Assessment of frailty in patients with heart failure: A new Heart Failure Frailty Score developed by Delphi consensus
Abstract
Aims
The Heart Failure Frailty Score (HFFS) is a novel, multidimensional tool to assess frailty in patients with heart failure (HF). It has been developed to overcome limitations of existing frailty assessment tools while being practical for clinical use. The HFFS reflects the concept of frailty as a multidimensional, dynamic and potentially reversible state, which increases vulnerability to stressors and risk of poor outcomes in patients with HF.
Methods and results
The HFFS was developed through a Delphi consensus process involving 54 international experts. This approach involved iterative rounds of questionnaires and interviews, where a panel of experts provided their opinions on specific questions prepared by the Steering Committee. The experts were invited to vote and share their views anonymously, using a 5-point Likert scale over iterative rounds. An 80% threshold was set for agreement or disagreement for each statement. Twenty-two variables from four domains (clinical, functional, psycho-cognitive and social) have been selected for inclusion in the HFFS after the third round of the Delphi process. A shorter version (S-HFFS), including 10 variables, has also been developed for daily clinical use.
Conclusions
The HFFS is a new multidimensional tool for the identification of frailty in patients with HF. It should also enables healthcare providers to identify potential ‘red flags’ for frailty in order to develop personalized care plans. The next step will be to validate the new score in patients with HF.
Introduction
Frailty is a significant global health challenge with significant implications for patients, clinical practice and public health. In patients with heart failure (HF), frailty is highly prevalent (45% overall)1 and is independently associated with poorer clinical outcomes.2-4 Patients with HF who are frail experience lower quality of life (QofL), along with increased risks of disability, dependence and cognitive decline compared with their nonfrail counterparts.2, 5
Several operational instruments6, 7 are available for assessing frailty in clinical practice, which can be categorized into those based on the Fried phenotype8 and those following the Rockwood multidimensional approach.9, 10 However, the practical application of these instruments in busy clinical settings is often hampered by their time-consuming nature and the need for specific tools. Consequently, despite guideline recommendations to monitor frailty in patients with HF,11, 12 assessments are frequently conducted using more subjective methods, such as the ‘eyeball test’ or ‘foot-of-the-bed’ assessment, or the semi-quantitative Clinical Frailty Score.6, 13
Additionally, the clinical and pathophysiological overlap between frailty and HF increases the risk of misclassification when using general frailty assessment tools not specifically designed for patients with HF. This misclassification can significantly impact patient management, especially since frail patients are at high risk of experiencing ‘frailtyism’,14 resulting in lack of consideration of indicated therapies despite evidence of benefit.
To overcome these problems, an international and multidisciplinary group of experts, including those from the Heart Failure Association, the Korean Heart Failure Society, and the Chinese Heart Failure Society, developed the new multidimensional Heart Failure Frailty Score (HFFS), agreeing on specific items to include in the four main domains (clinical, functional, psycho-cognitive and social), of frailty,11 using the consensus Delphi method.
Methods
The HFFS has been developed using a Delphi consensus methodology. This approach involved iterative rounds of questionnaires and interviews, where a panel of specialist experts in relevant fields provided their opinions on specific questions prepared by a Steering Committee.15-17 The goal was to achieve consensus through mutual sharing of opinions over multiple rounds.
The expert were selected on the basis of their clinical work with frail HF patients and/or on their publication record on frailty, from Europe, Asia and the Americas. The project included seven members of the Steering Committee (SA, AJSC, LH, EJ, GR, PS and CV) three of whom (LH, EJ and CV) responsible for drafting the initial round of questions. Another member (MT) assisted the Steering Committee in drafting and analysing the first round results.
An invitation letter outlining the project's aims and the Delphi process methodology was sent electronically to the group of those who accepted the invitation joined the Heart Failure Frailty Score group.
According to the Delphi methodology, to establish consensus on the parameters for constructing the HFFS, the participating experts were invited to share their views anonymously, using a 5-point Likert scale (‘1’: strong disagreement, ‘2’: disagreement, ‘3’: neutral stance, ‘4’: agreement and ‘5’: strong agreement). Consensus for each statement was defined as being achieved if the combined percentage of responses in categories ‘4’ and ‘5’ for positive agreement, or ‘1’ and ‘2’ for negative agreement, was equal or greater than 80%, in line with previous research utilizing this methodology.15 As other previous studies used a 70% agreement threshold, statements with consensus levels between 70% and 79% were further deliberated and reassessed.18 Statements that received less than 70% agreement were excluded from the HFFS.
