCWP),10 a non-invasive estimate of LV filling pressure, which has shown prognostic significance.11 LV and LA structural and functional changes are fairly common in HCM patients. Previous research has suggested that LV and LA strain are prognostic markers in LV diastolic dysfunction patients,12 particularly in those with HCM.8 LA and LV are directly connected in physiological structure, and their functions are closely coupled during the diastolic phase of the LV.13, 14 There is a lack of evidence in clinical practice for non-invasive assessment of LV diastolic assessment in HCM. Given the interaction between LV and LA function, a novel and simple parameter left atrioventricular coupling index (LACI), defined by the ratio between the LA volume and the LV volume in LV end-diastolic phase, serving as a potential marker of LV diastolic dysfunction.15-17 A study has demonstrated an association between LACI and prognosis in patients with heart failure.17 It has also been introduced as a predictor of cardiovascular events in multi-ethnic healthy population15 and of new-onset atrial fibrillation in HCM patients.18
Study on the predictive value of CMR-derived LACI for adverse clinical outcomes in HCM is limited, and its prognostic utility has not been thoroughly explored. We assumed that this simple indicator may provide incremental prognostic value in HCM patients. Therefore, this study aims to explore the association between LACI and adverse clinical outcomes in HCM patients.
Methods
Study population
CMR image acquisition
All participants for CMR examination were performed on a 3.0 T MR scanner (Magnetom Skyra, Siemens Healthineers, Erlangen, Germany) with an 18-channel phased-array coil in the supine position. The CMR protocol included short-axis and long-axis (two-, three- and four-chamber) cine images and late gadolinium enhancement (LGE). A steady-state free-precession sequence with respiratory gating and electrocardiograph gating were used for imaging acquisition. Three slices for long-axis plane and 8–10 slices covering the whole left ventricle for short-axis plane. The main imaging parameters were as follows: repetition time 3.1 ms, echo time 1.4 ms, flip angle 55°, field of view 360 × 360 mm2, matrix 192 × 146, slice thickness 8 mm, 25 phases per heartbeat. LGE image acquisition was performed 10 to 15 min after gadobutrol (Gadovist, Bayer Schering Pharma AG, Germany) injection of 0.1 mmol/kg and a 20 mL normal saline rinse. A two-dimensional phase-sensitive inversion recovery sequence was used to obtain the LGE images at the same position as short-axis cine images. The main imaging parameters were as follows: repetition time 3.5 ms, echo time 1.2 ms, flip angle 55°, inversion time 220 to 350 ms, field of view, 360 × 360 mm2; matrix, 256 × 168; slice thickness, 8 mm.
CMR image analysis
Image analysis was performed using cardiovascular business software CVI42 (v.5.12, Circle Cardiovascular Imaging Inc., Calgary, Canada). LV endocardial and epicardial contours on the short-axis cine images were automatically tracked in the end-systolic and end-diastolic phases, and manual corrections were made if needed. The LV papillary muscles were included in LV volumes and excluded from LV mass.20 Most of LV parameters were standardized by body surface area (BSA), including end-diastolic volume index (EDVI), end-systolic volume index (ESVI), cardiac index (CI) and LV mass index (LVMI). To access LV global longitudinal strain (GLS), LV epicardial and endocardial borders were manually tracked in short-axis, and long-axis (two-, four- and three-chamber) cines. BSA was calculated by the Mosteller formula,21 .
LACI
LACI is defined by the ratio between the LA volume and the LV volume in LV end-diastolic phase, as previous studies described.15, 16 LA volume was measured from two- and four-chamber cines, LV volume was measured from short-axis cine. LA volume and LV volume were measured in the same end-diastolic phase (Figure 1).
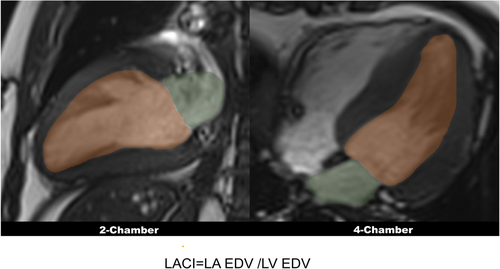
Non-invasive CMR-derived PCWP
LAV is LA volume in end-ventricular systole, and LVM is LV mass in end diastole.
