Investigation of left ventricular ejection fraction in a Swiss heart failure population: Insights into mortality and sex differences
Rubén Fuentes Artiles and Renald Meçani contributed equally to this work.
Abstract
Aims
Understanding heart failure (HF) characteristics is essential to improve patient outcomes. Categorizing HF beyond left ventricular ejection fraction (LVEF) is challenging due to heterogeneous clinical presentation and aetiologies. Despite global studies on HF, the role of LVEF on mortality remains controversial. We explored the association of LVEF with mortality, considering sex differences and comorbidities in a cohort from the largest tertiary cardiovascular centre in Switzerland.
Methods
HF patients admitted to the University Hospital of Bern from January 2015 to December 2019 were evaluated. LVEF was used to classify patients into HF with preserved ejection fraction (HFpEF), HF with mid-range ejection fraction (HFmrEF) and HF with reduced preserved ejection fraction (HFrEF) categories. Cox proportional hazard models and time-stratified analyses adjusted for potential confounders were employed.
Results
A total of 5824 HF patients were included, and 2912 died over a median follow-up time of 3.39 years. Mortality rates across LVEF categories showed no significant differences, while overall, women showed significantly higher mortality; 30 day mortality was lower in the HFpEF category [hazard ratio (HR) 0.67, 95% confidence interval (CI): 0.52–0.88, P = 0.003], with persistent effects upon stratification in males (HR 0.59, 95% CI: 0.42–0.81, P < 0.001) and non-diabetics (HR 0.62, 95% CI: 0.44–0.87, P = 0.005). An isolated reduction in HFpEF mortality was observed in females after 1 year (HR 0.72, 95% CI: 0.53–0.98, P = 0.035).
Conclusions
The prognostic role of LVEF on all-cause mortality remains unclear, while differences in mortality rate distribution between women and men mirror established HF pathophysiological sex differences. Future HF studies should focus on HF aetiology and include measures beyond LVEF for comprehensive characterization.
Introduction
Heart failure (HF) is recognized as a major public health problem and affects over 64 million people worldwide with increasing tendency.1 Understanding the characteristics of HF has become imperative for clinical and research practice alike to enhance diagnostic precision, optimize therapeutic approaches and improve patient outcomes.2 The well-established, international classification subdivides patients based on their left ventricular ejection fraction (LVEF), which has been ascertained to ideally reflect underlying pathophysiology.3 More specifically, patients are categorized as having HF with reduced (HFrEF; LVEF ≤ 40%), mid-range (HFmrEF; LVEF 41%–49%) or preserved (HFpEF; LVEF ≥ 50%) ejection fraction.
LVEF classification has been criticized to oversimplify the disease pattern of HF.4 However, categorization of HF patients beyond LVEF is challenging, as the clinical presentation of the disease is heterogeneous and patients can exhibit significant differences both in terms of aetiology and with respect to comorbidities.5 Lack of epidemiological data for HF further restricts categorization.6 Although several studies have globally investigated the profile of HF patients in other countries,7-10 data on the demographics, clinical characteristics and prognosis of HF in Switzerland are scarce, given the lack of national registries or centralized data collection of HF patients.11
In our ambispective cohort study, we therefore aimed to describe the profile of HF patients based on medical records at the University Hospital of Bern and to investigate the association of LVEF with mortality, taking into consideration follow-up time, sex differences and comorbidities within our cohort.
Methods
Study design
In this ambispective cohort study, we followed up and characterized HF patients admitted to the University Hospital of Bern, the largest tertiary cardiology centre in Switzerland, between January 2015 and December 2019. All variables were obtained from the clinical data warehouse, which contains administrative and medical data of all patients. Diagnoses were in line with the International Statistical Classification of Diseases and Related Health Problems 10th version (ICD-10). This study was approved by Bern Cantonal Ethics Commission (KEK) under Project ID 2020-00980.
Participants
Our study population encompassed adult inpatients (≥18 years) with an ICD-10 diagnosis of HF (I50, I11.0, I09.81, I13.0 and I13.2). Included participants had to have at least one hospital admission since January 2015 and not object to the use of their health-related data for research purposes. We excluded patients with missing LVEF measurements and erroneous or missing survival data.
