Baseline kidney function and the effects of dapagliflozin on health status in heart failure in DEFINE-HF and PRESERVED-HF
Abstract
Aims
Sodium–glucose co-transporter-2 (SGLT2) inhibitors improve health status and outcomes in the setting of heart failure (HF) across the range of ejection fraction (EF). Baseline kidney disease is common in HF, complicates HF management and is strongly linked to worse health status. This study aimed to assess whether the treatment effects of dapagliflozin on health status vary based on estimated glomerular filtration rate (eGFR).
Methods and Results
We conducted a pooled participant-level analysis of two double-blind, randomized trials, DEFINE-HF (n = 236) and PRESERVED-HF (n = 324), which evaluated dapagliflozin versus placebo. Both multicentre studies enrolled adults with HF, New York Heart Association Class II or higher, elevated natriuretic peptides, and an EF < 40% in DEFINE-HF or >45% in PRESERVED-HF. The primary exposure was eGFR. The main outcome was the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) at 12 weeks. Across both trials, there were 583 (99.3%) participants with a baseline eGFR. The median (25th, 75th) eGFR was 59 (46, 77) mL/min/1.73 m2. Dapagliflozin improved KCCQ-CSS at 12 weeks [placebo-adjusted difference, +5.0 points, 95% confidence interval (CI) 2.6–7.5; P < 0.001], and this was consistent in participants with an eGFR ≥ 60 (+6.0 points, 95% CI 2.4–9.7; P = 0.001) and eGFR < 60 (+4.1 points, 95% CI 0.5–7.7; P = 0.025) (P interaction = 0.46). The benefits of dapagliflozin on KCCQ-CSS remained robust across eGFR when modelled as a continuous variable (P interaction = 0.48).
Conclusions
Dapagliflozin led to early and clinically meaningful improvements in health status in HF patients, regardless of EF or baseline eGFR.
Background
Sodium–glucose co-transporter-2 (SGLT2) inhibitors are now strongly endorsed by international guidelines1, 2 for heart failure (HF) with reduced ejection fraction (HFrEF), HF with mildly reduced ejection fraction (HFmrEF) and HF with preserved ejection fraction (HFpEF) based on multiple landmark clinical trials.3-6 In contrast to other foundational pillars of guideline-directed medical therapy (GDMT) for HF, SGLT2 inhibitors have a significant benefit on short- and intermediate-term health status that appears consistent within different subgroups and robust across multiple domains.7-10 However, baseline kidney disease is common in HF irrespective of ejection fraction (EF) and strongly linked to worse health status, functional capacity and adverse HF events.11, 12
Aims
The objective of this pooled analysis of the DEFINE-HF and PRESERVED-HF trials was to assess whether the treatment effects of dapagliflozin on health status vary based on estimated glomerular filtration rate (eGFR).
Methods
The methods and primary results of the DEFINE-HF (NCT02653482)9 and PRESERVED-HF (NCT03030235)10 trials have been previously reported. Briefly, both studies were double-blind, randomized trials of dapagliflozin versus matching placebo enrolling participants with HF, New York Heart Association (NYHA) Functional Class II or higher symptoms, elevated natriuretic peptides, and an EF ≤ 40% (DEFINE-HF) or an EF ≥ 45% (PRESERVED-HF). The eGFR cutoff for exclusion was <30 mL/min/1.73 m2 in DEFINE-HF and <20 mL/min/1.73 m2 in PRESERVED-HF.