LGE extent
LGE extent was assessed in the CVI42 signal intensity module. The epicardial and endocardial borders of the LV myocardium were manually traced on the short-axis LGE images. A region of normal myocardium was manually delineated, and the software automatically identified areas with signal intensity exceeding five standard deviations above the normal region,24 which were defined as the extent of LGE. The extent of LGE was expressed as the percentage of the extent of enhancement relative to the overall LV mass.
Follow-up and endpoints
The period of follow-up was the duration from the first CMR examination to January 2024. Follow-up information was collected by medical records and telephone contact with patients or their family members. Three types of events were defined as composite endpoint, including death-related, heart failure-related and arrhythmia-related events. All-cause mortality, resuscitated cardiac arrest, SCD aborted by appropriate implantable cardioverter-defibrillator (ICD) discharge and heart transplantation were identified as death-related events. Heart failure hospitalization, heart failure aggravation and implantation of LV assist device were listed as heart failure-related events. Heart failure hospitalization refers to the need for inpatient treatment due to the onset of heart failure symptoms.25 Heart failure aggravation refers to the progression and worsening of symptoms in a patient who already has a diagnosis of heart failure.25 Besides, arrhythmia-related events included new-onset or recurrent atrial fibrillation, haemodynamic disturbance caused by episodes of tachycardia or ventricular fibrillation, and implantation of ICD. The arrhythmic events are typically assessed through Holter or electrocardiogram results by electronic medical records or self-report. In case of multiple events occurrences within one patient, prioritization of recording time was made as follows: death prior to heart failure and heart failure prior to arrhythmia. Of note, each patient only accounts for one event.
Statistical analysis
Results
Study population
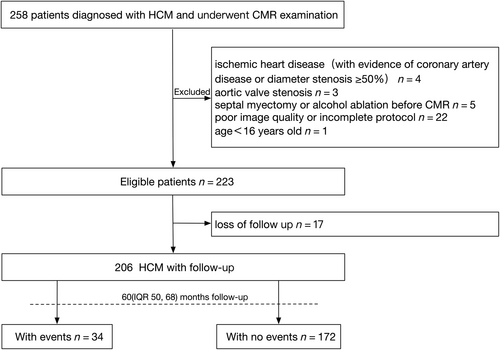
A total of 258 HCM patients who underwent CMR examination were reviewed. Patients were excluded due to ischaemic heart disease (n = 4), aortic valve stenosis (n = 3), septal myectomy or alcohol ablation before CMR (n = 5), poor image quality or incomplete protocol (n = 22) and age under 16 years (n = 1). After 17 patients were lost to follow-up, 206 patients were ultimately included in the analysis. Thirty-four HCM patients reached the composite outcome during a median follow-up time of 60 (IQR 50, 68) months, including death-related (n = 14), heart failure-related (n = 7), arrhythmia-related (n = 13), as shown in Figure 2. The death-related events included SCD (n = 10), non-cardiac death (n = 2) and heart transplantation (n = 2). Heart failure-related events included heart failure hospitalizations (n = 5) and heart failure aggravation (n = 2). Arrhythmia-related events included new-onset atrial fibrillation (n = 5), recurrent atrial fibrillation (n = 5) and ICD implantation (n = 3). The clinical and echocardiography characteristics of our study population are summarized in Table 1. The average age of the study population was 51 ± 13 years with mainly male patients (75.7%). Higher age was found in HCM patients with composite outcome (57 ± 13 years vs. 50 ± 13 years, P = 0.003). Other typical cardiovascular risk factors, such as gender, NYHA functional class, atrial fibrillation and LA diameter also showed statistically significant differences between the two groups. No significant differences were also observed on HCM-SCD score, LVOTG, the proportion of obstructive patients and SCD family history between the two groups.
