Incidence of atrial fibrillation in patients with an insertable cardiac monitor and symptomatic heart failure
Abstract
Aims
We aim to evaluate the incidence of atrial fibrillation (AF) in a large real-world cohort of patients implanted with an insertable cardiac monitor (ICM) who had a clinical history of symptomatic heart failure (HF) with reduced or preserved left ventricular ejection fraction (LVEF).
Methods
Patients with an ICM and a history of HF events were identified from the Optum® de-identified Electronic Health Record dataset merged with an ICM device dataset collected during 2007–2021. All ICM-detected AF episodes that were available with ≥30-s of ECG at onset were adjudicated using artificial intelligence (AI model). Episodes with AI model probability of AF ≥ 0.9 were analysed. The Kaplan–Meier incidence of AF as a function of episode duration, history of AF, and LVEF were assessed.
Results
A total of 1020 patients with ICM were identified of whom 911 had ≥180 days of follow-up and were included. According to the AI model, 358 patients had 8407 episodes of true AF. Incidence of AF at 42 months was 45.6% (44.1% vs. 46.8% in reduced vs. preserved LVEF). Incidence of new-onset AF was 23.2% (23.3% vs. 22.2% in reduced vs. preserved LVEF) in patients with no clinical history of AF. Patients with new-onset AF had a higher HF event rate compared with patients who had no clinical history of AF and did not develop AF during follow-up [OR = 2.73 (1.47–5.09); P = 0.002]. Patients with preserved LVEF had more longer duration paroxysmal AF compared with those with reduced LVEF (44.5% vs. 33.9%, P = 0.02).
Conclusions
AF was observed in almost half of patients with ICM and symptomatic HF. One-fourth of the patients had new onset AF and a higher rate of HF events compared with patients without AF. AF incidence was similar in patients with preserved and reduced LVEF.
Introduction
The global prevalence of atrial fibrillation (AF) is approximately 50 million, and it is estimated that in the US alone, 16 million people may have AF by 2050.1 As almost one third of the AF population is asymptomatic and has paroxysmal episodes, the worldwide AF burden is most likely under-estimated.2, 3 AF frequently co-exists with heart failure (HF) with each disease predisposing to the other. AF and HF share multiple common risk factors, and various studies have shown that the combination of AF and HF results in substantially worse morbidity and mortality compared with either condition alone.4-6
The pathogenesis of AF may be different in HF with reduced (HFrEF) versus preserved ejection fraction (HFpEF) in relation to neuro-hormonal activation, systemic inflammation and shared risk factors resulting in important differences.7, 8 Prior studies have shown a higher prevalence of AF at baseline in HFpEF compared with HFrEF, with some reports showing that almost two-thirds of the patients with HFpEF develop AF simultaneously or in close proximity to the HFpEF diagnosis.9 However, it is important to note that the prevalence and incidence of AF in HFrEF and HFpEF in prior reports have been reported using mostly short-term monitoring techniques, such as Holters or 7- to 14-day patch monitors.9-12 The reported incidence of AF in HF patients with no prior history of AF has varied from 9% to 44%, depending on the type of electrocardiographic monitoring and whether the patient had HFrEF versus HFpEF.10-12
Continuous long-term monitoring using subcutaneous insertable cardiac monitors (ICM) has been used to diagnose cardiac arrhythmias in various settings, such as unexplained syncope, intermittent palpitations, or suspected arrhythmias.13, 14 ICMs have been shown to detect incidence of subclinical AF in patients with a high risk for stroke,15 after cryptogenic stroke16 or strokes attributed to large or small vessel disease,17 and after AF ablation procedures.18 However, limited data exist regarding the incidence of AF in patients implanted with an ICM who have a clinical history of HF, and how the incidence and burden of AF vary with different HF subtypes. Therefore, in this study, we investigated the incidence of AF in a large real-world cohort of patients implanted with an ICM device who had a clinical history of HFrEF or HFpEF.