Variables
The following variables were obtained from the clinical data warehouse and considered for our statistical analysis: sex, age, body mass index, New York Heart Association (NYHA) functional class, LVEF, presence of intracardiac device therapy, some medications [beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), insulin and thiazolidinediones] and comorbidities [type 2 diabetes mellitus (T2DM) (ICD-10 E11.-), chronic kidney disease (CKD) (N18.-), atherosclerotic cardiovascular disease (ASCVD) (I25.1-), hypertension (I10.-), atrial fibrillation (I48.-), dyslipidaemia (E78.-) and chronic obstructive pulmonary disease (COPD) (J44.-)]. In addition, all-cause mortality with the date of event was assessed.
LVEF measurement
LVEF data were obtained by cardiac ultrasound. For patients with multiple LVEF entries, the mean value was considered for the analysis, while the single measurement was considered for patients with one LVEF entry.
Survival data
Survival status was assessed by linking it with the national mortality record. Follow-up of each participant began on the date of the first echocardiography measurement and ended on the date of death or loss to follow-up, whichever occurred first.
Statistical analysis
Data were summarized as medians for continuous variables and as counts for categorical variables. To assess normality of continuous variables, Q–Q plots, histograms and the Shapiro–Wilk test were used. Survival by LVEF category for the overall inpatient population was investigated by applying Kaplan–Meier curves. Cox proportional hazard regression models were used to estimate the association of LVEF categories and the risk of all-cause mortality. Two statistical models were built. Model 1 was adjusted for age and sex, and Model 2 additionally for CKD, ASCVD, hypertension, atrial fibrillation, COPD and dyslipidaemia. To explore whether the association between LVEF categories and mortality risk differed by sex and T2DM status, stratification and interaction terms involving LVEF with sex or T2DM were defined. A sensitivity analysis was added by investigating whether LVEF as a continuous measure was linked to mortality risk and testing a quadratic term to explore non-linear associations. In a further sensitivity analysis, patients with multiple LVEF measurements were excluded, as they may have had a worse prognosis that could potentially have affected overall study findings. Proportionality of hazards (PH) was tested through Cox regression with time-dependent covariates using P > 0.05 as the cut-off for significance. When PH assumptions were not met, analyses were further stratified by time period: 30 days (Period 1), 1 year (Period 2), 1–2 years (Period 3) and >2 years (Period 4), and reported as hazard ratios (HRs) per period.
Statistical analyses were performed using IBM SPSS Statistics for Windows,13 and the Kaplan–Meier curves were generated using RStudio.14 Graphs, tables and plots were created with Microsoft Excel.15
Results
Baseline characteristics
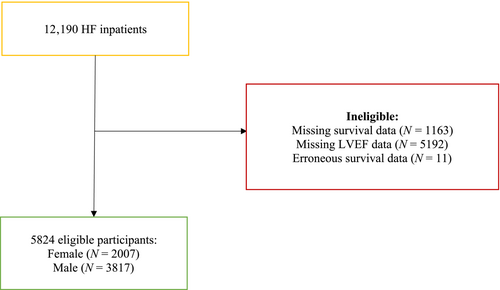
We included a total of 12 190 HF inpatients who were hospitalized between January 2015 and December 2019 (Figure 1). Baseline characteristics are depicted in Table 1.