| Variables | All patients (n = 206) | With composite outcome (n = 34) | Without composite outcome (n = 172) | P value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Males, n (%) | 156 (75.7) | 21 (61.8) | 135 (78.5) | 0.032 |
| Age, years | 51 ± 13 | 57 ± 13 | 50 ± 13 | 0.003 |
| BMI, kg/m2 | 25.1 (23.4, 27.3) | 24.7 (21.9, 26.1) | 25.2 (23.5, 25.4) | 0.224 |
| Hypertension, n (%) | 124 (60.2) | 20 (58.8) | 104 (60.5) | 0.851 |
| Diabetes, n (%) | 28 (13.6) | 4 (11.8) | 24 (14) | 0.999 |
| Dyslipidaemia, n (%) | 139 (67.5) | 22 (64.7) | 117 (68) | 0.694 |
| Smoking, n (%) | 74 (35.9) | 10 (29.4) | 64 (37.2) | 0.439 |
| Alcohol consumption, n (%) | 37 (18.0) | 5 (14.7) | 32 (18.6) | 0.807 |
| NYHA functional class, n (%) | 0.028 | |||
| I/II | 186 (90.3) | 27 (79.4) | 159 (92.4) | |
| III/IV | 20 (9.7) | 7 (20.6) | 13 (7.6) | |
| Unexplained syncope, n (%) | 10 (4.9) | 1 (2.9) | 9 (5.2) | 0.999 |
| Atrial fibrillation, n (%) | 14 (4.4) | 11 (32.4) | 3 (1.7) | < 0.001 |
| SCD family history, n (%) | 1 (0.5) | 0 | 1 (0.6) | 0.999 |
| NSVT, n (%) | 13 (6.3) | 4 (11.8) | 9 (5.2) | 0.236 |
| HCM-SCD score, % | 1.5 (1.2, 1.9) | 1.6 (1.3, 1.8) | 1.4 (1.1, 2.0) | 0.174 |
| Medication | ||||
| Beta-blockers, n (%) | 160 (77.7) | 27 (79.4) | 133 (77.3) | 0.999 |
| ACEI, n (%) | 101 (49) | 19 (55.9) | 82 (47.7) | 0.454 |
| Diuretics, n (%) | 18 (8.7) | 9 (26.5) | 9 (5.2) | < 0.001 |
| Calcium-antagonists, n (%) | 13 (6.3) | 6 (17.7) | 7 (4.1) | 0.001 |
| Echocardiography characteristics | ||||
| LVHmax, mm | 16.4 (15.6, 18.5) | 17.4 (16, 19) | 16.3 (15.5, 18.2) | 0.137 |
| LA diameter, mm | 36 (31, 46) | 42 (37, 47) | 35 (30, 39) | < 0.001 |
| Apical aneurysm, n (%) | 1 (0.5) | 0 | 1 (0.6) | 0.999 |
| LVOTG, mmHg | 16 (10, 24) | 16.5 (10, 24) | 14.5 (8.3, 22.8) | 0.515 |
| LVOT obstruction, n (%) | 43 (20.9) | 7 (20.6) | 36 (20.9) | 0.999 |
- Note: Values are means ± SD, n (%) or median (interquartile range).
- Abbreviations: ACEI, angiotensin-converting enzyme inhibitors; BMI, body mass index; HCM, hypertrophic cardiomyopathy; LA, left atrial; LVHmax, maximum left ventricular wall thickness; LVOT, left ventricular out-flow tract; LVOTG, left ventricular out-flow tract gradient; NSVT, non-sustained ventricular tachycardia; NYHA, New York Heart Association; SCD, sudden cardiac death.
CMR characteristics and CMR-derived LACI
CMR characteristics and CMR-derived LACI of study population are presented in Table 2. The median LACI for the entire cohort was 29.0% (IQR 21.2, 38.8). In patients with composite outcome, the LACI was significantly higher compared with patients without composite outcome [55.1% (IQR 41.8, 75.0) vs. 25.7% (IQR 19.3, 33.4), P < 0.001]. Additionally, HCM patients with composite outcome demonstrated significantly higher LAVImax, higher LAVImin, higher LACI, higher non-invasive PCWP, higher LGE extent, lower LV GLS, lower LAEF and lower LA strains compared with the group without composite outcome.