The Delphi Consensus began on 5 March 2020 but was paused due to the COVID-19 pandemic and resumed in January 2023 (Figure 1) with the inclusion of additional international experts and using a digital format.
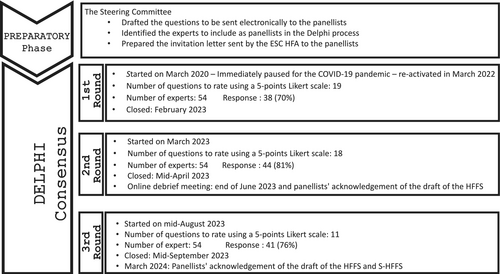
In total, 54 of the 63 invited experts agreed to join the project. They represented multiple specialties, settings and geographical areas (Table S1).
First round of the Delphi process
The first round of the Delphi process included 19 questions/statements. Four general statements addressed the definition of frailty and the need for an instrument tailored for patients with HF. The remaining questions pertained to the variables to be included in each of the four domains of the HFFS. Specifically, six statements were related to the clinical domain, while three statements each were proposed for the functional, psycho-cognitive and social domains (Table S2). Each question was accompanied by an open-ended comment box, allowing panellists to provide additional insights, express reservations or raise critical points, supported by relevant literature references whenever possible. The Steering Committee analysed the answers to the first round of questions and used them to prepare those for the second round. During the first round, there was a strong consensus on the definition of frailty in patients with HF and the need for a specific assessment instrument. However, the inclusion of age as a parameter to include in the clinical domain of the HFFS elicited some criticisms that required further discussion. Panellists were also asked to express their preferences regarding the time frame for assessing specific variables, such as unintentional weight loss, falls and unplanned hospitalizations.
Second round of the Delphi process
In March 2023, the second round of questions was sent to the panellists along with a summary of the results of the first round. This round included 18 questions/statements. Two general statements addressed the revised definitions of frailty and its four domains. Eight new statements introduced additional variables, while the remaining statements were revisions based on feedback from the first round (Table S3). These revisions aimed to finalize the operational definitions of the variables used in the HFFS, in order to facilitate their assessment in clinical practice, reduce misunderstandings and identify different degrees of frailty.
Three new statements—regarding a new definition of the stage of HF, life expectancy and percutaneous endoscopic gastrostomy—did not reach the consensus threshold and, therefore, were excluded from the score and not further discussed.
In June 2023, the outcomes and key issues from the first two rounds were presented in an online meeting. During this meeting, the draft HFFS with the agreed variables was presented. Prior to the online meeting, six members of the study group confidentially tested the score in their HF outpatient clinics to verify its applicability and the time needed to complete it. The estimated time to complete the score ranged from 7 to 25 min.
Although the draft HFFS received significant consensus and adequately covered the four domains of frailty, concerns were raised about the completion time and the feasibility of performing certain tests, such as the timed up and go test (TUG) or the Abbreviated Mental Test (AMT 4 test). The panellists concluded that the score did not meet one of its primary objectives: being time-efficient and easy to administer. Consequently, it was decided to create alongside the HFFS a shorter version (S-HFFS) for routine clinical practice. During the online meeting, the panellists deliberated and reached a consensus on which variables to retain in the S-HFFS to ensure the score remained user-friendly and practical to implement.
Third round of the Delphi score
In August 2023, the third and final round of the Delphi process (including 11 questions/statements), aimed to refine the operational definitions of the agreed variables and to finalize the S-HFFS, without losing its holistic approach and ability to identify patients with varying degrees of frailty (Table S4).
To reduce the time required to complete the score, patient-directed questions were removed from the S-HFFS and retained only in the HFFS.
In mid-September 2023, following the conclusion of the third round, the draft of the HFSS and of the S-HFFS were distributed online to the HFFS group for review. The final document, reflecting broad consensus, was drafted in February 2024 (Graphical abstract, Table S5). By the end of March 2024, the HFFS group provided their final approval of the HFSS (Figure 2) and S-HFFS (Figure 3).
