Early genetic screening and cardiac intervention in patients with cardiomyopathies in a multidisciplinary clinic
Abstract
Aims
Patients with cardiomyopathies are a heterogeneous group of patients who experience high morbidity and mortality. Early cardiac assessment and intervention with access to genetic counselling in a multidisciplinary Cardiomyopathy Clinic may improve outcomes and prevent progression to advanced heart failure.
Methods and results
Our prospective cohort study was conducted at a multidisciplinary Cardiomyopathy Clinic with 421 patients enrolled (42.5% female, median age 58 years), including 224 patients with dilated cardiomyopathy (DCM, 42.9% female, median age 57 years), 72 with hypertrophic cardiomyopathy (HCM, 43.1% female, median age 60 years), 79 with infiltrative cardiomyopathy (65.8% female, median age 70 years) and 46 who were stage A/at risk for genetic cardiomyopathy (54.3% female, median age 36 years). Patients were seen in follow-up at a median of 18 months. A pathogenic/likely pathogenic variant was identified in 28.5% of the total cohort, including 33.3% of the DCM cohort (28% TTN mutations) and 34.1% of the HCM cohort (60% MYBPC3 and 20% MYH7) who underwent genetic testing. The use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers/angiotensin receptor neprilysin inhibitor (48.3–69.5% of total cohort, P < 0.001), β-blockers (58.4–72.4%, P < 0.001), mineralocorticoid receptor antagonists (33.9–41.4%, P = 0.0014) and sodium/glucose cotransporter-2 inhibitors (5.3–27.9%, P < 0.001) all increased at follow-up. Precision-based therapies were also implemented, including tafamidis for transthyretin amyloidosis (n = 21), enzyme replacement therapy for Fabry disease (n = 14) and mavacamten (n = 4) for HCM. Optimization of medications and devices resulted in improvements in left ventricular ejection fraction (LVEF) from 27% to 43% at follow-up for DCM patients with reduced LVEF at baseline (P < 0.001) and reduction in left ventricular mass index (LVMI) from 156 g/m2 to 128 g/m2 at follow-up for HCM patients with abnormal LVMI at baseline (P = 0.009). Optimization of therapies was associated with stable plasma biomarkers in stage B patients while lowering levels of BNP (619–517.5 pg/mL, P = 0.048), NT-proBNP (777.5–356 ng/L, P < 0.001) and hsTropT (31–22 ng/L, P = 0.005) at follow-up relative to baseline values for stage C patients. Despite stage B patients having overt cardiomyopathy at baseline, stage A and B patients had a similarly high probability of survival (χ2 = 0.204, P = 0.652). The overall cardiovascular mortality rate was low at 1.7% for the cohort (0.5% for stage B and 3.3% for stage C) over a median of 34-month follow-up.
Conclusion
Our study demonstrates that a multidisciplinary cardiomyopathy clinic can improve the clinical profiles of patients with diverse genetic cardiomyopathies.
Introduction
Genetic cardiomyopathies are inherited diseases of the myocardium that often progress to left ventricular dysfunction and heart failure (HF).1, 2 This includes primary cardiomyopathies such as dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM) and secondary cardiomyopathies such as infiltrative cardiomyopathies.1-3 Early genetic testing is crucial in identifying disease in asymptomatic individuals for protective cardiac interventions, including device and medical therapies. Specifically, starting reverse remodelling therapies, such as guideline-directed medical therapy (GDMT) and primary prevention implantable cardiac defibrillators (ICDs) in high-risk individuals may prevent progression to more advanced stages of HF and reduce the risk of sudden cardiac death.3-5
Cardiovascular genetics is a relatively new field; many healthcare sites have yet to adopt this combined multidisciplinary approach.6 To address this gap, our Cardiomyopathy Clinic (CMC) at the Mazankowski Alberta Heart Institute was established in 2016 to provide cardiovascular care and streamline genetic testing for patients with suspected genetic cardiomyopathies. We hypothesized that early interventions may prevent or delay the progression of American Heart Association (AHA) stage A/at-risk and stage B/pre-HF patients to more advanced stages of disease.
Methods
The CMC occurs weekly and consists of a multidisciplinary team of cardiologists, a nurse practitioner, a clinical pharmacist, a registered dietician, a social worker and genetic counsellors. Inclusion criteria included patients with suspected cardiomyopathy (CM), including a family history of CM/sudden unexplained death, or patients with suspected genetic CM per the European Society of Cardiology (ESC) working group definition of CM (n = 421). They were recruited from the multidisciplinary CMC between January 2016 and November 2023. The ESC working group definition of CM for patients with suspected genetic CM included patients with myocardial disorder not explained by coronary artery disease, hypertension, valvular disease, drug toxicity or congenital heart disease.7 Patients referred to the CMC meeting the preceding criteria were approached for enrolment into our prospective study at their first clinic visit. Patients who were less than 18 years old at the time of enrolment were excluded from the study. All patients provided informed written consent before enrolment.