| CMR characteristics | All patients (n = 206) | With composite outcome (n = 34) | Without composite outcome (n = 172) | P value |
|---|---|---|---|---|
| CI, mL/m2 | 2.7 (2.4, 3.2) | 2.7 (2.4, 3.2) | 5.0 (4.2, 5.8) | 0.554 |
| LVEF, % | 60.2 (53.9, 64.7) | 60 (51.8, 65.0) | 60.3 (54.3, 64.4) | 0.609 |
| LVEDVI, mL/m2 | 70.7 (61.6, 79.8) | 70.1 (62.4, 74.3) | 70.7 (61.5, 80.4) | 0.611 |
| LVESVI, mL/m2 | 27.4 (23.2, 34.4) | 26.9 (24.8, 36.8) | 28.0 (22.7, 33.7) | 0.740 |
| LV mass index, g/m2 | 68.9 (54.6, 85.3) | 76.7 (61.9, 85.3) | 67.0 (52.7, 84.4) | 0.055 |
| LV GLS, % | −13.5 (−10.4, 15.7) | −10.6 (−7.0, 13.8) | −13.8 (−11.1, 16.1) | <0.001 |
| LAVImax, mL/m2 | 43.1 (54.6, 73.2) | 62.1 (54.6, 73.2) | 41.3 (33.6, 47.8) | <0.001 |
| LAVImin, mL/m2 | 19.4 (14.7, 26.7) | 37.5 (27.1, 49.0) | 18.1 (13.5, 23.8) | <0.001 |
| LACI, % | 29.0 (21.2, 38.8) | 55.1 (41.8, 75.0) | 25.7 (19.3, 33.4) | <0.001 |
| LAEFtotal, % | 54.4 (46.3, 59.5) | 40.6 (33.7, 48.3) | 55.3 (49.6, 60.4) | <0.001 |
| LAEFactive, % | 36.8 ± 11.2 | 27.1 ± 11.6 | 38.7 ± 10.1 | <0.001 |
| LAEFpassive, % | 23.7 ± 8.5 | 17.7 ± 6.4 | 24.9 ± 8.4 | <0.001 |
| εs, % | 25.2 (18.9, 33.6) | 15.1 (10.6, 22.4) | 27.1 (21.0, 34.7) | <0.001 |
| εa, % | 13.8 (9.4, 18.7) | 7.3 (5.5, 13.1) | 14.5 (10.8, 20.1) | <0.001 |
| εe, % | 11.0 (7.3, 15.8) | 7.5 (4.7, 10.4) | 12.0 (7.9, 16.4) | <0.001 |
| PCWP, mmHg | 16.6 (14.4, 19. 7) | 20.3 (18.1, 21.2) | 15.9 (13.9, 18.8) | <0.001 |
| LGE extent, % | 10.0 (6.3, 13.7) | 9.4 (5.7, 13.3) | 11.2 (10.1, 16.3) | 0.006 |
- Note: Values are means ± SD or median (interquartile range).
- Abbreviations: CI, confidence interval; CMR, cardiac magnetic resonance; LACI, left atrioventricular coupling index; LAEFactive, left atrial active ejection fraction; LAEFpassive, left atrial passive ejection fraction; LAEFtotal, left atrial total ejection fraction; LAVImax, maximum of left atrial volume index; LAVImin, minimum of left atrial volume index; LGE, late gadolinium enhancement; LV GLS, left ventricular longitudinal strain; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume index; LVMI, left ventricular mass index; PCWP, pulmonary capillary wedge pressure; εa, active strain; εe, passive strain; εs, total strain.
Clinical outcome and prognostic value of LACI
The results of univariate and multivariate proportional hazard analyses for the composite outcome are presented in Tables 3 and 4. In univariate regression analyses, all variables were significantly associated with the occurrence of composite outcome, including LACI (HR = 1.056, P < 0.001). Given the number of outcome events, we developed a multivariable Cox model incorporating two clinical indicators and one LV/LA variable. Upon initial inclusion of age alone in the Cox model, neither sex nor NYHA classification reached statistical significance. Therefore, they were not included in the final model to maintain its parsimony and predictive accuracy. Medication was excluded from the model based on clinical interpretation, as its inclusion did not contribute significantly to the model's predictive power. Eventually, a clinical model was built by integrating age and atrial fibrillation. When adding LACI to the clinical model, only age (HR = 1.046) and LACI (HR = 1.054) were identified as independent predictors (Model 2). Similarly, when LA diameter, LAVI, LAEF, non-invasive PCWP, LGE extent and LV GLS were separately included in the clinical model, each was independently associated the composite outcome (Model 1, Models 3–10).