Methods
Study population
In this retrospective cohort study, patients with cardiovascular diseases were included from the Optum® de-identified Electronic Health Records (EHR) database from 2007 to 2021. Patients with Medtronic Reveal LINQ™ family of ICMs were identified who had at least 6 months of EHR data prior to implant. Patients were only included if they had HF events (HFE) before implant. HFEs were defined as inpatient with primary diagnosis of HF, or observation unit or emergency stay with primary diagnosis of HF and IV diuresis administration. Primary diagnosis of HF was ascertained based on ICD9/ICD10 codes: 428.X, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, I50.X, I11.0, I13.0, and I13.2. Patients with at least one LVEF (left ventricular ejection fraction) available measurement were categorized into LVEF <50%; LVEF ≥35% and <50%; and LVEF ≥50%. All LVEF measurements available in the patients were considered and the median value was used to ascertain HFrEF (LVEF <50%) versus HFpEF (LVEF ≥50%). This study used de-identified data and hence was exempt from institutional review board approval.
Outcome of interest
The primary outcome of interest was the incidence of AF. Secondary outcomes were AF events, HFE and all-cause mortality. AF detected by ICMs is considered as subclinical device-detected AF, as opposed to clinical AF which can be detected by electrocardiogram.19 ICMs detect AF based on incoherence RR intervals and absence of single p-wave between two R-waves over non-overlapping 2-min periods.20, 21 ICMs send automatic wireless nightly transmissions and the longest duration AF episode detected during the day is sent up with 30 s of ECG every night. Additionally, manual transmission with up to 14 detected episodes of AF with 2 min of ECG at onset may be transmitted by patients for physician review, which occurs on average every 1–3 months. All ICM detected AF episodes that were available with at least 30 seconds of ECG at onset were adjudicated using an artificial intelligence (AI) model. The AI model used an ensemble of features based 2D input to a 6-layer sequential convolution neural network to classify the rhythm as AF and not AF. The features that form the 2D input were based on the p-wave morphological characteristics during AF, incoherence of RR intervals in the form of Lorenz plots of difference of RR intervals, episode duration, RR interval time series histograms, and the historical occurrence of AF burden. The AI model was previously trained and validated using over 60 K manually adjudicated episodes and was shown to have an accuracy of over 97%.22 For each ICM detected AF episode, the AI model generates a probability value of the episode likely to be true AF. ICM detected AF episodes with AI model-based probability of true AF > 0.9 were adjudicated to be true AF. Clinical history of AF prior to ICM implant was ascertained based on ICD9/ICD10 codes: 427.31, I48.0, I48.1, I48.2, or I48.91. Patients were also considered to have a clinical history of AF if the patient had true AF on day 1 after implant and persistent AF for the first 30 days after implant.
ICM detects AF continuously and stores the total cumulative duration of detected AF episodes during a day as the daily AF burden. AF burden over entire follow-up was categorized into four categories based on previously reported definitions: (1) No AF: no days with AF burden >10 min; (2) Paroxysmal AF: ≥1 day with AF burden >10 min; (3) Persistent AF: ≥7 continuous days with AF burden >22.5 h; and (4) Permanent AF: ≥90 continuous days with AF burden >22.5 h. Paroxysmal AF was further divided into three groups based on maximum daily AF burden: 10 min–1 h, 1–5.5 h, and ≥5.5 h. All the groups were mutually exclusive with the largest AF burden category being the classification for each patient. AF burden was not corrected for inappropriate AF episode detection; however, it has been observed in earlier studies that inappropriate detections are primarily short duration episodes and do not affect burden significantly.23
Statistical analysis
Kaplan–Meier curves for AF episode incidence as a function of episode duration, clinical history of AF, and left ventricular ejection fraction (LVEF) were assessed. Proportion of patients in the AF burden groups were reported for LVEF <50% and LVEF ≥50% and were compared using the chi-square test. Patients with LVEF <50% were combined into one group to have sufficient patients in both groups for this comparison. Patients were stratified into AF groups using two components of information, one with a temporal aspect post-implant and one without. First, patients were separated by history of AF at ICM implant. This component had no temporal aspect. Then, patients with no clinical history of AF were further separated into those who were detected with AF during follow-up and those with no device detected AF. This resulted in three AF groups: (1) patients with a clinical history of AF; (2) patients with no clinical history of AF and device detected AF during ICM follow-up; and (3) patients with no clinical history of AF and no device detected AF during available ICM follow-up. AF events, HF events and all-cause mortality were evaluated in all three cohorts. HF events were defined similarly as what was considered for history of HFEs. AF events were defined as inpatient with primary diagnosis of AF, or observation unit or emergency stay with primary diagnosis of AF based on ICD9/ICD10 codes—same as what was used for evaluating clinical history of AF. The first occurrence of AF or HF events was used to compute the AF and HF event rates in the different patient groups. A logistic regression model using backward selection was performed to assess the effect of AF group on the AF and HF event rate. AF group was always included in the model, but other baseline variables were only included if their P-value was below 0.10 after non-significant variables were eliminated. The odds ratio and P-value for group was reported after adjusting for all variables in the final model. The resulting analysis was cross-sectional in nature as the time period for detecting AF and the time period for incidence of HF events and death were concurrent. Temporal association of AF burden, as measured by implantable devices, with HF event risk was recently shown in a similar large real-world dataset.24 Because of the concurrence of these events with HF, the analysis was supplemented by two Kaplan–Meier analyses determining time to the first composite event of HF or death. The first was performed in patients with no device detected AF after baseline and assessed the time to HF or death from baseline stratified by baseline AF history. The second was performed in patients with device detected AF, again stratified by AF history, but instead assessed the time to HF or death from the first post-baseline detection of AF. All statistical analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc., Cary NC). Statistical significance was set at P < 0.05.