| Characteristics | Overall population (n = 5824) | Female (n = 2007) | Male (n = 3817) | Male vs. female (P value) | Missing values |
|---|---|---|---|---|---|
| Age (median, IQR) | 73 (64–81) | 77 (68–83) | 71 (62–79) | <0.001 | 0 |
| BMI (median, IQR) | 26.4 (23.3–30.1) | 25.5 (22.1–29.7) | 26.7 (23.9–30.2) | <0.001 | 1560 (26.8) |
| Ejection fraction (median, IQR) | 50 (35–60) | 55 (40–64) | 45 (32.5–60) | <0.001 | |
| Ejection fraction classification | |||||
| HFrEF (%) | 2173 (37.3) | 567 (28.3) | 1606 (42.1) | <0.001 | |
| HFmrEF (%) | 571 (9.8) | 152 (7.6) | 419 (11) | ||
| HFpEF (%) | 3080 (52.9) | 1288 (64.2) | 1792 (46.9) | ||
| NYHA | |||||
| NYHA I (%) | 198 (6.1) | 52 (4.6) | 146 (6.9) | <0.001 | 2580 (44.3) |
| NYHA II (%) | 582 (17.9) | 162 (14.3) | 420 (19.9) | ||
| NYHA III (%) | 1321 (40.7) | 449 (39.6) | 872 (41.3) | ||
| NYHA IV (%) | 1143 (35.2) | 470 (41.5) | 673 (31.9) | ||
| Medical history | |||||
| ASCVD (%) | 3932 (67.5) | 1153 (57.4) | 2779 (72.8) | <0.001 | 0 |
| Atrial fibrillation (%) | 2885 (51.5) | 966 (50.6) | 1919 (52) | 0.315 | 0 |
| Hypertension (%) | 4481 (80) | 1567 (82) | 2914 (78.9) | 0.006 | 0 |
| Dyslipidaemia (%) | 222 (4) | 65 (3.4) | 157 (4.3) | 0.122 | 0 |
| COPD (%) | 815 (14.6) | 241 (12.6) | 574 (15.6) | 0.003 | 0 |
| CKD (%) | 2485 (42.7) | 874 (43.5) | 1611 (42.2) | 0.325 | 0 |
| T2DM | 1601 (27.5) | 476 (23.7) | 1125 (29.5) | <0.001 | 0 |
| Medications | |||||
| Insulin (%) | 1813 (31.4) | 530 (26.7) | 1283 (33.9) | <0.001 | 0 |
| Oral antidiabetic medicine (%) | 1307 (22.7) | 400 (20.2) | 907 (24) | 0.001 | 0 |
| Antithrombotic medications (%) | 5681 (98.5) | 1947 (98.2) | 3734 (98.6) | 0.193 | 0 |
| Beta-blockers (%) | 4971 (86.2) | 1632 (82.3) | 3339 (88.2) | <0.001 | 0 |
| Digoxin and nitrates (%) | 1796 (31.1) | 700 (35.3) | 1096 (28.9) | <0.001 | 0 |
| RAAS inhibitors (%) | 5117 (88.7) | 1702 (85.8) | 3415 (90.2) | <0.001 | 0 |
| Diuretics (%) | 5194 (90) | 1812 (91.4) | 3382 (89.3) | 0.014 | 0 |
| Implantable cardiac devices (%) | 468 (8) | 73 (3.6) | 395 (10.3) | <0.001 | 0 |
| Deaths (%) | 2912 (50) | 1058 (52.7) | 1854 (48.6) | 0.003 | 0 |
| Observed time (years) (median, IQR) | 3.39 (1.63–5.05) | 3.25 (1.52–4.95) | 3.47 (1.72–5.10) | 0.001 | 0 |
- Abbreviations: ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HFmrEF, HF with mid-range ejection fraction; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; IQR, inter-quartile range; NYHA, New York Heart Association; RAAS, renin–angiotensin–aldosterone system; T2DM, type 2 diabetes mellitus.
A total of 5674 patients were excluded due to missing LVEF data; 1163 patients had missing survival data; and 11 patients had erroneous survival data. Our eligible population included in the final analyses consisted of 5824 patients, with 34% females and 65% males. The median age was 77 and 71, respectively. T2DM occurred with a prevalence of 27.1% across the whole population, with a higher prevalence in males (29.5% vs. 23.7% in females). Among the most prevalent comorbidities were hypertension (80%), ASCVD (72%), atrial fibrillation (51.5%) and CKD (42.7%). Based on LVEF, more than half of the population was classified as having an HFpEF (52.9%), followed by HFrEF (37.3%) and HFmrEF (9.8%). NYHA scores from NYHA I, II, III and IV were 6.1%, 17.9%, 40.7% and 35.2%, respectively. The median follow-up time was 3.39 years, with 2912 (50%) deaths observed, 1058 (52.7%) in the female and 1854 (48.6%) in the male subpopulation.