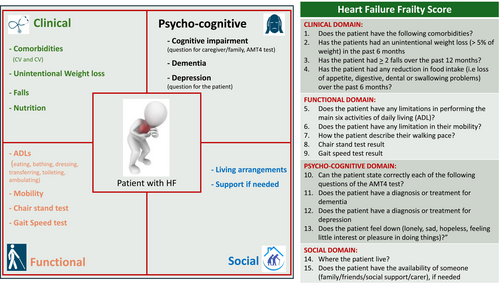
ADLs, activities of daily living; AMT4, Abbreviated Mini Mental Test; CV, cardiovascular.
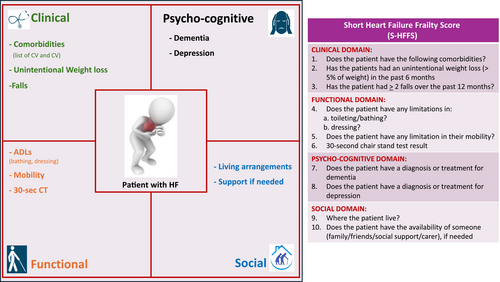
ADLs, activities of daily living; AMT4, Abbreviated Mini Mental Test; CV, cardiovascular; 30-sec CT, 30-s chair stand test.
Updated definition of frailty in heart failure
Frailty in HF has been defined as a ‘multidimensional dynamic state, independent age, that makes the individual with HF more vulnerable to the effects of stressors’.11 This definition has been further amended to include the potential reversibility of frailty and its association with poor outcomes, and its relationship with age. Therefore, the updated definition of frailty reads as follows: ‘a multidimensional, dynamic, and potentially reversible state, related to but independent of age, that makes individuals with HF more vulnerable to the effects of stressors and at increased risk of poor outcomes’.
The relationship between age and frailty is a crucial consideration. While aging is an unmodifiable risk factor for the development of both frailty and HF, as well as for adverse outcomes, frailty is not an inevitable consequence of aging.18 In HF patients, this state of vulnerability is primarily driven by the presence of HF and is more closely associated with biological age than chronological age. Indeed, frailty is also common (up to 30%) in young (<65 years) patients with HF.19, 20 Moreover, while some variables of frailty, such as cognitive and physical impairments, tend to correlate with chronological age, other domains, such as psychological and social aspects, are less inherently related to age. These domains are influenced more by individual's health status and specific adverse life events.21 For these reasons, chronological age has not been included in the HFFS. It will be a variable to be collected during the validation phase of the score. The concept that frailty should not be subordinated to chronological age is fundamental for clinical practice. It underscores the importance of assessing frailty in all patients, regardless of chronological age.
HFFS variables according to their domains
Clinical domain
Variables included by consensus in the clinical domain are co-morbidities, unintentional weight loss, falls in the previous year and nutrition.
Co-morbidities
People with HF have a number of multiple long term cardiac and noncardiac conditions with over 40% of such patients having at least five co-morbid diseases.22-24 These co-morbidities can significantly impact quality of life (e.g., diabetes, previous stroke and depression), overall life expectancy (e.g., cancer), and the use of HF guideline-directed medical therapy (e.g., chronic kidney disease).
After multiple discussion regarding the number and type of co-morbid conditions to include, and based on the literature on frailty and its association with co-morbidities in HF patients, it has been agreed to record co-morbidities that consistently correlate with unfavourable patient outcomes or influence therapeutic decisions.25, 26 After the 2023 online meeting, the list of co-morbidities was finalized and shared with the panellists in the third round (Table S4).
Unintentional weight loss (>5% of weight) in the past 6 months
Unintentional weight loss (UWL) is a variable included in most instruments for evaluating frailty and is associated with negative outcomes both in patients with HF27, 28 and in the general population.29 A 5% change in weight (10 lbs or 4.5 kg in less than 1 year) is a significant predictor of frailty, indicating a general catabolic status and reduced muscle strength and fatigue.30 However, in patients with HF, distinguishing weight loss due to frailty from other causes is challenging. Weight loss can result from nonclinical causes such as medications (e.g., diuretics in decompensated HF patients), socio-economic factors (e.g., malnutrition, anorexia and isolation), and psycho-cognitive factors (e.g., depression, cognitive impairment and dementia).31
In the first round, 94% of the panellists agreed to include UWL in the clinical domain of the HFFS. However, only in the third round, the operational definition of ‘unintentional’ WL was agreed as ‘not the expected consequence of treatment (e.g., not associated with intensification of diuretic therapy) or known illnesses’, according to Wong CJ.32
Falls over the past 12 months
Falls are an important yet often overlooked health problem associated with high risks of physical injuries, such as fractures and brain injuries, as well as psychological effects, including fear of falling, depression and social isolation, even after noninjurious falls.33 Falls negatively impact QofL, increase morbidity and elevate medical care costs.34, 35 The risk factors for falls are multifactorial, including aging, fatigue, physical weakness, postural hypotension, disability and polypharmacy.33, 36 These factors contribute to the higher incidence of falls in HF patients (43%) compared with those with other chronic diseases (≈30%).37 During the third round, it was agreed to record the variable falls as the occurrence of ‘≥2 unintentional falls over the past year’, similar to the formulation in the SARC-F (strength (S), assistance walking (A), rising from a chair (R), climbing stairs (C) and falls (F) questionnaire).38 The 12-month period was chosen over 12 months to mitigate the influence of seasonal variations.