For the purposes of this pooled participant-level analysis, the main outcome of interest was the change in Kansas City Cardiomyopathy Questionnaire (KCCQ) Clinical Summary Score (KCCQ-CSS) at 12 weeks, which was a component of the primary endpoint in DEFINE-HF and the primary endpoint in PRESERVED-HF.13 The KCCQ is a standardized 23-item, self-administered instrument that quantifies HF-related symptoms, physical function, quality of life and social function.14 Scores range from 0 to 100, and higher scores reflect better health status. KCCQ-CSS includes the symptom and physical function domains.15
For the present analysis, participant-level data were pooled from both trials, and subjects were stratified by eGFR ≥ 60 versus <60 mL/min/1.73 m2. Clinical characteristics were compared using the chi-squared test or Fisher's exact test for categorical variables and the Student's t test or Wilcoxon rank-sum test for continuous variables as appropriate. All outcomes were evaluated using the modified intention-to-treat data sets in both trials, which included all randomized subjects who received at least one dose of study medication and had KCCQ assessed at 12 weeks. Heterogeneity of treatment effect (i.e., dapagliflozin vs. placebo) by baseline eGFR was assessed as a categorical variable (i.e., eGFR ≥ 60 vs. <60) using analysis of covariance (ANCOVA) and continuous variable using cubic splines adjusted for baseline KCCQ value, sex, left ventricular EF (LVEF), type 2 diabetes (T2D) status, atrial fibrillation (AF) status and baseline urine albumin/creatinine ratio (UACR). In the responder analysis, the proportion of dapagliflozin- and placebo-treated participants who had deterioration, as well as at least small (≥5 points), at least moderate (≥10 points) and large (≥20 points) improvements in KCCQ-CSS, was estimated using logistic regression models adjusted for the same variables as ANCOVA models.
Both DEFINE-HF and PRESERVED-HF were academically-led investigator-initiated studies funded by AstraZeneca (Cambridge, UK). Institutional review boards (IRBs) approved the study for all sites in both trials, and all subjects provided written informed consent for research participation. SP and MNK take full responsibility for the manuscript's integrity and had full access to source data, complete control and authority over its preparation and the decision to publish.
Results
Across both trials, there were 583 (99.3%) participants with an available eGFR (Table 1). The median (25th, 75th) eGFR was 59 (46, 77) mL/min/1.73 m2, and there were 287 (49.2%) participants with an eGFR ≥ 60 and 296 (50.8%) participants with an eGFR < 60. Subjects with an eGFR < 60 were older (69.2 ± 10.0 vs. 62.6 ± 12.4 years, P < 0.001), were more likely to be female (47.6% vs. 39.0%, P = 0.035) and had a higher prevalence of type 2 diabetes mellitus (64.2% vs. 53.7%, P = 0.009). This subgroup also tended to have a higher EF (48.3 ± 17.8 vs. 41.1 ± 18.0, P < 0.001). Participants with a reduced eGFR were less likely to be prescribed with an angiotensin receptor–neprilysin inhibitor (ARNi) (11.8% vs. 18.5%, P = 0.025) or a mineralocorticoid receptor antagonist (MRA) (42.6% vs. 52.3%, P = 0.019). They also had a lower baseline KCCQ-CSS (64.4 ± 20.5 vs. 67.8 ± 21.6, P = 0.028), KCCQ-Physical Limitation Score (KCCQ-PLS) (61.4 ± 22.9 vs. 65.2 ± 24.8, P = 0.023) and 6 min walk distance (240.8 ± 111.6 vs. 295.9 ± 106.4, P < 0.001).