| Variables | Univariate analysis | ||
|---|---|---|---|
| HR | 95% CI | P value | |
| Male | 2.476 | (1.226, 5.001) | 0.011 |
| Age, year | 1.050 | (1.021, 1.080) | <0.001 |
| NYHA functional class | 2.467 | (1.070, 5.688) | 0.034 |
| Atrial fibrillation | 14.576 | (6.799, 31.250) | <0.001 |
| Diuretics | 5.119 | (2.357, 11.117) | <0.001 |
| Calcium-antagonists | 2.594 | (1.052, 6.397) | 0.038 |
| LA diameter, mm | 1.124 | (1.069, 1.081) | <0.001 |
| LACI, % | 1.056 | (1.044, 1.068) | <0.001 |
| LV GLS, % | 1.126 | (1.044, 1.214) | 0.002 |
| LAVImax, mL/m2 | 1.043 | (1.031, 1.056) | <0.001 |
| LAVImin, mL/m2 | 1.055 | (1.041, 1.070) | <0.001 |
| LAEFtotal, % | 0.923 | (0.902, 0.946) | <0.001 |
| LAEFactive, % | 0.929 | (0.901, 0.957) | <0.001 |
| LAEFpassive, % | 0.900 | (0.861, 0.940) | <0.001 |
| εs, % | 0.884 | (0.844, 0.926) | <0.001 |
| εa, % | 0.848 | (0.786, 0.915) | <0.001 |
| εe, % | 0.841 | (0.776, 0.911) | <0.001 |
| PCWP, mmHg | 1.096 | (1.045, 1.149) | <0.001 |
| LGE extent, % | 1.037 | (1.005, 1.070) | 0.023 |
- Abbreviations: CI, confidence interval; HR, hazard ratio; LA, left atrial; LACI, left atrioventricular coupling index; LAEFactive, left atrial active ejection fraction; LAEFpassive, left atrial passive ejection fraction; LAEFtotal, left atrial total ejection fraction; LAVImax, maximum of left atrial volume index; LAVImin, minimum of left atrial volume index; LGE, late gadolinium enhancement; LV GLS, left ventricular longitudinal strain; NYHA, New York Heart Association; PCWP, pulmonary capillary wedge pressure; εa, active strain; εe, passive strain; εs, total strain.
| Variables | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 | Model 9 | Model 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| HR | HR | HR | HR | HR | HR | HR | HR | HR | HR | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Age, year | 1.045** | 1.046** | 1.054*** | 1.052*** | 1.049*** | 1.053*** | 1.030* | 1.070*** | 1.052*** | 1.038* |
| (1.015, 1.077) | (1.021, 1.088) | (1.023, 1.086) | (1.022, 1.084) | (1.019, 1.080) | (1.023, 1.084) | (1.000, 1.061) | (1.035, 1.105) | (1.021, 1.183) | (1.008, 1.068) | |
| Atrial fibrillation | 6.600*** | 1.045 | 4.802** | 3.908** | 5.463*** | 7.671*** | 7.513*** | 5.689*** | 9.962*** | 13.159*** |
| (2.285, 15.416) | (0.438, 4.795) | (1.921, 12.002) | (1.440, 10.605) | (2.287, 13.051) | (3.307, 17.792) | (2.252, 13.003) | (2.439, 13.268) | (4.539, 21.876) | (5.983, 28.945) | |
| LA diameter, mm | 1.085** | |||||||||
| (1.026, 1.147) | ||||||||||
| LACI, % | 1.054*** | |||||||||
| (1.037, 1.071) | ||||||||||
| LAVImax, mL/m2 | 1.040*** | |||||||||
| (1.023, 1.056) | ||||||||||
| LAVImin, mL/m2 | 1.048*** | |||||||||
| (1.029, 1.068) | ||||||||||
| LAEFtotal, % | 0.935*** | |||||||||
| (0.910, 0.961) | ||||||||||
| LAEFactive, % | 0.942*** | |||||||||
| (0.914, 0.971) | ||||||||||
| LAEFpassive, % | 0.929** | |||||||||
| (0.883, 0.978) | ||||||||||
| PCWP, mmHg | 1.313*** | |||||||||
| (1.179, 1.462) | ||||||||||
| LV GLS, % | 1.146*** | |||||||||
| (1.059, 1.241) | ||||||||||
| LGE extent, % | 1.041* | |||||||||
| (1.005, 1.078) |
- Abbreviations: CI, confidence interval; HR, hazard ratio; LA, left atrial; LACI, left atrioventricular coupling index; LAEFactive, left atrial active ejection fraction; LAEFpassive, left atrial passive ejection fraction; LAEFtotal, left atrial total ejection fraction; LAVImax, maximum of left atrial volume index; LAVImin, minimum of left atrial volume index; LGE, late gadolinium enhancement; LV GLS, left ventricular longitudinal strain; PCWP, pulmonary capillary wedge pressure.