Nutrition
During the first round, some panellists identified ‘nutrition’ as a critical factor in frailty. Patients with HF often experience disruptions in food intake and absorption due to various factors, including changes in taste linked to polypharmacy, early satiety, malabsorption from intestinal oedema, depression and cognitive dysfunction.39-41
During the second round, a new statement regarding nutrition as a clinical domain parameter was formulated with the question: ‘In the past 6 months, has there been a reduction in the patient's food intake (e.g., due to loss of appetite, digestive, dental, or swallowing problems)?’ This question achieved consensus (80%) and was then included in the HFFS.
Functional domain
Variables included by consensus in the functional domain are the 30-second Chair Stand Test (CST), limitations in activities of daily living (ADLs), patient-reported movement description and the Timed Up and Go (TUG) test.
Reduced exercise capacity and strength are characteristic features of HF, partly due to its direct impacts like reduced cardiac output, abnormal ventilatory response and skeletal muscle dysfunction,42 and partly due to concurrent presence of cardiac and noncardiac co-morbidities,43, 44 such as iron deficiency, neurological disorders and peripheral vascular diseases. Physical impairment in HF patients strongly predicts adverse health outcomes,43, 45-49 including social and care dependence,47 increased risk of falls,33 hospitalization,48 and mortality,49 independently of chronological age.
Activities of daily living
Activities of daily living (ADLs) encompass both basic (ADL) and instrumental ADL (IADL) categories. They serve as key indicators of an individual's functional status, being essential for maintaining independence in physical and cognitive functions. Impairment in ADLs significantly correlates with a higher risk of hospital readmission and mortality, regardless of other prognostic markers.50, 51
During the initial and subsequent rounds of discussion, consensus was reached to include all six ADLs (toilet use, feeding, dressing, bathing, transferring from bed to chair and ambulating) in the functional domain of the HFFS. Each ADL's assessment in the score involved grading the patient's capability (able, partially able or unable). In the S-HFFS, to satisfy the needs to keep the score easy to apply and not time-consuming, only bathing and dressing were included, as these are more frequently lost in HF patients and previously used in studies like OPERA.52
During the third round, none of the IADL reached the consensus threshold for inclusion in the HFFS. This because, in contrast to ADLs, dependency in performing IADLs is highly prevalent (75%) among patients with HF and influenced more by external factors such as gender, geography and cultural backgrounds.53
Mobility
Considering that a patient's mobility capacity and their reliance on aids like wheelchairs can significantly impact functional ability and access to social activities and rehabilitation programs, mobility was proposed as a new parameter during the second round. This variable gathered high consensus and was incorporated into the HFFS. A retrospective cohort study involving 114 553 adults diagnosed with HF demonstrated that impaired mobility (indicated by the use of a wheelchair, cane or walker) independently increased the risk of hospitalization or mortality, especially in patients under 65 years old.54 During the third round, a question on self-reported walking pace (slow – normal – fast) was included in the HFFS.
Timed up and go test and 30-s chair stand test
The timed up and go (TUG) test assesses how quickly a patient can rise from a chair, walk 3 m, turn around, return and sit down, providing a reliable measure of balance and mobility in patients with HF and requires a short duration to be performed.55, 56 A modified protocol combining the five times sit to stand test (measuring the time to stand five times from a seated position)57 and gait speed test (measuring speed over 3 m)58, to better assess lower limb strength was agreed during the second round.