LVEF and mortality risk
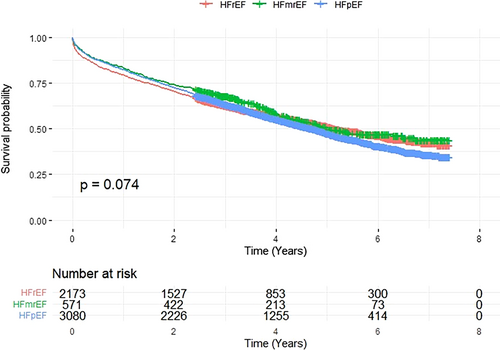
Overall survival analysis displayed patients with HFpEF to have the lowest survival rates, as depicted on Kaplan–Meier curves (Figure A1). Survival rates did not differ significantly between LVEF categories. The HFrEF category was used as a reference for regression analyses. Cox proportional hazard regression Model 1 (adjusted for age and sex) showed no significant differences in the risk of all-cause mortality for HFmrEF [HR 0.874, 95% confidence interval (CI): 0.763–1.002, P = 0.054] (Table 2) and HFpEF (HR 0.942, 95% CI: 0.870–1.020, P = 0.140). Model 2, adjusted for comorbidities, revealed lower overall mortality in the HFpEF category (HR 0.869, 95% CI: 0.800–0.944, P < 0.001) and upon further stratification in patients with T2DM (HR 0.788, 95% CI: 0.684–0.907, P < 0.001) (Table 2). No significant interaction terms were observed between LVEF categories, sex and T2DM status (P = 0.859).
| Model 1 | Model 2 | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Overall population | ||||||
| HFrEF | Reference | Reference | ||||
| HFmrEF | 0.874 | 0.763 | 1.002 | 0.890 | 0.776 | 1.022 |
| HFpEF | 0.942 | 0.870 | 1.020 | 0.869 | 0.800 | 0.944 |
| Females | ||||||
| HFrEF | Reference | Reference | ||||
| HFmrEF | 1.123 | 0.880 | 1.434 | 1.144 | 0.894 | 1.465 |
| HFpEF | 0.979 | 0.852 | 1.126 | 0.914 | 0.791 | 1.057 |
| Males | ||||||
| HFrEF | Reference | Reference | ||||
| HFmrEF | 0.790 | 0.670 | 0.932 | 0.799 | 0.677 | 0.944 |
| HFpEF | 0.929 | 0.843 | 1.023 | 0.851 | 0.769 | 0.941 |
| T2DM | ||||||
| HFrEF | Reference | Reference | ||||
| HFmrEF | 0.874 | 0.687 | 1.113 | 0.878 | 0.689 | 1.118 |
| HFpEF | 0.837 | 0.730 | 0.959 | 0.788 | 0.684 | 0.907 |
| No diabetes | ||||||
| HFrEF | Reference | Reference | ||||
| HFmrEF | 0.886 | 0.751 | 1.045 | 0.903 | 0.764 | 1.068 |
| HFpEF | 0.987 | 0.895 | 1.088 | 0.906 | 0.818 | 1.003 |
- Abbreviations: CI, confidence interval; HFmrEF, HF with mid-range ejection fraction; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; HR, hazard ratio; T2DM, type 2 diabetes mellitus.
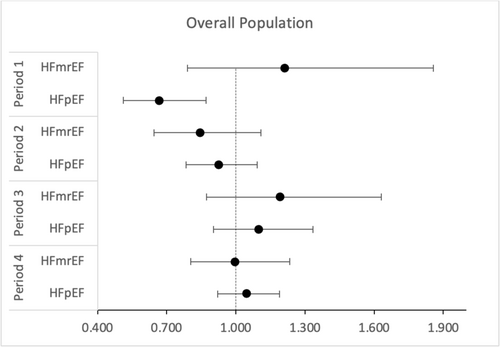
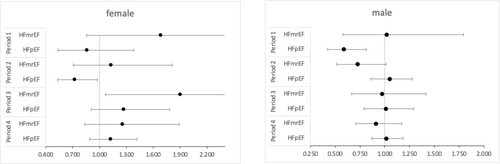
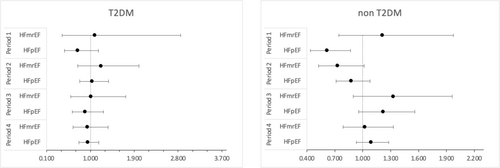
Cox regression models did not fulfil the proportionality of the hazard assumption. Subsequent time-stratified analyses (Table 3) showed that the lower risk of mortality for HFpEF patients was found during the initial 30 days of observation (HR 0.67, 95% CI: 0.52–0.88, P = 0.003) (Figure 2). After stratification by sex, the effect persisted in males (HR 0.59, 95% CI: 0.42–0.81, P < 0.001) but was no longer statistically significant in females (HR 0.86, 95% CI: 0.54–1.38, P = 0.533) (Figure 3). Stratification by T2DM status maintained the effect in non-T2DM patients only (HR 0.62, 95% CI: 0.44–0.87, P = 0.005) (Figure 4). After 1 year, women showed an isolated reduced HFpEF mortality (HR 0.72, 95% CI: 0.53–0.98, P = 0.035) (Figure 3). After 2 years, no significant mortality risk differences were observed across LVEF categories, nor upon further stratification by T2DM and sex.