Clinical characterization
Patients were categorized into cohorts of cardiomyopathies utilizing guideline-based definitions.7 DCM was defined as LV dilation (LVIDd > 5.6 cm)/systolic dysfunction (LVEF ≤ 49%) not explained by abnormal loading conditions (hypertension, valvular disease, congenital heart disease) or ischemic heart disease alone. HCM was defined as the presence of increased LV mass (>95 g/m2 for women and >115 g/m2 for men) or wall thickness (≥1.5 cm) not explained by abnormal loading conditions alone. Infiltrative CMs were defined by abnormal deposition of substances (on cardiac imaging) in heart tissue, leading to diastolic dysfunction. Infiltrative CM patients with suspected Fabry disease (FD) based on imaging underwent genetic testing to confirm the diagnosis, while transthyretin amyloidosis (ATTR) was confirmed with nuclear medicine pyrophosphate scans and all patients with confirmed ATTR underwent genetic testing to look for hereditary vs. wild-type disease. Patients were also categorized based on the American Heart Association (AHA) stages of HF as follows: patients at risk for CM/HF (stage A), patients with early CM/pre-HF (stage B), patients with overt CM/HF (stage C) and patients with advanced CM/HF (stage D).5 A prospective chart review was performed to obtain patient demographics, medication/device utilization, medical history, genetic testing results and longitudinal clinical data for our cohort of patients at patient enrolment (baseline) and follow-up visits. An automatic sphygmomanometer was utilized to measure blood pressure at baseline and follow-up clinic visits. Biochemical (BNP, NT-proBNP, hsTropT), electrocardiogram (ECG), transthoracic echocardiography (TTE) and cardiac magnetic resonance imaging (CMR) data were reviewed to form detailed patient profiles. Follow-up was not standardized across the cohort and rather varied pending clinical status; the median time to follow-up clinic visit was 18 months (Table 1).
| Patient | DCM | HCM | Infiltrative CM | Stage A/at risk for CM | Total |
|---|---|---|---|---|---|
| Characteristics | (n = 224) | (n = 72) | (n = 79) | (n = 46) | (n = 421) |
| Follow-up, months | 18 (12–24) | 18 (9.5–25.5) | 21 (10.5–29.8) | 20.5 (17.5–26) | 18 (12–26) |
| Age, years | 57 (44–66) | 60 (51–70.8) | 70 (59–79) | 36 (30–49.5) | 58 (44–69) |
| Sex, n | |||||
| Male | 128 (57.1%) | 41 (56.9%) | 27 (34.2%) | 21 (45.7%) | 242 (56.8%) |
| Female | 96 (42.9%) | 31 (43.1%) | 52 (65.8%) | 25 (54.3%) | 179 (42.5%) |
| AHA stage | |||||
| Stage A | 0 (0%) | 0 (0%) | 0 (0%) | 46 (100%) | 46 (10.9%) |
| Stage B | 105 (46.9%) | 46 (63.9%) | 36 (45.6%) | 0 (0%) | 187 (44.4%) |
| Stage C | 117 (52.2%) | 26 (36.1%) | 40 (50.6%) | 0 (0%) | 183 (43.5%) |
| Stage D | 2 (0.9%) | 0 (0%) | 3 (3.8%) | 0 (0%) | 5 (1.2%) |
| Race | |||||
| White | 192 (85.7%) | 63 (87.5%) | 70 (88.6%) | 40 (87.0%) | 365 (85.7%) |
| Black | 8 (3.6%) | 3 (4.2%) | 5 (6.3%) | 2 (4.3%) | 18 (4.2%) |
| East Asian | 7 (3.1%) | 1 (1.4%) | 1 (1.3%) | 0 (0%) | 9 (2.1%) |
| South Asian | 4 (1.8%) | 4 (5.6%) | 1 (1.3%) | 1 (2.2%) | 10 (2.3%) |
| Latin | 3 (1.3%) | 1 (1.4%) | 1 (1.3%) | 0 (0%) | 5 (1.2%) |
| Middle Eastern | 5 (2.2%) | 0 (0%) | 1 (1.3%) | 1 (2.2%) | 7 (1.6%) |
| Indigenous | 5 (2.2%) | 0 (0%) | 0 (0%) | 2 (4.3%) | 7 (1.6%) |
| Comorbidities, n | |||||
| Hypertension | 45 (20.1%) | 23 (31.9%) | 23 (29.1%) | 6 (13%) | 97 (23%) |
| Dyslipidaemia | 42 (18.8%) | 20 (27.8%) | 19 (24.1%) | 5 (10.9%) | 86 (20.4%) |
| Diabetes mellitus | 43 (19.2%) | 10 (13.9%) | 12 (15.2%) | 3 (6.5%) | 68 (16.2%) |
| CAD | 22 (9.8%) | 4 (5.6%) | 7 (8.9%) | 0 (0%) | 33 (7.8%) |
| CKD | 13 (5.8%) | 8 (11.1%) | 11 (13.9%) | 0 (0%) | 32 (7.6%) |
| Atrial fibrillation | 44 (19.6%) | 13 (18.1%) | 29 (36.7%) | 2 (4.3%) | 88 (20.9%) |
| Family history of CM/SCD | |||||
| Positive | 66 (29.5%) | 16 (22.2%) | 4 (5.1%) | 46 (100%) | 132 (31.4%) |
| Negative | 158 (70.5%) | 56 (77.8%) | 75 (94.9%) | 0 (0%) | 289 (68.6%) |
| Medications, n | |||||
| ACEi/ARB/ARNI | 136 (60.7%) | 24 (33.3%) | 38 (48.1%) | 4 (8,7%) | 202 (48%) |
| BB | 169 (75.4%) | 42 (58.3%) | 34 (43%) | 1 (2.2%) | 246 (58.4%) |