| eGFR ≥ 60 (N = 287) | eGFR < 60 (N = 296) | P value | |
|---|---|---|---|
| Treatment group | 0.968 | ||
| Dapagliflozin | 144 (50.2%) | 149 (50.3%) | |
| Placebo | 143 (49.8%) | 147 (49.7%) | |
| Demographics | |||
| Age (years), mean ± SD | 62.6 ± 12.4 | 69.2 ± 10.0 | <0.001 |
| Gender | 0.035 | ||
| Male | 175 (61.0%) | 155 (52.4%) | |
| Female | 112 (39.0%) | 141 (47.6%) | |
| Race | 0.173 | ||
| White | 164 (59.9%) | 193 (66.3%) | |
| Black | 102 (37.2%) | 94 (32.3%) | |
| Other | 8 (2.9%) | 4 (1.4%) | |
| Unknown | 13 (4.5%) | 5 (1.7%) | |
| Medical history | |||
| Duration of heart failure (years), median (25th, 75th) | 3.9 (1.2, 8.2) | 3.9 (1.5, 8.7) | 0.719 |
| Ejection fraction (%), mean ± SD | 41.1 ± 18.0 | 48.3 ± 17.8 | <0.001 |
| Ischaemic heart disease | 0.138 | ||
| Yes | 89 (31.0%) | 109 (36.8%) | |
| No | 198 (69.0%) | 187 (63.2%) | |
| Type 2 diabetes mellitus | 0.009 | ||
| Yes | 154 (53.7%) | 190 (64.2%) | |
| No | 133 (46.3%) | 106 (35.8%) | |
| Atrial fibrillation | 0.221 | ||
| Yes | 129 (44.9%) | 148 (50.0%) | |
| No | 158 (55.1%) | 148 (50.0%) | |
| ICD | 0.333 | ||
| Yes | 92 (32.1%) | 84 (28.4%) | |
| No | 195 (67.9%) | 212 (71.6%) | |
| Baseline HF/CV medications | |||
| ACEi/ARB | 0.493 | ||
| Yes | 169 (58.9%) | 166 (56.1%) | |
| No | 118 (41.1%) | 130 (43.9%) | |
| ARNi | 0.025 | ||
| Yes | 53 (18.5%) | 35 (11.8%) | |
| No | 234 (81.5%) | 261 (88.2%) | |
| Beta-blocker | 0.347 | ||
| Yes | 243 (84.7%) | 242 (81.8%) | |
| No | 44 (15.3%) | 54 (18.2%) | |
| Hydralazine | 0.090 | ||
| Yes | 36 (12.5%) | 52 (17.6%) | |
| No | 251 (87.5%) | 244 (82.4%) | |
| Long-acting nitrates | 0.029 | ||
| Yes | 60 (20.9%) | 85 (28.7%) | |
| No | 227 (79.1%) | 211 (71.3%) | |
| MRA | 0.019 | ||
| Yes | 150 (52.3%) | 126 (42.6%) | |
| No | 137 (47.7%) | 170 (57.4%) | |
| Loop diuretics | 0.789 | ||
| Yes | 249 (86.8%) | 259 (87.5%) | |
| No | 38 (13.2%) | 37 (12.5%) | |
| Lipid-lowering agents | 0.051 | ||
| Yes | 220 (76.7%) | 246 (83.1%) | |
| No | 67 (23.3%) | 50 (16.9%) | |
| Anticoagulant agent | 0.929 | ||
| Yes | 125 (43.6%) | 130 (43.9%) | |
| No | 162 (56.4%) | 166 (56.1%) | |
| Glucose-lowering medications among participants with type 2 diabetes mellitus | |||
| Insulin | <0.001 | ||
| Yes | 65 (22.6%) | 111 (37.5%) | |
| No | 222 (77.4%) | 185 (62.5%) | |
| GLP-1RA | 0.0 ± 0.1 | 0.0 ± 0.2 | 0.098 |
| DPP4 inhibitors | 0.786 | ||
| Yes | 17 (5.9%) | 16 (5.4%) | |
| No | 270 (94.1%) | 280 (94.6%) | |
| Sulfonylurea | 0.792 | ||
| Yes | 32 (11.1%) | 31 (10.5%) | |
| No | 255 (88.9%) | 265 (89.5%) | |
| Metformin | 0.007 | ||
| Yes | 80 (27.9%) | 55 (18.6%) | |
| No | 207 (72.1%) | 241 (81.4%) | |
| Physical exam | |||