However, after the online meeting, concerns were raised regarding the logistical space needed to perform these functional tests. Given that mobility was also evaluated through other functional tests, panellists decided to prioritize the chair stand test due to its feasibility in restricted spaces. To avoid a floor effect of the five times sit to stand test, in less frail patients, the 30-s (CST) was included in the S-HFFS.47, 59 This test reliably assesses lower body strength, balance and endurance, correlating well with other measures of functional capacity (e.g., 6-min walk test, peak oxygen uptake during exercise testing) and predicting negative outcomes in HF patients.60
Psycho-cognitive domain
Variables included by consensus in the psycho-cognitive domain are depression, dementia and clinical suspicion of cognitive impairment.
Cognitive impairment and dementia
Patients with HF face a significantly increased risk of developing cognitive impairment and dementia compared with those without HF, even after adjusting for age, sex and co-morbidities.61, 62 This risk appears to be particularly heightened in patients younger than 65 years at HF onset,63 with prevalence rates around 41% for cognitive impairment and 20% for dementia.63-65 The mechanisms linking HF to cognitive decline and dementia are multifaceted, potentially involving cerebral hypoperfusion, ischaemic insults, chronic inflammation, neurohormonal activation and shared risk factors such as hypertension, diabetes and atrial fibrillation.65-67
Cognitive impairment adversely affects self-care abilities, treatment adherence and QofL, while clinical dementia correlates strongly with dependence, disability and heightened risks of negative outcomes.68, 69
During the initial round of discussions, it was agreed to include cognitive impairment as a parameter in the psycho-cognitive domain. Among the proposed screening tests (Table S6), only the Abbreviated Mental Test (AMT 4)70 reached consensus and was included in the score during the second round. After the online meeting, the AMT 4 was included exclusively only in the HFFS.
The inclusion of dementia as a variable was unanimously agreed upon from the outset. Dementia ranked high by panellists due to its significant impact on frailty and subsequent impairment across multiple domains.
Depression
Depression affects approximately 30% of patients with HF, a rate considerably higher than in the general population.71 The higher prevalence of depression can be a direct consequence of the burden of HF with symptoms limiting the daily activities, recurrent episodes of decompensation often requiring hospitalisations and the negative contribution of noncardiac co-morbidities.71-73 Patients with HF with concomitant depression experience an amplified severity of HF symptoms, poorer QofL, increased morbidity and mortality, higher healthcare service utilization and costs. They have also a poorer adherence to guideline-directed medical therapy and healthy behaviours, thus increasing the risk of negative outcomes.74-77
During the first round, there was an agreement to include depression as one of the parameters of the psycho-cognitive domain. The question ‘Does the patient feel down (lonely, sad, hopeless, feeling little interest or pleasure in doing things)?’ to identify likelihood of depression was included only in the HFFS after the online meeting.
Although the following question ‘Does the patient feel down (lonely, sad, hopeless, feeling little interest or pleasure in doing things)?’ reached 80% of the agreement at the end of the second round, it was included only in the HFFS after the online meeting.
Social domain
Variables included by consensus in the social domain are the patient's living arrangements and the availability of support when needed. Research on the social domain of frailty in patients with HF has been relatively sparse compared with other domains, which may have contributed to the initial challenge in reaching consensus for its inclusion in the HFFS during the first round of the Delphi process. The use of the broad term ‘social impairment’ might have influenced the percentage of agreement obtained. Various indicators have been utilized in the literature to explore social components of frailty,78, 79 defined by Bunt et al. as a spectrum involving risks related to social resources (such as social network and marital status), general resources (like living arrangements, lifestyle, environment and education) and activities (including social participation, volunteering and religious engagement) that fulfil basic social needs over a lifetime.80
A meta-analysis of Gorji H et al. evaluated patients with HF and social frailty in relation to social isolation, living alone, low social support and a limited social network, reported that they were prevalent in 37%, 32%, 33% and 40%, respectively.81 The prevalence may be higher (66.4%) among patients requiring hospitalization, as shown in the FRAGILE-HF study.82
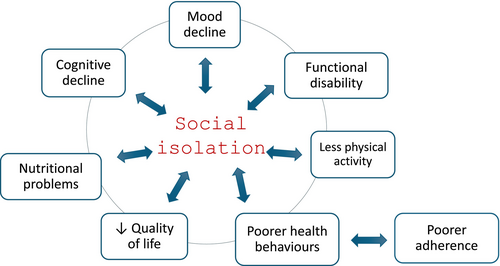
Social isolation is a stressor directly impacting structural, immune and neuroendocrine systems and indirectly promoting unhealthy behaviours such as smoking and physical inactivity.47 These factors cause a cascade of negative events (Figure 4) that explains the link between social aspects of frailty and the occurrence of adverse clinical outcomes, including increased hospitalizations and all-cause mortality.83
Patient living arrangements
Living alone is a significant risk factor for social isolation and frailty. Patients who live alone are considered socially vulnerable, facing a higher likelihood of psychological distress, such as increased risk of depression, feelings of loneliness and physical impairment.84, 85 Consequently, they are more susceptible to stressors and the development of frailty.86
During the second round, panellists reached consensus on expanding the operational definition of living arrangements to include both the type of residence (home vs. residential or hospice setting) and whether the patient lives alone or not.