Results
Patient cohort and baseline demographics
A total of 1020 patients with a Reveal LINQ ICM and a history of HF admission prior to implant were identified. The baseline characteristics of the included patients are shown in Table 1. The mean age was 68±13 years and 48% were women. LVEF was available in 889 (87%) of the 1020 patients, of whom 394 (44%) had LVEF <50%, and 495 (56%) had LVEF ≥50%. NYHA class was available in 296 (29%) of the 1020 patients, of whom 39 (13%) were class I, 126 (43%) were class II, 112 (38%) were class III, and 19 (6%) were class IV. A total of 911 patients with a minimum of 180 days of follow-up and a mean follow-up duration of 25.8±11.4 months were included. Of the 516 (57%) patients with AF, 505 patients had an EHR-documented clinical history of AF prior to implant and 11 had AF detected on the first day after implant with longstanding persistent AF for the first 30 days after implant.
| All patients with history of HF events (n = 1020) | Patients with history of HF events and median LVEF < 50% (n = 394) | Patients with history of HF events and median LVEF ≥ 50% (n = 495) | |
|---|---|---|---|
| Mean age (SD) | 68 (13) | 65 (13) | 69 (12) |
| LVEF recorded | 889 | 394 | 495 |
| Mean LVEF | 48.1 | 33.7 | 59.6 |
| Male sex | 535 (52%) | 251 (64%) | 221 (45%) |
| Hypertension | 967 (95%) | 372 (94%) | 476 (96%) |
| Diabetes | 571 (56%) | 210 (53%) | 285 (58%) |
| Coronary artery disease | 766 (75%) | 320 (81%) | 356 (72%) |
| Myocardial infarction | 403 (40%) | 175 (44%) | 194 (39%) |
| Vascular disease | 312 (31%) | 126 (32%) | 157 (32%) |
| Atrial fibrillation | 586 (57%) | 229 (58%) | 287 (58%) |
| Renal dysfunction | 539 (53%) | 192 (49%) | 290 (59%) |
| Stroke/transient ischemic attack | 525 (51%) | 196 (50%) | 274 (55%) |
| Medications | |||
| ACE-I/ARB | 855 (84%) | 361 (92%) | 396 (80%) |
| Beta-blockers | 797 (78%) | 303 (77%) | 409 (83%) |
| Loop diuretics | 915 (90%) | 362 (92%) | 445 (90%) |
| Spironolactone | 532 (52%) | 221 (56%) | 257 (52%) |
| Sacubitril/valsartan | 26 (3%) | 26 (7%) | 0 (0.0%) |
| Vasodilator/nitrate | 256 (25%) | 143 (36%) | 91 (18%) |
| AAD Class I | 72 (7%) | 14 (4%) | 49 (10%) |
| AAD Class III/IV | 382 (37%) | 167 (42%) | 184 (37%) |
| Digoxin | 134 (14%) | 78 (20%) | 56 (11%) |
| Anticoagulation | 547 (54%) | 222 (56%) | 266 (54%) |
| ICM reason for monitoring | |||
| AF ablation monitoring | 52 (5%) | 21 (5%) | 21 (4%) |
| AF management | 172 (17%) | 74 (19%) | 80 (16%) |
| Cryptogenic stroke | 240 (24%) | 93 (24%) | 126 (25%) |
| Palpitations | 47 (5%) | 13 (3%) | 26 (5%) |
| Suspected AF | 71 (7%) | 27 (7%) | 40 (8%) |
| Syncope | 388 (38%) | 138 (35%) | 185 (37%) |
| Ventricular tachycardia | 27 (3%) | 18 (5%) | 8 (2%) |
| Other/unknown | 23 (2%) | 10 (3%) | 9 (2%) |
- Abbreviations: AAD, anti-arrhythmic drugs; ACE-I, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; HF, heart failure; LVEF, left ventricular ejection fraction; SD, standard deviation.