| Body mass index (kg/m2), median (25th, 75th) | 32.2 (27.5, 39.1) | 34.0 (29.7, 39.1) | 0.020 |
| Heart rate (b.p.m.), mean ± SD | 70.6 ± 11.6 | 69.0 ± 1.4 | 0.082 |
| Systolic blood pressure (mmHg), mean ± SD | 127.4 ± 22.5 | 130.8 ± 21.6 | 0.062 |
| Baseline laboratory studies | |||
| NT-proBNP (pg/mL), median (25th, 75th) | 788 (409, 1473) | 871 (476, 1951) | 0.042 |
| BNP (pg/mL), median (25th, 75th) | 192 (112, 354) | 172 (104, 324) | 0.137 |
| Urine albumin/creatinine ratio (mg/g), median (25th, 75th) | 17 (7, 68) | 30 (8, 114) | 0.025 |
| Haemoglobin A1c (%), mean ± SD | 6.8 ± 1.8 | 6.9 ± 1.7 | 0.072 |
| Haemoglobin (g/dL), mean ± SD | 13.3 ± 1.7 | 12.7 ± 1.9 | <0.001 |
| Functional measures | |||
| NYHA class | 0.097 | ||
| I | 182 (63.4%) | 164 (55.4%) | |
| II | 104 (36.2%) | 130 (43.9%) | |
| III | 1 (0.3%) | 2 (0.7%) | |
| KCCQ Overall Summary Score, mean ± SD | 65.4 ± 21.8 | 64.0 ± 20.3 | 0.263 |
| KCCQ Clinical Summary Score, mean ± SD | 67.8 ± 21.6 | 64.4 ± 20.5 | 0.028 |
| KCCQ Total Symptom Score, mean ± SD | 70.2 ± 23.1 | 67.3 ± 22.3 | 0.073 |
| KCCQ Physical Limitation Score, mean ± SD | 65.2 ± 24.8 | 61.4 ± 22.9 | 0.023 |
| KCCQ Quality of Life Score, mean ± SD | 61.3 ± 24.2 | 63.0 ± 24.1 | 0.362 |
| KCCQ Social Limitation Score, mean ± SD | 64.1 ± 28.8 | 63.9 ± 27.1 | 0.764 |
| 6 min walk distance, mean ± SD (m) | 295.9 ± 106.4 | 240.8 ± 111.6 | <0.001 |
- Note: All values are listed as a number (N) and percentage (%) unless otherwise specified.
- Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNi, angiotensin receptor–neprilysin inhibitor; BNP, B-type natriuretic peptide; CV, cardiovascular; DPP4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; GLP-1RA, glucagon-like peptide-1 receptor agonist; HF, heart failure; ICD, implantable cardioverter-defibrillator; KCCQ, Kansas City Cardiomyopathy Questionnaire; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
Dapagliflozin improved KCCQ-CSS at 12 weeks [placebo-adjusted difference, +5.0 points, 95% confidence interval (CI) 2.6–7.5; P < 0.001], and this was consistent in participants with an eGFR ≥ 60 (+6.0 points, 95% CI 2.4–9.7; P = 0.001) and eGFR < 60 (+4.1 points, 95% CI 0.5–7.7; P = 0.025) (P interaction = 0.46). The benefits of dapagliflozin on KCCQ-CSS and other KCCQ domains were robust across eGFR modelled as a continuous variable (all P interaction = NS) (Figure 1). In the responder analysis, fewer dapagliflozin- versus placebo-treated subjects experienced worsening of KCCQ-CSS at 12 weeks, and greater proportions of dapagliflozin-treated individuals had at least small, at least moderate and large improvements, and these results remained consistent when stratified by eGFR categories (all P interaction = NS) (Figure 2).