| MRA | 116 (51.8%) | 13 (18.1%) | 15 (19%) | 0 (0%) | 144 (34.2%) |
| SGLT2i | 19 (8.5%) | 1 (1.4%) | 4 (5.1%) | 0 (0%) | 24 (5.7%) |
| Vitals | |||||
| SBP, mmHg | 119 (109–127) | 122.5 (114.3–133.8) | 120 (108–135) | 121 (110–128) | 120 (110–130) |
| DBP, mmHg | 69 (63–76) | 73.5 (65–80) | 70 (62–77) | 73 (65.5–80) | 70 (63–78) |
| Biomarkers | |||||
| BNP, pg/mL | 314 (43.5–933) | 417 (92.5–691) | 411.5 (235–762.5) | 21 (16–50.5) | 324 (70–795) |
| NT-proBNP, ng/L | 467 (104.3–1039.5) | 373 (116–882) | 357 (136.5–830.75) | 43.5 (11–407.25) | 414 (109–946) |
| hsTropT, ng/L | 21 (9–46) | 24 (11.5–59) | 34.5 (24.5–96.75) | 5.5 (5–13.25) | 26 (9–51.3) |
| 12-lead ECG | |||||
| Heart rate, bpm | 67 (60–82) | 63 (57.8–69) | 69 (60–78.3) | 68 (59.5–78) | 66.5 (59–79) |
| QRS duration, ms | 110 (98–142) | 105 (94–154.5) | 105.5 (95–146.8) | 91 (85–101) | 106 (94–141) |
| QTc interval, ms | 454 (429–485) | 455.5 (432–487.5) | 464.5 (438–489) | 414 (398.5–441) | 452 (426–483) |
| First-degree AVB | 44 (19.6%) | 19 (26.4%) | 17 (21.5%) | 3 (6.5%) | 83 (19.7%) |
| LBBB | 43 (19.2%) | 14 (19.4%) | 6 (7.6%) | 0 (0%) | 63 (15%) |
| RBBB | 10 (4.5%) | 3 (4.2%) | 7 (8.9%) | 0 (0%) | 20 (4.8%) |
| Echocardiography | |||||
| LVIDd, cm | 5.7 (5.1–6.3) | 4.5 (3.9–5.2) | 4.5 (4.1–5) | 4.7 (4.1–4.9) | 5.2 (4.5–5.9) |
| LVIDs, cm | 4.5 (3.9–5.4) | 3 (2.4–3.5) | 3.2 (2.8–3.8) | 2.7 (2.6–3.5) | 3.9 (3–4.8) |
| LVPWd, cm | 1.0 (0.8–1.1) | 1.2 (1–1.3) | 1.4 (1.1–1.6) | 0.9 (0.8–1) | 1 (0.9–1.2) |
| LVEF, % | 38 (25–49) | 60 (55–60) | 50 (40–60) | 60 (57.5–65) | 47.5 (32–57.5) |
| LVMI, g/m2 | 105.7 (90.1–122) | 129.1 (95–161.1) | 126 (109.1–151.4) | 73.1 (60.8–86.9) | 108.1 (88.9–135.3) |
| E/e′ ratio | 10.7 (8.2–14.5) | 14.3 (10.1–16.7) | 14.4 (11–18.3) | 7.9 (5.6–9.8) | 11.6 (8.4–15.4) |
| LAVI, mL/m2 | 33.5 (25.9–42.8) | 38.2 (28.7–49.3) | 38 (29.7–49.6) | 23.4 (19.0–28.7) | 34 (26.0–43) |
| TAPSE, cm | 2 (1.6–2.4) | 2.2 (1.8–2.6) | 1.6 (1.4–2) | 2.2 (2–2.5) | 2 (1.6–2.4) |
| Cardiac MRI | |||||
| LAVI, mL/m2 | 54.2 (40.0–67.0) | 44.5 (30.2–49.9) | 43.5 (37.7–69.5) | 35.2 (30.7–49.1) | 34 (26.0–43) |
| LVEDVi, mL/m2 | 111 (95–137) | 72.5 (59–82) | 81.5 (70–93) | 73 (68–92) | 92 (72.5–114.5) |
| LVESVi, mL/m2 | 78.5 (50.3–107.5) | 28 (20.5–37.5) | 34.5 (29–47.3) | 31 (25.3–37.5) | 44 (30.8–79) |
| LVEF, % | 34.5 (20.3–47.8) | 61.5 (55–67) | 56.5 (47.5–61.8) | 59 (53.5–63.8) | 50 (34–59) |
| LVMI, g/m2 | 65 (54–84) | 75 (61–103) | 82 (67.5–102.3) | 51.5 (47–64) | 68 (55–88.3) |
| RVEDVi, mL/m2 | 81 (67–100) | 65 (53–72) | 77.5 (68.5–85.5) | 75 (59–83.3) | 76 (65–93) |
| RVESVi, mL/m2 | 45 (33–59) | 21 (20–33) | 39.5 (24.8–46.8) | 29 (22.3–38.5) | 38.5 (26–50) |
| RVEF, % | 47 (31–53) | 60.5 (53.3–65.5) | 55 (41.3–63.8) | 58 (51.3–66.5) | 52 (41.8–59) |
- Data are expressed as number (percentage) or median (interquartile range).
- ACEi/ARB/ARNI, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers/angiotensin receptor-neprilysin inhibitor; AVB, atrioventricular block; BB, β-blockers; BNP, brain natriuretic peptide; CAD, coronary artery disease; CKD, chronic kidney disease; CM, cardiomyopathy; DBP, diastolic blood pressure; DCM, dilated cardiomyopathy; ECG, electrocardiogram; HCM, hypertrophic cardiomyopathy; hsTropT, high-sensitivity troponin T; LAVI, left atrial volume index; LBBB, left bundle branch block; LVEDVi, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVi, left ventricular end-systolic volume index; LVIDd, left ventricular internal dimension at end-diastole; LVIDs, left ventricular internal dimension at end-systole; LVMI, left ventricular mass index; LVPWd, left ventricular posterior wall thickness at end-diastole; MRA, mineralocorticoid receptor antagonists; NT-proBNP, N-terminal pro-brain natriuretic peptide; RBBB, right bundle branch block; RVEDVi, right ventricular end-diastolic volume index; RVEF, right ventricular ejection fraction; RVESVi, right ventricular end-systolic volume index; SBP, systolic blood pressure; SCD; sudden cardiac death; SGLT2i, sodium-glucose co-transporter-2 inhibitors; TAPSE, tricuspid annular plane systolic excursion.