Incidence of atrial fibrillation in the overall cohort
A total of 8407 ICM detected AF episodes from 358 patients were adjudicated as true AF at the chosen probability threshold of 0.9 in the AI model. A total of 35 episodes (1.0%) were symptomatic as measured by at least one patient symptom marker activation during the AF episode. The incidence of AF in the overall patient cohort as a function of episode duration is shown in Figure 1. Incidence of AF, as detected by an ICM and adjudicated by an AI model over 42 months of follow-up, was estimated to be 37.6% for episode duration ≥1 h and 45.6% for episode duration ≥2 min.
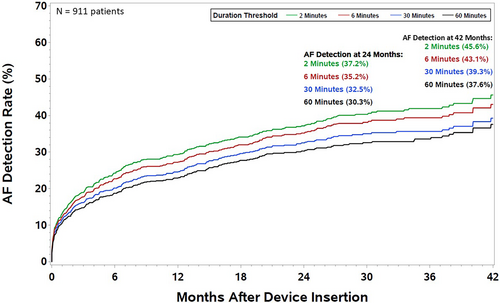
Incidence of new-onset atrial fibrillation in the overall cohort
There were 395 (43%) patients who had no clinical history of AF, of whom 74 (19%) developed new onset AF, as detected by the ICM during follow-up and adjudicated by the AI model. Figure 2 shows the incidence of AF stratified by clinical history of AF. The estimated 42-month new onset AF incidence, that is, in patients with no clinical history of AF prior to implant of the ICM, was 23.2% (Figure 2). Patients with new onset AF had no significant difference in AF event rates compared with patients who had a clinical history of AF (Table 2).
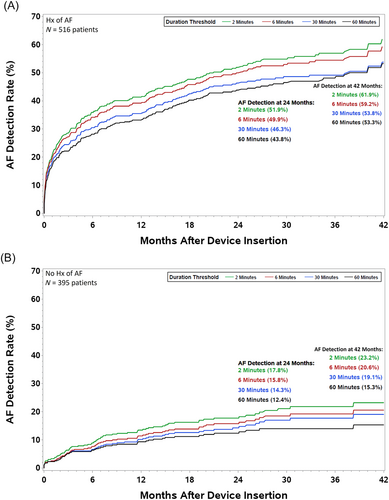
| N | Number of patients with AF events (%) | Number of patients with HF events (%) | Number of patient deaths: all-cause (%) | |
|---|---|---|---|---|
| Clinical history of AF prior to ICM implant | 516 | 161 (31.2%) | 115 (22.3%) | 84 (16.3%) |
| Patients with recorded LVEF | 516 | 161 (31.2%) | 115 (22.3%) | 84 (16.3%) |
| LVEF < 50% | 229 | 66 (28.8%) | 41 (17.9%) | 33 (14.1%) |
| LVEF < 35% | 107 | 31 (29.0%) | 14 (13.1%) | 11 (10.3%) |
| 35% ≤ LVEF < 50% | 122 | 35 (28.7%) | 27 (22.1%) | 22 (18.0%) |
| LVEF ≥ 50% | 287 | 95 (33.1%) | 74 (25.8%) | 51 (17.8%) |
| New-onset AF | 74 | 20 (27.0%) | 20 (27.0%) | 14 (18.9%) |
| Patients with recorded LVEF | 68 | 17 (25.0%) | 19 (27.9%) | 11 (16.2%) |
| LVEF < 50% | 32 | 6 (18.8%) | 12 (37.5%) | 2 (6.3%) |
| LVEF < 35% | 14 | 2 (14.3%) | 7 (50.0%) | 1 (7.1%) |
| 35% ≤ LVEF < 50% | 18 | 4 (22.2%) | 5 (27.8%) | 1 (5.6%) |
| LVEF ≥ 50% | 36 | 11 (30.6%) | 7 (19.4%) | 9 (25.0%) |