- * P value < 0.05.
- ** P value < 0.01.
- *** P value < 0.001.
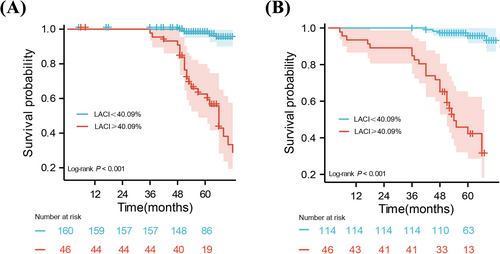
For discrimination performance, the area under the curve of LACI was the highest among all evaluated parameters, including LA diameter, LAVI, LAEF, non-invasive PCWP, LV GLS and LGE extent, with all DeLong tests showing statistical significance (Figure 3, Table S1). An optimal cut-off of 40.09% for LACI and 17.58 mmHg for non-invasive PCWP were respectively used to classify patients into low- and high-risk groups. Kaplan–Meier survival curve manifested HCM patients with LACI ≥ 40.09% had a lower cumulative event-free survival rate compared with those with LACI < 40.09% (Figure 4A). The significance was also maintained in patients with LA dilatation (Figure 4B).
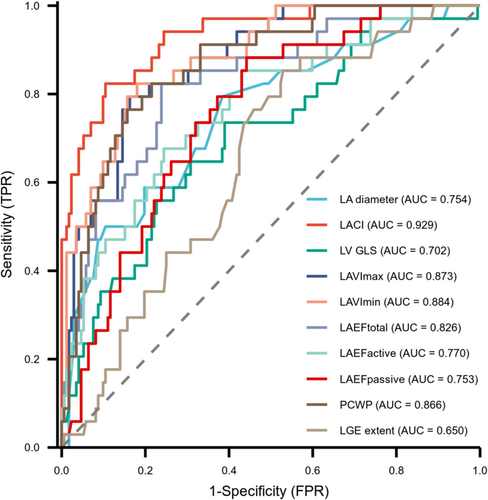
To evaluate the discriminatory capability of LACI, we added LA diameter, LAVI and LAEF to clinical models, respectively. The multivariable models incorporating LACI yielded the highest C-statistic 0.892 (95% CI: 0.861, 0.922), outperforming models with LA diameter, LAVImax, LAVImin, LAEF or LGE extent (Table S2). Adding LACI to the clinical model significantly improved the reclassification index (NRI = 0.627, 95% CI: 0.112–0.934, P < 0.001) and discrimination index (IDI = 0.295, 95% CI: 0.016–0.709, P < 0.001). Additionally, when LACI was added to the clinical model including LV GLS, the IDI and NRI were significantly enhanced (IDI = 0.249, 95% CI: 0.020–0.611, P = 0.02; NRI = 0.603, 95% CI: 0.000–0.934, P = 0.02).