Cardiac imaging
Patients underwent cardiac imaging typically within 6 months of enrolment. Two-dimensional transthoracic echocardiography (TTE) was utilized to assess left ventricular size and function (Simpson's biplane formula with echocardiography enhancing agents), left ventricular mass (Teichholz formula) and left atrial volume (biplane formula). Right ventricular function was assessed by tricuspid annular plane systolic excursion (TAPSE) and diastolic function by Doppler echocardiography (E/e′). The American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) guideline definitions were used for chamber quantification and subsequent sub-group classification and analyses.8 Cardiac magnetic resonance (CMR) utilizing conventional cine imaging and standardized local CMR imaging protocols were used to assess biventricular volumes, mass and function.
Genetic counselling and testing
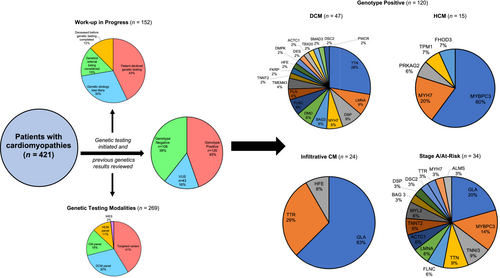
Genetic counsellors at our CMC provided pre-test counselling to review family history, discuss genetic testing modalities and review possible results/implications from testing. Various genetic testing methods were utilized, and previous results were reviewed for patients with genetic testing before clinic enrolment (Figure 1). Genetic testing included single gene, targeted variant, dedicated cardiology gene panels or whole exome sequencing as determined by the genetic counsellors based on family history and in conjunction with each patient. Standardized CM (217 genes), DCM (130 genes) and HCM (92 genes) panels were utilized, and the complete list of genes tested can be accessed via Blueprint Genetics. Patients with HCM and typical symptoms for ATTR-CM, including unexplained neuropathy, carpal tunnel syndrome, distal biceps tendon tear or cardiac imaging findings suggestive of amyloidosis went for nuclear medicine pyrophosphate scanning and genetic testing to rule out ATTR-CM. Post-test counselling was provided to disclose the genetics results and cascade screening was offered where indicated (pathogenic/likely pathogenic or variants of unknown significance), which included early cardiac testing (ECGs and cardiac imaging through TTE) and targeted variant testing for family members. Family studies were considered for variants of uncertain significance if there was a significant family history or where establishing de novo status would be helpful in variant reclassification. Variant classifications used were in line with the American College of Medical Genetics and Genomics (ACMG) 2015 guidelines for the interpretation of sequence variants.9 Specifically, evidence was based on the type of variant (i.e. missense, nonsense, frameshift, slice site), allele frequency in the gnomAD database, functional domain affected, in silico analysis and if the variant has been reported in ClinVar or other published literature.9, 10 In addition, disease-specific variant classification was used for dilated cardiomyopathy.11 Patients who underwent genetic testing with negative results received post-test counselling, and whole exome sequencing was considered for certain patients at the discretion of our genetic counsellors and medical geneticists in conjunction with our cardiologists.
Clinical outcome data
The province of Alberta has an integrated healthcare system, whereby a patient's unique lifetime identifier can link patient encounters anywhere with the healthcare system.12, 13 Health administrative data including the Discharge Abstract Database (DAD), National Ambulatory Care Reporting System (NACRS), Provincial Registry (REG) and Vital Statistics (VS) were used to ascertain clinical outcome data. The DAD utilizes the International Classification of Diseases, Tenth Revision, and Canadian Enhancement (ICD-10-CA) codes to collect information on acute care hospitalizations and includes up to 25 ICD-10 codes for diagnoses, including a Most Responsible Diagnosis and up to 24 secondary codes. The NACRS utilizes up to 10 ICD-10 codes to classify emergency department (ED) visits and hospital-based ambulatory care. REG and VS data were used to determine the cardiovascular (CV) or all-cause death date. Patient outcome data were collected from their first clinic visit until death or when censored at the end of the follow-up duration.
Data analysis
Continuous variables are expressed as median (interquartile range) and categorical variables as number (%) unless otherwise specified. Categorical variables were compared at baseline and follow-up using Pearson chi-square tests. Continuous variables were analysed at baseline and follow-up using the Wilcoxon signed-rank test. Clinical outcome data were analysed for our patient cohorts using Kaplan–Meier survival curve analyses and log-rank testing. Two-sided P < 0.05 was considered significant, and analyses were conducted using IBM SPSS Statistics 28.0.
Ethics
The University of Alberta Health Research Ethics Board approved this study (Pro00077124), and our work was performed per the Declaration of Helsinki and was reported as recommended by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Figure S1A).
Results
Baseline clinical characteristics
Patients (n = 421) were referred to the multidisciplinary CMC from various sources, including medical genetics, heart rhythm device clinic, paediatric cardiology and adult cardiology clinics (Figure S1B). Most patients were diagnosed with DCM (n = 224), followed by infiltrative CM (n = 79) and HCM (n = 72) (Table 1). At baseline, most of our cohort was classified as stage B/pre-HF (n = 187) or stage C/HF (n = 183), followed by stage A/at risk for HF (n = 46), while only five patients were at the end stage of HF (stage D).5, 14
HCM patients had a high frequency of first-degree atrioventricular (AV) block (26.4%, Table 1). The prevalence of atrial fibrillation was similar between DCM and HCM cohorts at 19.6% and 18.1%, respectively. TTE demonstrated that DCM patients had left ventricular (LV) dysfunction (LVEF 38%) without evidence of diastolic dysfunction (Table 1). In contrast, HCM patients had preserved function (LVEF 60%), increased LVMI (129.1 g/m2), increased LAVI (38.2 mL/m2) and impaired diastology (E/e′ ratio of 14.3). Similarly, infiltrative CM patients had increased LVMI (126 g/m2), increased LAVI (38 mL/m2) and impaired diastology (E/e′ ratio 14.4).