| Patients with no history of AF and did not develop AF as per continuous monitoring by ICM | 321 | 20 (6.2%) | 44 (13.7%) | 56 (17.4%) |
| Patients with recorded LVEF | 305 | 20 (6.7%) | 42 (13.8%) | 55 (18.0%) |
| LVEF < 50% | 133 | 12 (9.0%) | 22 (16.5%) | 33 (24.8%) |
| LVEF < 35% | 55 | 4 (7.3%) | 8 (14.6%) | 12 (21.8%) |
| 35% ≤ LVEF < 50% | 78 | 8 (10.3%) | 14 (11.6%) | 22 (26.9%) |
| LVEF ≥ 50% | 172 | 8 (4.7%) | 20 (11.6%) | 22 (12.8%) |
| Total | 911 | 201 (22.1%) | 179 (19.6%) | 154 (16.9%) |
| Patients with recorded LVEF | 889 | 198 (22.3%) | 176 (19.8%) | 150 (16.9%) |
| LVEF < 50% | 394 | 84 (21.3%) | 75 (19.0%) | 68 (17.3%) |
| LVEF < 35% | 176 | 37 (21.0%) | 29 (16.5%) | 24 (13.6%) |
| 35% ≤ LVEF < 50% | 218 | 47 (21.6%) | 46 (21.1%) | 44 (20.1%) |
| LVEF ≥ 50% | 495 | 114 (23.0%) | 101 (20.4%) | 82 (16.6%) |
- Abbreviations: AF, atrial fibrillation; HF, heart failure; ICM, insertable cardiac monitor; LVEF, left ventricular ejection fraction.
Incidence of atrial fibrillation in HFrEF versus HFpEF
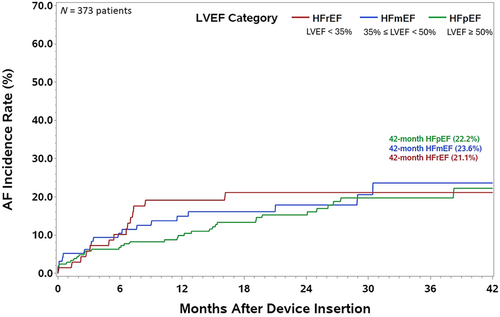
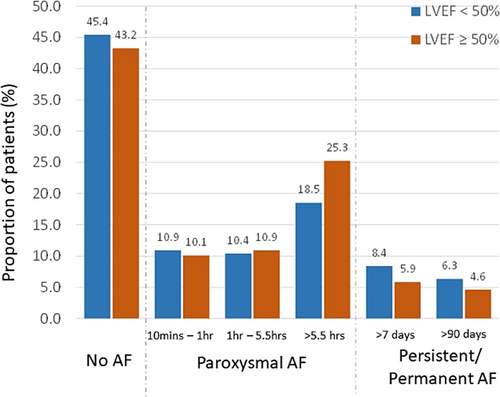
Figure 3 shows the incidence of AF stratified by LVEF. Of the 394 patients with LVEF below 50%, 176 were less than 35% while 218 were greater than or equal to 35% but less than 50%. The remaining 495 patients with recorded LVEF were greater than or equal to 50%. The 42-month estimated AF incidence in patients with a clinical history of HF was 46.8% for those with LVEF ≥50% (panel A), 43.1% for LVEF between 35% and 50% (panel B), and 46.2% for LVEF <35% (panel C). Further, combining patients with LVEF <50%, the 42-month estimated AF incidence was 44.1% (panel D). The incidence of new-onset AF at 42-month was 23.3% for LVEF<50%, 22.2% for LVEF ≥50%, 23.6% for LVEF between 35% and 50%, and 21.1% for LVEF <35% (Figure 4). The proportion of patients in the different AF burden groups stratified by LVEF <50% and ≥50% is shown in Figure 5. More than 50% of patients with LVEF ≥50% or <50% had ≥1 day of >10 min of device detected AF (54.6% vs. 56.8%, P = 0.51). Among patients with AF (≥1 day of >10 min of device detected AF), a larger proportion with LVEF <50% had persistent or permanent AF compared with patients with LVEF ≥50% (27.0% vs. 18.5%, P = 0.02) and a smaller proportion had longer duration paroxysmal AF (33.9% vs. 44.5%, P = 0.02).