Availability of support (family/friends/carer/social support)
Living with HF entails numerous challenges, including severe symptoms, functional limitations, frequent decompensations and adherence to specific lifestyle behaviour and complex treatment regimens.5, 87, 88 The availability of a caregiver (whether family members, friends or social support services) plays a crucial role in mitigating these challenges and reducing the vulnerability of patients with HF.89-91 Recognizing its importance, panellists agreed to incorporate the availability of someone to provide support when needed as a component of the social domain.
How to use the frailty score in clinical practice before its validation
In clinical practice, assessment and identification of HFFS variables in patients with HF, irrespective of their chronological age, are a crucial step in promoting a holistic and multidimensional approach to management.
The definition of agreed parameters to include into the HFFS has represented the second and essential step to the development of the score.11 The next and final step will be its validation in HF cohort studies (chronic first and then acute HF patients) in order to assess the role and the relative weight of the individual variables in determining frailty and the ability of both frailty scores to accurately distinguish between frail and nonfrail patients.
Before its validation, panellists believe that the new HFFS as a straightforward, multidimensional tool to capture a snapshot of a patient's health status at a given moment.
By using the HFFS, healthcare professionals can identify potential red flags indicating simultaneous impairments across the four main domains of frailty. This enables the development of more personalized and effective care plans.5, 92
Indeed, a recent study examining the predictive value of the four frailty domains93 in 854 patients with HF found that the number of frailty domains correlated with a proportional increased risk of adverse events within 1 year.
Although the HFFS has been presented by discussing the four main domains and their components separately, it is essential to evaluate frailty domains collectively, recognizing their intrinsic links and reciprocal interactions. Understanding these interconnections, not only within each domain but also across domains, can assist healthcare professionals in prioritizing interventions that address variables central to frailty or those most amenable to intervention and reversal. This approach aids in identifying patients with HF at risk of becoming frail or who are already frail, facilitating tailored therapies and services.
The score is designed for use by healthcare professionals. Most variables can be extrapolated from medical records. While potentially applicable in both chronic and acute care settings, panellists suggest that the variables included are particularly useful for evaluating patients at discharge, outpatients or those in chronic care settings rather than upon admission. Assessing frailty during acute HF episodes can be challenging, particularly regarding the physical and psychological domains, potentially leading to misinterpretation. Therefore, the HFFS will be validated against other frailty scores in patients with HF in different clinical settings (outpatient setting first and then acute setting).
Monitoring and application of the HFFS
For patients deemed at risk or presumed frail, HFFS monitoring should be conducted longitudinally, as frailty status may either deteriorate or improve over time. The S-HFFS has already been tested by members of the Steering Committee across different countries in a small patient sample, and feedback indicates it is easy to apply, straightforward and brief (about 5 min to administer).
Although during the Delphi process, the Heart Failure Frailty Score group attempted to rank the HFFS components, this was deferred to the validation phase, as the ranking is related to the weight of the different variables. This upcoming stage will determine the relative importance of each HFFS component and establish cut-point values for distinguishing between nonfrail, pre-frail and varying degrees of frailty.
Chronological age (collected as date of birth), HF stage, New York Heart Association (NYHA) functional class, and the frequency of unplanned hospitalisations will be collected and used as correction factors during the validation phase.
Limitations
Anonymity during the Delphi process prevents identification of which panellists participated and whether they contributed to all three rounds of feedback. Although the panel included experts from Europe, Asia and the Americas, a preponderance of experts from Europe and cardiologists may have influenced responses due to differences in healthcare systems and cultural backgrounds. The preponderance of cardiologists was related to the fact that this is a score to be used primarily in cardiology clinics and by cardiologists or by healthcare professionals working in cardiovascular units. Other factors influencing agreement on variables included expert perceptions of time required for test administration, such as cognitive assessments, and challenges related to availability of suitable spaces for conducting functional tests.