Genetic testing
After being seen at the CMC, 63.9% (n = 269) had genetic testing completed (compared to only 25.4% before enrolment, P < 0.001) (Figure 1). The remaining 152 patients (36.1%) did not have genetic results, as 65 (15.4%) declined testing, 23 (5.4%) are being considered for a genetics referral and 19 (4.5%) were deceased before submitting a sample for testing. The remaining 45 patients (10.7%) were found to have alternative explanations for their cardiac disease, including toxin-mediated, undiagnosed hypertension and undiagnosed arrhythmia burden in combination with a negative family history following a detailed review by a cardiologist and genetic counsellor. Patients being considered for a genetics referral included patients still undergoing additional investigations, including 12-lead ECG, Holter monitoring and CMR to elucidate aetiology before proceeding with genetic testing. For patients with completed genetic testing (n = 269), 39.4% (n = 106) had negative genotypes, while 44.6% (n = 120) and 16.0% (n = 43) had positive genotypes (pathogenic/likely pathogenic) and variants of unknown significance (VUS), respectively (Figure 1 and Table S1). Various genetic testing modalities were used for each cohort, with targeted variant testing being the most common (Table S2). In patients with DCM, 47 (33.3%) had positive genotypes and 33 (23.4%) had VUS, while 61 (43.3%) had negative results. DCM patients with positive genotypes were heterogeneous, but TTN mutations were the most common (28%). For HCM patients, 15 (34.1%) had positive genotypes, 5 (11.4%) had VUS, and 24 (54.5%) had negative results. HCM patients with positive genotypes were more homogenous, with MYBPC3 (60%) and MYYH7 (20%) mutations being most common. Amongst patients at-risk for CM, 34 (82.9%) had positive genotypes, two (4.9%) had VUS, and five (12.2%) had negative results. Amongst the infiltrative CM cohort, 15 patients had genetically confirmed (GLA mutations) FD, and there were seven patients with hereditary transthyretin amyloidosis out of 29 patients with transthyretin amyloidosis (ATTR).
Optimization of medications, cascade screening and devices
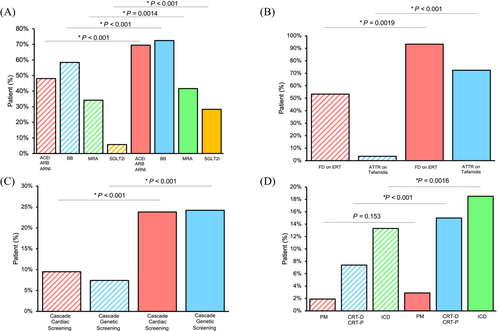
The initiation and/or up-titration of pharmacological therapies, cascade family screening and cardiac devices were implemented at clinician discretion rather than by protocol (Figure 2). The ESC 2023 HF guidelines were utilized to guide clinical decision-making.15 Notably, patients with HFrEF (LVEF ≤ 40%) were initiated and up-titrated on quadruple therapy (ACEi/ARB/ARNI, β-blockers, MRA, and SGLT2i). While patients with HFmrEF (LVEF 41–49%) were started on SGLT2i (class I indication), ACEi/ARB/ARNI (IIb), MRA (IIb) and β-blockers (IIb). Finally, patients with HFpEF (LVEF ≥ 50%) were started on started on SGLT2i (class I), while the aetiology of HF and comorbidities were aggressively managed in these patients, including hypertension, with ACEi/ARB/ARNI, β-blockers and MRA (class I). Patients at risk for CM/HF (stage A) and patients with early CM/pre-HF (stage B) had their comorbidities managed quite aggressively (typically hypertension) with early initiation of ACEi/ARB/ARNI or β-blockers, which is not yet reflected universally in HF or CM guidelines. The use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers/angiotensin receptor neprilysin inhibitor (ACEi/ARB/ARNI, P < 0.001), β-blockers (BB, P < 0.001), mineralocorticoid receptor antagonists (MRA, P = 0.0014) and sodium/glucose cotransporter-2 inhibitors (SGLT2i, P < 0.001) all increased over baseline at follow-up clinic visits (median of 18 months; Figure 2). Enzyme replacement therapy (ERT) was initiated on 14 of the 15 patients (93.3%) with FD, and tafamidis was initiated on 21 of the 29 patients (72.4%) with ATTR (Figure 2). 59.6% of stage C and D patients (112 out of 188 patients) were on scheduled furosemide after their baseline clinic visit with a median dose of 40 mg of furosemide daily. Stage A and B patients had not developed overt HF and were not on maintenance diuretic therapies. Cascade family screening, which includes performing genetic testing, ECG or cardiac imaging on otherwise asymptomatic patients, has been a cornerstone of the management practices in our CMC. Forty patients (9.5%) had cascade cardiac screening at baseline, and another 100 patients (23.8%) had cascade cardiac screening completed after clinic attendance (P < 0.001; Figure 2). Additionally, cascade genetic screening (as reflected in the pedigrees in Figure S3–S7) has increased from 31 patients (7.4%) at baseline to 54 patients (12.8%) after their initial clinic visit (P < 0.001). The use of ICDs was common in our cohort of patients, primarily to reduce the likelihood of sudden cardiac death (SCD) in these patients, with 78 patients (18.5%) having an ICD implanted after their initial clinic visit relative to 56 patients (13.3%) at baseline (P = 0.0016; Figure 2). Patients received ICD and CRT-D implantation for both primary and secondary indications per Canadian Cardiovascular Society (CCS) Heart Rhythm Society guidelines and the 2022 ESC guidelines for managing patients with ventricular arrhythmias and preventing sudden cardiac death.16, 17 CRT-D devices were implanted in patients with LVEF < 35% and QRSd > 130 ms due to left bundle branch blocks and following an adequate trial of guideline-directed medical therapy. Standard primary indications included implantation for patients with LVEF < 35% after 3 months of optimal medical therapy or patients. ICDs were also implanted for primary prevention in patients with clinical risk factors, specifically massive LVH ≥ 30 mm, history of suspected cardiac syncope, strong family history of sudden cardiac death and specific high-risk genetic mutations (lamin A/C being the most common). Secondary indications included cardiac arrest (VT-VF) and sustained VT in patients with significant structural heart disease in which there is no other clear reversible cause. Seventy-eight patients received ICDs, with 47 patients receiving an ICD for primary indications (Table S3), whereas 31 patients received an ICD for secondary indications. CRT-D devices were implanted in 63 patients.