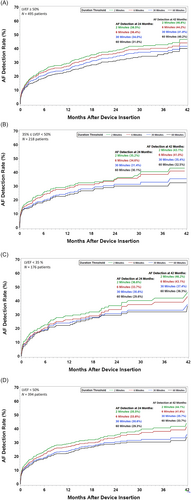
Heart failure events and all-cause mortality
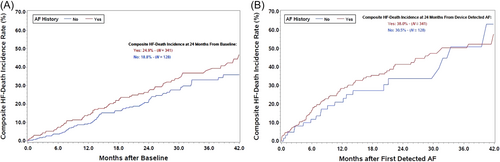
There was a total of 179 patients with HF events and 154 all-cause deaths. HF events and all-cause mortality rates for the three group of patients are shown in Table 2. Patients with new onset AF during follow-up had a similar rate of HF events compared with patients with a prior clinical history of AF [adjusted odds ratio = 1.16 (0.65–2.04); P = 0.619]. Patients with new onset AF had more than twice as many HF events as compared with patients who had no clinical history of AF and had not developed AF during follow-up [adjusted odds ratio = 2.73 (1.47–5.09) P = 0.002]. Patients with clinical history of AF had a significantly higher rate of HF events compared with patients who had no clinical history of AF and had not developed AF during follow-up [adjusted odds ratio = 2.36 (1.58–3.55) P < 0.001]. No significant differences in mortality rates were observed between the groups. The evaluation of the temporal relationship of occurrence of HF events or death with respect to AF occurrence is shown in the K-M curves in Figure 6. In patients with no device detected AF, K-M estimates of time to first HF event or death from baseline AF stratified by clinical history of AF are shown in Figure 6, with a higher incidence of HF in patients with clinical history of AF. In patients with device detected AF, K-M estimates of time to first HF event or death from onset of device detected AF stratified by clinical history of AF are shown in Figure 6 with higher incidences compared with patients without device detected AF and over 30% incidence at 24 months in patients with new onset AF.
Discussion
In this large real-world cohort of patients with ICM and a history of symptomatic HF, we show several key findings. First, 57% of the HF patients had a clinical history of AF. Second, the incidence of new-onset AF among patients with HF was 23% during ICM follow-up. Third, patients with new onset AF had a higher likelihood of HF events compared with patients who did not have a clinical history of AF. Fourth, the incidence of AF was similar (in the range of 43% to 47%) for patients with HFpEF, and HFrEF. Similarly, there was no significant difference in the incidence of new-onset AF for patients with HFpEF, and HFrEF. The group with HFrEF had a higher proportion of patients with persistent or permanent AF and a lower proportion of longer duration paroxysmal AF compared with the HFpEF group. These results have important clinical implications for the management of patients with symptomatic HF (Graphical Abstract).
This study shows that the incidence of subclinical AF is high among patients with HFrEF, and HFpEF. This can have potential impact on clinical practice especially considering the fact that most HF patients have other risk factors for stroke as well such as age, hypertension, diabetes, and vascular disease. Thus, detecting AF in patients with prior history of HF may lead to guideline directed anticoagulation management.25 Recent studies have indicated that the duration of subclinical AF may be an important determinant in evaluating the balance between stroke risk and bleeding risk during anticoagulation management.26, 27 However, the appropriate duration of subclinical AF for anticoagulation management in patients with HF remains unclear. Nevertheless, it raises an important question on whether all patients with HF should be screened for subclinical AF.
Detection of new-onset AF in patients with HF can also alter clinical management in terms of catheter ablation. AF ablation has been proven to be an effective rhythm control strategy in patients with HF, especially for those with reduced ejection fraction.28, 29 Studies have shown that catheter ablation can significantly reduce the risk of mortality and HF events in patients with co-existent AF and HF.28 ICM can potentially not only help in identifying HF patients who may benefit from AF ablation therapies, but also help in monitoring for recurrences of AF post AF ablation and planning for redo ablation procedures if needed. Incidence of AF ablation therapies in HF patients implanted with ICM will be investigated in future studies such as ALLEVIATE-HF (NCT04452149).30
Previous studies have shown that patients with HF who develop AF have worse prognosis compared with patients with AF who develop HF.31 Our results show that patients with new onset AF had a higher likelihood of HF events compared with patients who did not have AF but a similar likelihood was observed compared with patients who had clinical history of AF. This suggests that clinicians should pay close attention to HF patients who develop new onset AF, as they are patients at higher risk needing closer follow-up. It is important to highlight that the patient cohort included in this analysis had ICM implants for other indications, including unexplained syncope, post cryptogenic stroke or AF ablation, and AF management. Patients with long standing persistent or permanent AF are not often considered for ICM implants, as it was observed in the AF burden analysis in this study. Similarly, patients who are undergoing AF ablation mostly do not have permanent AF, which may not be amenable to rhythm control strategies like AF ablation. As was observed in our study results, there were very few patients who had longstanding persistent or permanent AF in the study cohort, and hence, they were grouped into patients with a clinical history of AF.