The correlation between LACI and LV/LA parameters
LACI showed no significant correlation with LVEF, LVEDVI, LVESVI (LVEF: r = 0.08, P = 0.231; LVEDVI: r = 0.01, P = 0.88; LVESVI: r = 0.01, P = 0.89), but it was significantly associated with LV GLS, LAEF and LA strain parameters (LA diameter: r = 0.58, P < 0.05; LV GLS: r = 0.24, P < 0.05; εs: r = −0.63, P < 0.05; εa: r = −0.50, P < 0.05; εe: r = −0.58, P < 0.05; LAEF total: r = −0.70, P < 0.05; LAEF active: r = −0.56, P < 0.05; LAEF passive: r = −0.48, P < 0.05). Other correlation findings were shown in Table S3.
Discussion
In our study, we investigated the prognostic value of LACI derived from CMR in HCM risk stratification. HCM patients with composite outcome exhibited higher LACI, which represented left atrioventricular uncoupling. LACI was identified as an independent risk factor for composite clinical outcomes after adjusting for age, and atrial fibrillation. Additionally, LACI is superior to other LA functional/structural parameters on the identification of HCM high-risk patients. Lastly, LACI demonstrated utility in stratifying the risk of adverse clinical outcomes in HCM patients, even with the presence of LA enlargement.
Risk stratification and prevention are the most important components of clinical management in patients with HCM. Recent guidelines focus on identifying those at high risk for SCD. However, other adverse clinical events also require more attention, such as heart failure, atrial fibrillation and other adverse arrhythmias. The main parameters used to assess the SCD of patients with HCM from CMR are LV wall thickness, LVEF and LA diameter.5, 6 In our study, there was no significant difference in HCM-SCD score between the subgroups, the endpoints in this study included not only SCD events but also heart failure and arrhythmic events, these facts indicated that HCM score may not be appropriate for predicting such a diverse outcome set. Beyond LVEF, few parameters reflecting myocardial function have been thoroughly investigated, which may limit the comprehensiveness of risk evaluation. Functional and structural impairments of LV/LA have been reported in HCM patients,30 confirming the presence of LV and LA remodelling during disease progression. Meanwhile, studies have explored the relationship between LV/LA structural and functional impairments and adverse clinical outcomes in HCM. Notably, LV strains accessed by speckle-tracking echocardiography or CMR have been identified as an independent predictor of SCD.31-33 Similarly, LAEF and LA strain have been recognized as independent risk factors,9, 34, 35 providing incremental prognostic value beyond LA size for predicting new-onset atrial fibrillation or other adverse outcomes.36 LV or LA parameters are considered as powerful predictors for atrial fibrillation and adverse clinical outcome.
Considering the intrinsic linkage of LV and LA throughout the whole cardiac cycle, LA and LV coupling can better reflect left atrioventricular dysfunction. The relation between LA and LV was reflected in our study, we found LACI has a high correlation with the functional parameters of LV and LA, as previous study demonstrated.37 LACI was also proposed to evaluate prognosis in cardiomyopathy. Pezel et al. firstly found that LACI, as a novel indicator, is a powerful predictor of incident heart failure, atrial fibrillation, hard cardiovascular events and cardiovascular death in multi-ethnic population.15, 16, 38, 39 Other studies showed that LACI can be applied to evaluate the prognosis in heart failure17, 40 and myocardial infarction.37 In our study, HCM patients with adverse clinical outcome had increased LACI than those without, showing that left atrioventricular uncoupling was associated with adverse clinical outcome. This is partly in accordance with the latest study from Meucci et al.18 Of interest, although LAVI was significantly different between patients with and without composite outcome, there was no significant difference in LVEDVI, suggesting differences in atrial response and compensatory capacity. Higher LACI suggests a mismatch between LA size and LV size and may also account for the decrease of LV size due to LV hypertrophy18 or the enlargement of LA size remodelling caused by remodelling in the progression of HCM.41 Moreover, LACI, LA diameter, non-invasive PCWP, LV GLS, LGE extent LAVI and LAEFmin were independently associated with adverse clinical outcomes of death, heart failure and arrhythmia in HCM patients after adjusting for other covariates, such as age and atrial fibrillation. These findings are similar to the results of several recent studies exploring the prognosis of LACI.18, 37 Based on these results, LACI represents a simple and effective approach to unmask complicated pathophysiological mechanisms of cardiac performance. Similarly, LA enlargement has long been considered a prognostic factor for adverse clinical outcome42 and is used to calculate SCD risk in guidelines.5 But our result proved that the predictive value of LA diameter was less robust compared with the LACI, and LACI can be further applied for risk stratification even in the presence of LA enlargement. Only assessing the LA diameter may underestimate the risk of the composite endpoint, and LACI may be more appropriate for evaluating the composite outcomes.