Conclusions
The HFFS has been specifically developed to identify frailty within the HF population, aiming to mitigate limitations of existing scores while remaining easy to apply and time-efficient. Each variable included in the HFFS has demonstrated individual prognostic value beyond routinely assessed risk factors.
The HFFS is a comprehensive score capable of identifying frail HF patients at high risk for adverse outcomes, such as early readmission or mortality, promises to optimize management strategies and reduce incidence of these events.
The HFFS will undergo a validation phase to ascertain its prognostic capacity and ease of use in clinical practice. The validation phase will also determine the relative weight of each component of the score.
Conflict of interest statement
S. Anker reports grants and personal fees from CSL Vifor, personal fees from Bayer, personal fees from Boehringer Ingelheim, personal fees from Servier, grants and personal fees from Abbott Vascular, personal fees from Cardiac Dimensions, personal fees from Actimed Therapeutics, personal fees from Astra Zeneca, personal fees from Amgen, personal fees from Bioventrix, personal fees from V-Wave, personal fees from Brahms, personal fees from Cordio, personal fees from Occlutech, personal fees from Cardior, personal fees from CVRx, personal fees from Cytokinetics, personal fees from Edwards, personal fees from Farraday Pharmaceuticals, personal fees from GSK, personal fees from HeartKinetics, personal fees from Impulse Dynamics, personal fees from Pfizer, personal fees from Repairon, personal fees from Medical Sensible, personal fees from Vectorious, from V-Wave, outside the submitted work; and Dr. Anker is named co-inventor of two patent applications regarding MR-proANP (DE 102007010834 & DE 102007022367), but he does not benefit personally from the related issued patents. B. Bozkurt reports consulting for Abbott, Abiomed, American Regent, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Janssen, Liva Nova, Merck, Novo Nordisk, Regeneron, Respicardia/Zoll, Roche, Sanofi-Aventis, Vifor. P. Brooks reports honoraria from AstraZeneca. R. Ferrari reports personal fees from Servier International, Merck Serono, Lupin, Boehringer, Astrazeneca, Sunpharma, outside the submitted work. F. Gustafsson reports grants and personal fees from Abbott, personal fees from Novartis, personal fees from Astra Zeneca, personal fees from Pharmacosmos, grants and personal fees from Pfizer, personal fees from Ionis, personal fees from Alnylam, outside the submitted work. L Hill reports personal fees from AstraZeneca, personal fees from Novartis, outside the submitted work. Y. Matsue reports personal fees from Otsuka Pharmaceutical Co., personal fees from Novartis Pharma K.K., personal fees from Bayer Inc., personal fees from AstraZeneca, grants from Pfizer Japan Inc., grants from Otsuka Pharmaceutical Co., grants from EN Otsuka Pharmaceutical Co., Ltd., grants from Nippon Boehringer Ingelheim Co., Ltd., outside the submitted work. G. Rosano reports grants from Astra Zeneca, personal fees from Anlylam, grants from Boheringer Ingelheim, grants from CSL Vifor, other from Menarini, other from Servier, personal fees from Cipla, grants from Medtronic, outside the submitted work. GR work was supported by funding of the Italian Ministry of Health [Ricerca corrente 20/1819]. Dr. Theou reports that he has asserted copyright of the Pictorial Fit-Frail Scale, which is made freely available for education, research and not-for-profit health care. Licences for commercial use are facilitated through the Dalhousie Office of Commercialization and Industry Engagement. O. Tkaczyszyn reports personal fees (for the sub-investigation in clinical trials) from Takeda, Impulse Dynamics, Cytokinetics, Alnylam Pharmaceuticals, Eidos Therapeutics and V-Wave Ltd., outside the submitted work. H. Tsutsui reports personal fees from Novartis Pharma K.K., personal fees from Otsuka Pharmaceutical Co., Ltd., personal fees from Ono Pharmaceutical Co., Ltd., personal fees from Nippon Boehringer Ingelheim Co., Ltd., personal fees from Bayer Yakuhin, Ltd., personal fees from Pfizer Japan Inc., and Honoraria from AstraZeneca, outside the submitted work. The other co-authors have nothing to declare.
Funding
None.
Permission Note
No material hereby uploaded has been previously published. All material is original to this submission.