Methodologies for detection of subclinical AF are different for most pacemakers and defibrillators compared with ICMs. For pacemakers and defibrillators with a lead in the atrium, AF is detected based on high atrial rate in the atrium sensed by an atrial lead along with atrio-ventricular conduction ratio ≥2:1.32, 33 So AF, atrial flutter and atrial tachycardia are detected by the same mechanism and not discriminated. Further, data on subclinical AF detection using defibrillators mostly includes HF patients with reduced EF. In contrast, an ICM detects AF based on incoherence of RR intervals21 and HF patients with ICMs implanted span the spectrum of LVEFs, a large proportion being in the mid EF and preserved EF groups. Atrial flutter and atrial tachycardia can be detected by ICMs, but using a separate mode of operation which was only used in <10% of the patients. Thus, ICM detected atrial flutter and atrial tachycardia was not considered for this data analysis.
There are some limitations that should be considered. First, the retrospective nature of the analysis and the use of claims data as input for diagnoses, which relies on billing codes and is subject to coding errors. Second, the patients included in this study were implanted with an ICM indication other than HF and had a history of HF prior to implant, thus results observed may not be generalizable to a broader HF patient population. A high proportion of patients had clinical history of AF as some of the ICM implants were before or after AF ablation procedures. Third, the patient cohorts were ascertained based on EHR data. In some patients, EHR data may have been incomplete if the patient had visited medical providers outside the integrated delivery network of providers. Lastly, AF episodes were adjudicated with an AI model, which was tuned to have a high degree of specificity and thus might have underestimated incidence of AF episodes. Accuracy of manual adjudication of episodes is over 99%, however manual adjudication of over 25 000 episodes was not feasible for this analysis, and therefore, the use of the AI model was preferred.
Conclusion
Incidence of AF, as detected by an ICM and adjudicated by an AI model over 3 years of follow-up, was close to 50% in patients with symptomatic HF who had an ICM implanted. Approximately one fourth of the patients with HF and no prior AF had incidence of new onset AF. AF incidence, including new-onset AF, was similar in patients with HFpEF versus HFrEF. A higher proportion of longer duration paroxysmal AF was observed in patients with HFpEF compared with HFrEF.
Acknowledgements
We thank Nirav Patel and Verla Laager who served as scientific advisors.
Conflict of interest
M.S.K reports advisory board for Bayer. M.R.Z serves as a consultant to Abbott, Adona Medical, Aria CV, Avery Therapeutics, Inc., Boehringer-Ingelheim, Boston Scientific, Cardiovascular Research Foundation (CRF) Clinical Trials Center, CVRx, DIASTOL Therapeutics, LLC, EBR, Edwards, Eli Lilly, GenKardia, Innoventric, KestraMedical, Medtronic, Merck, Morphic Therapeutics, Novartis, Pulnova, Salubris Biotherapeutics, Sonata, SRNALYTICS, INC, V-WAVE, and Vectorious. R.K. reports consultancies from Medtronic, Cardionomic, Edwards Lifesciences, Impulse Dynamics, and scPharmaceuticals. S.S., B.V.D., J.K., N.F., and B.G. are Medtronic employees and shareholders. J.V. reports consultancies from Medtronic, Abbott, American Regent, Amgen, Applied Therapeutic, AskBio, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardiac Dimension, Cardiocell, Cardior, CSL Bearing, CVRx, Cytokinetics, Daxor, Edwards, Element Science, Faraday, Foundry, G3P, Innolife, Impulse Dynamics, Imbria, Inventiva, Ionis, Lexicon, Lilly, LivaNova, Janssen, Merck, Occlutech, Owkin, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Pharmain, Prolaio, Regeneron, Renibus, Roche, Salamandra, Sanofi, SC Pharma, Secretome, Sequana, SQ Innovation, Tenex, Tricog, Ultromics, Vifor, and Zoll.
Funding
This work was supported by Medtronic.