Dilated cardiomyopathy, hypertrophic cardiomyopathy and infiltrative cardiomyopathy patients
To better characterize changes in imaging, guideline-based definitions for TTE chamber quantification were used to classify patients as ‘abnormal’ or ‘normal’ at baseline across imaging parameters, and follow-up cardiac imaging was performed at a median time of 24 months.8 Most DCM patients were on GDMT, with 29% of patients on triple therapy (ACEi/ARB/ARNI + BB + MRA) and 27.2% on quadruple therapy (ACEi/ARB/ARNI + BB + MRA + SGLT2i), and 44.8% had device therapies as well (Figure S2A). DCM patients with reduced ejection fraction (LVEF < 40%) at baseline showed marked improvements in LVEF from 27% at baseline to 43% at follow-up (P < 0.001; Figure 3). DCM patients with abnormal TAPSE (≤1.6 cm) at baseline, TAPSE improved from 1.3 to 1.9 cm at follow-up (P < 0.001; Figure 3). For DCM patients, there was an improvement in LVEF for both stage B (P = 0.004) and C patients (P < 0.001; Figure 3) and a reduction in LVIDd for stage C patients (P = 0.002), which reached borderline significance in stage B patients (P = 0.056; Figure 3).
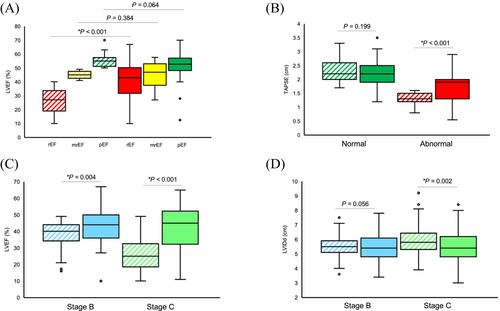
Fewer HCM patients were on quadruple (2.8%) or triple therapy (6.9%), with most of these patients being on some combination of ACEi/ARB/ARNI and/or β-blockers (Figure S2B). However, 52.8% of HCM patients received device therapy (31.9% had an ICD), and four patients were started on mavacamten. HCM patients had a median resting left ventricular outflow tract (LVOT) gradient of 10.4 mmHg, with 14 of 72 HCM patients (19.4%) being obstructive (LVOT ≥ 30 mmHg), while 11 patients (15.3%) had provocable gradients with Valsalva. Additionally, HCM patients with abnormal LVMI (normal range: 43–95 g/m2 for women and 49–115 g/m2 for men) at baseline had a reduction in LVMI improving from 156 to 128 g/m2 at follow-up (P = 0.009; Figure 4). HCM patients with abnormal E/e′ ratio (>15) showed a reduction in median E/e′ from 16.5 to 14.5 (P = 0.020; Figure 4). Stage B patients with abnormal LVMI at baseline had improvement in LVMI at follow-up from 156 to 123 g/m2 at follow-up (P = 0.013), while stage C patients had no further progression (Figure 4). In HCM patients with two clinic visits, a reduction in mean arterial pressure (MAP) from a median of 90 mmHg at baseline to 86.8 mmHg at follow-up (P = 0.047) was observed (Figure 4). HCM patients with abnormal LVMI at baseline but strict blood pressure control (SBP ≤ 120 mmHg) at follow-up had significant reductions in their LVMI from 165.9 g/m2 at baseline to 142.3 g/m2 at follow-up (P = 0.032). In comparison, those with more lenient SBP control (>120 mmHg) did not demonstrate such robust improvements (P = 0.194).
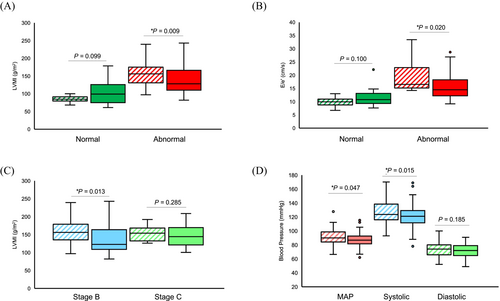
Most infiltrative CM patients were initiated on disease-specific therapies, such as ERT (93.3% of FD patients) and tafamidis (72.4% of ATTR patients; Figure S2C). There was a trend towards statistical significance for improvements in LVEF (P = 0.088) and LVMI (P = 0.064) for ATTR patients on tafamidis, while FD patients on ERT showed stability on imaging. Although CMR was not performed for all patients, 132 patients received CMR, and amongst these patients, 62 patients (47%) had evidence of late gadolinium enhancement.