In terms of model effectiveness, we found that LACI demonstrated better risk stratification at this endpoint compared with conventional LA parameters, non-invasive PCWP, LGE extent and LV GLS. Our work demonstrates that this physiological parameter has independent significance in any clinical setting and LACI may be a powerful diagnostic tool to elicit ventricular-atrial coupling. Notably, CMR-modelled non-invasive PCWP, an alternative to LVFP, has proven valuable in predicting poor prognosis in heart failure patients.11 Dilated LA and afterload related LV hypertrophy contribute to a physiological model of raised LV filling pressure.11 HCM patients are at increased risk of LV diastolic dysfunction leading to heart failure with preserved ejection fraction. Our study demonstrates for the first time that the threshold for CMR-derived non-invasive PCWP should be higher in HCM patients with composite outcomes compared with those without composite outcomes, potentially serving as a diagnostic marker for prognostically relevant higher non-invasive PCWP. Besides, it is noteworthy that previous studies have demonstrated that LV and LA strain assessment can provide incremental prognostic value to patients with HCM, better than myocardial volume analysis.43-45 Strain changes precede myocardial geometry/volume changes, enabling earlier and more precise diagnosis of cardiac functional deterioration.34, 36 In our study, we also find LA strains were associated with the composite outcomes, this result is consistent with that of Yang et al.,36 which explored the relationship between fast LA strain and prognosis. Although LACI assessment alone cannot completely replace LA or LV strain parameters in terms of prognostic ability, it is important to recognize in measuring atrioventricular ratio and volume mismatch. As the results of this study shows, LACI has a higher predictive value than LV GLS in assessing risk stratification and further improves model discrimination on the basis of adding LV GLS. These facts may imply the ability of LACI in indicating myocardial dysfunction may not be fully captured by isolated decreases in LA or LV strain. The atrioventricular coupling parameters may perform than the strain parameters that reflect LV function alone. Therefore, in addition to evaluation of strain, assessing LA and LV volume ratio may provide additional prognostic value to identify patients at high risk. Besides, the processing of strain data is relatively time-consuming compared with LACI. These advantages make LACI an attractive and cost- and time-saving imaging parameter with important prognostic implications. Our results also showed that LGE extent provided a prognostic value in HCM, which is consistent with previous studies,46-48 but we found that LACI is superior in this respect. This suggests that LACI may integrate multiple structural and functional aspects of the LA, providing a more comprehensive prognostic marker compared with the isolated evaluation of LGE extent.
Several limitations needed to be mentioned in this study. First, patient selection bias cannot be completely excluded due to this single-centre retrospective longitudinal design, the absence of an external validation cohort is a major limitation, and the cut-off values for the novel parameters require validation in independent cohorts to confirm their generalizability and prognostic significance. Second, recall bias may exist in the follow-up of arrhythmia-related events, potentially leading to an underestimation of event incidence. Additionally, the papillary muscles were excluded from the myocardial mass analysis but were included in the volume analysis; this may lead to a slight underestimation of LVMI and a slight overestimation of LVEDVI/LVESVI. Lastly, we did not include native T1 and extracellular volume fraction in this study, which have been shown to be associated with the prognosis of patients with HCM.49 Because we aimed to use LACI as a simple and practical indicator to assess the prognosis of HCM, this study did not utilize tissue characterization parameters. Further studies are warranted to incorporate these tissue characterization parameters to provide a more comprehensive assessment of myocardial tissue characteristics and its impact on clinical outcomes in HCM patients.
In conclusion, LACI is independently associated with adverse clinical outcomes in HCM and is superior to other LA functional/structural parameters and LGE extent on identifying HCM high-risk patients. LACI provides a simple approach to identify HCM patients at high risk for clinical adverse outcomes in clinical practice.
Conflict of interest statement
Xinwei Tao is a current employee of Bayer. The other authors have no conflicts of interest to declare.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No. 82272109).