| Period 1 | Period 2 | Period 3 | Period 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||||
| Overall population | ||||||||||||
| HFrEF | Reference | Reference | Reference | Reference | ||||||||
| HFmrEF | 1.211 | 0.790 | 1.856 | 0.846 | 0.646 | 1.109 | 1.193 | 0.873 | 1.630 | 0.996 | 0.804 | 1.233 |
| HFpEF | 0.669 | 0.513 | 0.872 | 0.925 | 0.783 | 1.092 | 1.098 | 0.903 | 1.334 | 1.046 | 0.921 | 1.189 |
| Females | ||||||||||||
| HFrEF | Reference | Reference | Reference | Reference | ||||||||
| HFmrEF | 1.683 | 0.857 | 3.305 | 1.131 | 0.707 | 1.811 | 1.902 | 1.066 | 3.394 | 1.256 | 0.836 | 1.888 |
| HFpEF | 0.861 | 0.537 | 1.380 | 0.722 | 0.534 | 0.977 | 1.273 | 0.908 | 1.784 | 1.124 | 0.890 | 1.420 |
| Males | ||||||||||||
| HFrEF | Reference | Reference | Reference | Reference | ||||||||
| HFmrEF | 1.018 | 0.578 | 1.792 | 0.722 | 0.515 | 1.013 | 0.972 | 0.667 | 1.415 | 0.911 | 0.709 | 1.172 |
| HFpEF | 0.586 | 0.422 | 0.815 | 1.049 | 0.861 | 1.278 | 1.012 | 0.792 | 1.293 | 1.015 | 0.870 | 1.184 |
| T2DM | ||||||||||||
| HFrEF | Reference | Reference | Reference | Reference | ||||||||
| HFmrEF | 1.095 | 0.421 | 2.849 | 1.216 | 0.741 | 1.995 | 1.015 | 0.597 | 1.726 | 0.938 | 0.646 | 1.362 |
| HFpEF | 0.740 | 0.469 | 1.165 | 1.035 | 0.782 | 1.371 | 0.895 | 0.631 | 1.270 | 0.949 | 0.766 | 1.175 |
| No diabetes | ||||||||||||
| HFrEF | Reference | Reference | Reference | Reference | ||||||||
| HFmrEF | 1.212 | 0.743 | 1.976 | 0.729 | 0.523 | 1.017 | 1.327 | 0.898 | 1.961 | 1.023 | 0.788 | 1.329 |
| HFpEF | 0.616 | 0.437 | 0.868 | 0.877 | 0.713 | 1.079 | 1.222 | 0.956 | 1.562 | 1.091 | 0.930 | 1.280 |
- Abbreviations: CI, confidence interval; HFmrEF, HF with mid-range ejection fraction; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; HR, hazard ratio; T2DM, type 2 diabetes mellitus.
Sensitivity analysis
A very slight yet significant association between LVEF and mortality was found when the former was considered as a continuous variable (HR 0.996, 95% CI: 0.994–0.999, P = 0.002). Excluding HF patients with multiple LVEF measurements did not alter our results significantly (Tables A1 and A2). Application of a quadratic term showed that the relationship between LVEF and mortality was not linear (P < 0.001). The exclusion of patients with multiple LVEF measurements (N = 3617; 62.1%) as well as conducting the analyses with the minimal, maximal or baseline LVEF values assessed over the observed period did not significantly alter results (data not shown). In a model adjusted for sex, age and the number of comorbidities as a continuous variable, we found that the risk of mortality increased by approximately 15.9% for each additional comorbidity (HR 1.159, 95% CI: 1.121–1.197, P < 0.001). Further adjustment for medications did not alter our main findings (data not shown).
Discussion
In this study of HF patients receiving inpatient care, we showed no consistent association of LVEF categories with mortality. However, our findings suggest the possible presence of a time-dependent prognostic significance of LVEF. Our results were not significantly affected by sex or diabetes status, although women in general presented with higher age and mortality.