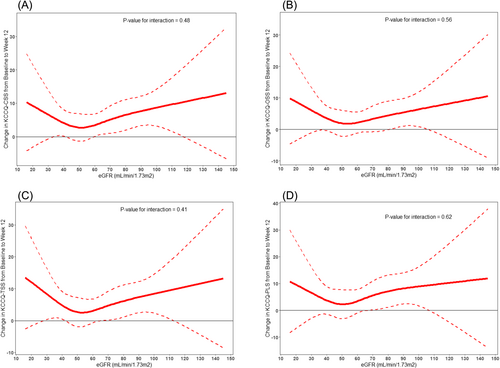
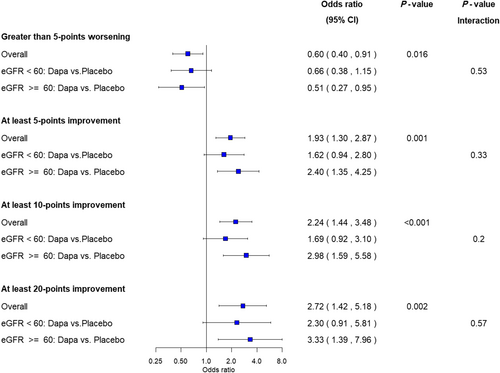
Conclusions
In this pooled participant-level analysis, subjects with a reduced eGFR were older, were more likely to be female, had a higher EF, were less likely to be prescribed with an ARNi or MRA, and had greater impairments in health status and functional capacity. Despite baseline differences, dapagliflozin- versus placebo-treated participants experienced greater improvement in KCCQ-CSS and other KCCQ domains at 12 weeks irrespective of eGFR when analysed as a categorical (i.e., eGFR ≥ 60 vs. <60) or continuous variable. This finding is consistent with the known beneficial effects of SGLT2 inhibitors on clinical outcomes across the full range of kidney function.3-6
The differences in clinical characteristics between participants with an eGFR ≥ 60 versus <60 mL/min/1.73 m2 are likely driven by a significantly higher proportion with HFmrEF and HFpEF among those with a reduced eGFR.13 It is notable that there is a risk-treatment mismatch in the setting of chronic kidney disease even though ARNis and MRAs are recommended for symptomatic HFmrEF (IIa) and HFpEF (IIb) in this eGFR range.1, 2
The observed disparity in health status by baseline eGFR was relatively small in magnitude and limited to the KCCQ-CSS and KCCQ-PLS subscales. In contrast, the ~60 m between-group difference in 6 min walk distance suggests that participants with a reduced eGFR have severe functional impairments that may not be fully captured even using validated disease-specific questionnaires for health status.
Importantly, dapagliflozin led to short-term improvements in multiple domains of health status including symptom burden, physical functioning and quality of life. This is a clinically relevant finding as other GDMTs have had a minimal impact on health status, particularly in HF subgroups including those with chronic kidney disease and/or preserved EF.1, 2 The meaningful benefit seen in DEFINE-HF and PRESERVED-HF may in part be explained by a greater burden of symptoms at baseline compared with landmark approval studies7, 8 priming this at-risk cohort to experience a more robust response after implementing an efficacious therapy.
There are several limitations that should be acknowledged including the post hoc nature of this analysis, the limited number of participants with an eGFR < 30, the short duration of follow-up and insufficient statistical power to evaluate clinical outcomes.
In conclusion, among participants with HF across the range of EF, treatment with dapagliflozin led to early and clinically meaningful improvements in HF-related health status across a wide range of kidney function.
Conflict of interest statement
APA has received relevant research support through grants to his institution from the National Heart, Lung, and Blood Institute (K23HL150159), the American Heart Association (Second Century Early Faculty Independence Award), The Permanente Medical Group, Northern California Community Benefits Programs, Garfield Memorial Fund, Abbott Laboratories, Amarin Pharma, Inc., Edwards Lifesciences LLC, Esperion Therapeutics, Inc. and Novartis. AJS receives research funding and/or is a consultant for Bayer, CSL Vifor, Abbott, Impulse Dynamics, Boston Scientific, Medtronic, Edwards Lifesciences, Biotronik, Story Health, General Prognostics and Acorai and owns stock with ISHI. MNK receives research funding and/or is a consultant for Alnylam Pharmaceuticals, Inc., Amgen Inc., Applied Therapeutics, Inc., AstraZeneca, Bayer Inc., Boehringer Ingelheim Inc., Cytokinetics, Inc., Dexcom, Inc., Lilly, Esperion Therapeutics, Inc., Janssen Pharmaceuticals, Inc., Lexicon Pharmaceuticals, Inc., Merck & Co., Inc., Novo Nordisk, Pharmacosmos A/S, Pfizer Inc., Sanofi, Vifor Pharma Management Ltd. and Youngene Therapeutics. The other authors report no disclosures.
Funding
The DEFINE-HF and PRESERVED-HF studied were investigator-initiated trials funded by AstraZeneca and conducted by Saint Luke's Mid America Heart Institute independent of the funding source.