Biomarkers
The levels of plasma biomarkers (BNP, NT-proBNP and hsTropT) increased in a stepwise fashion from pre-symptomatic (stage A) to symptomatic (stage C) patients across all CM phenotypes. Stage A patients had the lowest BNP (21 pg/mL), NT-proBNP (43.5 ng/L) and hsTropT (5.5 ng/L) values at baseline relative to stage B and stage C patients. The optimization of cardiac medications and device therapies was associated with stable plasma biomarkers in stage B patients while lowering levels of BNP (619–517.5 pg/mL, P = 0.048), NT-proBNP (777.5–356 ng/L, P < 0.001) and hsTropT (31–22 ng/L, P = 0.005) at follow-up relative to baseline values for stage C patients (Figure 5).
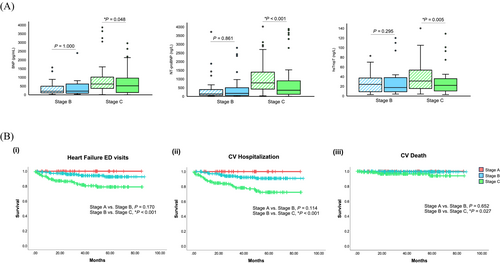
Clinical outcomes
Concordant with the improvements in plasma biomarkers, we observed low rates of cardiovascular hospitalization and ED visits. At the median follow-up time of 34 months, 35 patients (8.3% of the total cohort) had HF ED visits (8 stage B patients, 25 stage C patients and 2 stage D patients). Additionally, the mean time to first HF ED visit was 71.7 months for stage C (95% CI: 66.9–76.5) relative to 83.1 months (95% CI: 80.4–85.8) for stage B patients (χ2 = 13.304, P < 0.001), whereas there was no difference for stage B relative to stage A patients (χ2 = 1.882, P = 0.170; Figure 5). There were 46 patients (10.9%) with a cardiovascular hospitalization (CV hospitalization) at 34 months (11 stage B patients, 31 stage C patients and 4 stage D patients). The mean time to first CV hospitalization was 68.0 months (95% CI: 62.8–73.2) for stage C relative to 81.8 months (95% CI: 78.8–84.8) for stage B patients (χ2 = 16.710, P < 0.001), whereas there was no significant difference for stage B relative to stage A patients (χ2 = 2.495, P = 0.114; Figure 5). Stage B patients had a higher probability of survival relative to stage C patients (χ2 = 4.910, P = 0.027) and had a similar survival probability to stage A patients (χ2 = 0.204, P = 0.652; Figure 5). The CV mortality rate was low at 1.7% for the cohort (0.5% for stage B and 3.3% for stage C), and the all-cause mortality rate was 9.2% (4.8% for stage B and 13.7% for stage C) over a median follow-up time of 34 months. Additionally, only two patients (0.5%) in our cohort received heart transplantations throughout the follow-up period. Despite overall low hospitalization rates and mortality, there were 30 patients (7.1% of the total cohort) with a new incidence of HF (13 HFrEF patients, four HFmrEF patients and 13 HFpEF patients) across the entire cohort, indicating a progression from stage A/B to stage C at their follow-up clinic visits. This included 17 DCM patients (56.6%), three HCM patients (10.0%), nine (30.0%) infiltrative CM patients and one at risk for CM patient (3.3%). Additionally, nine patients who were at risk for CM/stage A progressed to stage B.
Discussion
Patients with genetic cardiomyopathies represent a precarious patient population since many remain asymptomatic for an extended time despite carrying a high risk for SCD or the development of HF. A multidisciplinary approach to the care of these patients has been recommended, leading to the establishment of cardiovascular genetics clinics worldwide.7 However, our study appears to be amongst the first to report longitudinal clinical outcomes within this diverse patient group. Additionally, previous work has focussed on clinical outcomes for CM patients with advanced HF at baseline (NYHA III/IV) with fewer studies for patients with ESC ‘at-risk’ and AHA stage A/B disease,5, 18-20 despite evidence that patients with early disease also exhibit higher mortality than the general population.21, 22 Therefore, we established a referral service for a large catchment in Western Canada to provide genetic testing and HF care for patients with cardiomyopathies.23, 24 Our prospective study outlines the yield of genetic testing, as well as the effectiveness of a multidisciplinary model to streamline genetic testing, cascade screening and the optimization of therapies. We also demonstrate substantial improvements in specific cardiac imaging parameters, reductions in levels of plasma biomarkers and overall low-event rates.
Our CM cohort contained a high proportion of DCM patients relative to HCM, which is in line with recent estimates that DCM is more common than previously recognized with prevalence rates approaching 1:250 in the general population,25 compared to the HCM prevalence of 1:200.26 Amongst patients with genetic testing completed, 120 patients (44.6%) had positive genotypes (pathogenic or likely pathogenic) likely owing to inclusion of patients who were at risk for CM where the prevalence of positive genetics results was relatively high (82.9% with positive genotypes). However, 47 DCM patients (33.3% of those tested) had positive genotypes, and 15 HCM patients (34.1% of those tested) had positive genotypes, which aligns with previous findings.21, 23 Of note, 65 patients (15.4% of the total cohort) declined genetic testing, and less than 25% of the cohort has had cascade screening completed at follow-up. Reasons for declining testing and cascade screening included fear/anxiety of results, insurance discrimination and patient disinterest. Attending pre-test counselling with our genetics counsellors has helped alleviate some of these concerns, but further patient education and research will be required to address the hesitancy surrounding genetic testing. However, we have enrolled five multi-generational families with distinct BAG3, LMNA, ACTC1, FLNC and MYH7 mutations, emphasizing the potential role of cascade screening in this vulnerable patient population.
Patients with DCM had high rates of atrial fibrillation, AV block and left bundle branch block, demonstrating the negative impact of myocardial disease in disrupting normal heart conduction and rhythm.27 Hypertension was common and, in combination with the underlying genetic architecture of these cardiomyopathies, can exacerbate patients from asymptomatic or pre-symptomatic HF into an overt HF phenotype.4, 28-30 Overall, patients with abnormal imaging parameters at baseline demonstrated improvement at follow-up. In contrast, patients who were within normal ranges at baseline did not have progression of their CM on imaging. Despite improvement in imaging parameters, 30 patients (7.1%) still developed new-onset HF at follow-up. We also recruited patients at risk for developing CM (stage A) but without overt CM at baseline. Many of these patients have been initiated on low-dose BB (i.e. bisoprolol 1.25 mg daily) and/or ACEi/ARBs (i.e. ramipril 1.25 mg daily) to prevent progression from at-risk for HF (stage A) to pre-HF (stage B), which is not yet recommended in the most recent CM clinical guidelines.5, 7, 14 Importantly, 10 (22%) patients within this at-risk cohort (n = 46) have developed evidence of overt CM at follow-up, further emphasizing the need to identify this group of at-risk patients earlier, and may need to be reflected in future guidelines.
Although patients with stage B disease do not have overt HF, they can experience substantial mortality risk, even when only mild LV impairment is present.31, 32 Our study demonstrated that early intervention in this population allowed for reversal or stability in biomarkers and cardiac imaging parameters, associated with low mortality risk. Specifically, there was evidence of LVEF recovery in stage B and stage C DCM patients and improvement in LVMI in stage B HCM patients. Initiation of medications facilitated aggressive BP control, which may have driven cardiac remodelling within our cohort of HCM patients. Importantly, hypertension may be quite common amongst individuals with HCM, and concomitant hypertension may play a pathogenic role in worsening the phenotype and prognosis.29, 30
The reverse remodelling seen on imaging also translated to improvement in plasma biomarkers at baseline compared to follow-up for stage C patients, while stage B patients showed stability in plasma biomarkers. With further longitudinal data, plasma biomarkers may be helpful in prognosticating disease trajectory amongst asymptomatic stage A and B patients to predict which patients may be at increased risk of rapid progression. Stage A/B patients with elevated BNP have a worse prognosis than stage C/D patients with lower BNPs, but our study was too small to investigate this hypothesis.33 Both imaging parameters and plasma biomarkers in our study suggested that stage B patients had more advanced disease relative to stage A patients at baseline. We suspect that initiating therapies early and aggressively in stage B patients delayed the progression of the disease, which is reflected in the low-event rate within this cohort. Previous natural history studies cited higher annual mortality rates between 2% and 6% for HCM patients and 12% and 15% for DCM patients with HF symptoms (stage C and higher).34, 35
As cardiovascular genetics evolves in the coming years, polygenic risk scores may eventually be used to quantify an individual's susceptibility to developing cardiomyopathy.28, 36 Disease-specific therapies, such as ERT for FD and tafamidis for ATTR, were up-titrated aggressively in our cohort of infiltrative CM patients. Novel therapies, including myosin inhibitors like mavacamten, represent an opportunity to continue advancing the precision-medicine approach for treating hypertrophic cardiomyopathy.37 Although our present work has demonstrated the preliminary benefits of a multidisciplinary approach to genetic cardiomyopathies, future work should aim to quantify the benefits of this approach in reducing long-term mortality, morbidity and quality-adjusted life years in this population.
Limitations
Our study does have several limitations. First, despite our cohort including a group of heterogeneous patients with suspected genetic cardiomyopathies, this is a single-site prospective study rather than a multicentre randomized control trial. There is no similar cohort of patients from our site that we could compare to our current cohort, and we, therefore, compared the outcomes in our clinic to previously described cohort studies. In a future study, it would be ideal to conduct a multi-site prospective randomized control trial comparing the current standard of care in a general cardiology clinic to that provided in a sub-specialized cardiovascular genetics clinic to draw more robust conclusions. Second, the patients enrolled in our study were low risk at baseline as many of our patients were referred from cardiology clinics or other specialist clinics where they were already receiving guideline-directed therapies. As a result, the number of adverse clinical outcomes may be partially underestimated in our cohort of patients. Third, given constraints on access to CMR at our site, not all patients routinely receive CMRs at baseline; thus, we cannot include a full complement of CMR data.
Conclusions
Patients with cardiomyopathies represent a diverse group of patients with an increased risk of morbidity and mortality. Identifying patients at risk for CM early in their disease and immediate cardiac assessment and interventions in this population can reduce CM-associated morbidity. This prospective cohort study demonstrates that a combined cardiovascular genetics clinic is associated with favourable clinical trajectories of cardiomyopathy patients.
Acknowledgements
The authors would like to recognize the physicians, nurses and other allied healthcare workers who provided and continue to provide care for the patients included in our study. We are also grateful to the patients and their families for their willing participation in this study.
Conflict of interest statement
None.
Funding
This work was supported by the Heart and Stroke Foundation of Canada. In-kind support was provided by the Alberta SPOR SUPPORT Unit for linkage to Alberta Health Services administrative health data.
Open Research
Data availability statement
To comply with Alberta's Health Information Act, the dataset used for this study cannot be made publicly available. The dataset from this study is held securely in coded form within the AbSPORU (Alberta Support for Patient Oriented Research Unit) Data Platform. While legal data sharing agreements between the investigators, AbSPORU and Alberta Health Services/Alberta Health prohibit us from making the dataset publicly available, access to de-identified data may be granted to those who meet pre-specified criteria for confidential access, available at www.absporu.ca, and on formal request to the corresponding author.