We showed that a lower mortality risk may be present among HFpEF patients, an effect possibly driven by the first days of follow-up in our cohort. In line with our findings, HFrEF was usually believed to be associated with the highest mortality compared with HFpEF,16 but more recent studies have shown conflicting results. One study analysing patients from the ASCEND-HF trial with acute decompensated HF found similar mortality rates across different LVEF categories. However, after adjusting for age and sex, mortality significantly increased in the population with LVEF < 35%, representing a large part of HFrEF patients in the study.17 Nonetheless, it primarily focused on short-term outcomes in acute HF, which may have missed important effects influenced by the dynamics of chronic HF.
Our observed lower mortality rate for HFpEF in time-stratified analyses within the first 30 days is likely attributable to the inclusion of HFrEF patients experiencing acute illness, a factor that has previously been demonstrated to be associated with higher 30 day mortality within this category.18 Beyond 2 years, there were no significant mortality rate differences across LVEF categories within our cohort. Shiga et al. also found comparable mortality rates across LVEF categories in their multicentre study.19 Contrary to those findings, Lam et al. showed significantly higher mortality rates for HFrEF compared with both HFpEF and HFmrEF following patients over 2 years in a prospective multicentre study.20 A potential explanation for the discrepancy in results might be the specific setting from which patients were recruited for the analyses. In the study of Lam et al., 30%–39% of HF patients were receiving outpatient care. Mortality rates have been criticized for significantly differing between inpatient and outpatient HF populations, given that the latter generally include younger, more stable patients with less comorbidities, thus limiting comparability.21 Therefore, the HFpEF and HFmrEF patients in our cohort may have experienced different impacts from their more serious condition compared with outpatients, potentially leading to a convergence in mortality rates with HFrEF patients over time.
The investigation of mortality in HFmrEF patients remains a topic of ongoing debate. Although Koh et al. demonstrated that HFmrEF and HFpEF exhibit comparable mortality rates,22 a finding further supported by a recent meta-analysis,23 the HFmrEF phenotype is distinct. It encompasses both HFrEF patients who recover their LVEF24 and HFpEF patients who experience a decline of their LVEF due to the natural progression of the disease.25 Therefore, it has been suggested to consider HFmrEF as a transitional zone, where patients with diverse HF aetiologies may coexist at varying stages in the progression of their disease, somewhat impairing assessment of mortality rates within this category by itself.
Regardless of the LVEF category, ample evidence has shown hospitalization to be of prognostic value. Shah et al. demonstrated a very high 5 year mortality rate of 75.4% for hospitalized patients, irrespective of LVEF.26 In line with this, Koudstaal et al. found the lowest mortality rates to be present in patients with newly recorded HF and no prior hospital admissions.27 The effect of hospitalizations on mortality burden has yet to be established.
Concerning sex differences, the overall mortality, regardless of LVEF category, was higher among women. Furthermore, a notable reduction in 1 year mortality was observed in females with HFpEF compared with females with HFrEF, which could suggest that women within this LVEF category in our cohort were more stable than those in the other categories at this time. Prior evidence exploring sex differences in HF showed conflicting results,28 which are partly rooted in epidemiological variations of the disease between the sexes. Not only do women generally exhibit a lower incidence of HF compared with men,29 but they also display a relatively higher prevalence of HFpEF.7 For instance, a study by Stolfo et al. investigating over 42 000 patients in the Swedish Heart Failure Registry reported that 55% of the HFpEF population was female, compared with 29% women within the HFrEF population.30 Initially, the disproportionate distribution was merely attributed to women's longer life expectancy and different ages of HF presentation.31 However, recent findings suggest that pathophysiological sex differences may play a significant role, as a recent review demonstrated.32 Men are predominantly affected by macrovascular disease, leading to myocyte necrosis and scar formation as the primary drivers of HF pathogenesis. In contrast, females may experience endothelial inflammation and coronary microvascular dysfunction, potentially explaining higher rates of HF in connection with emotional distress and chemotherapy. Additionally, these differences are presumed to account for HF manifestations exclusive to females, such as peripartum cardiomyopathy.33 Overall mortality was significantly higher for females in our cohort, emphasizing the need for attention to sex-specific factors in general, irrespective of any disparities in HF categorization between the sexes.
Our study results not only highlight the challenges in HF categorization, as evidenced by discrepancies in mortality analyses as well as epidemiological and pathophysiological sex differences, but they also underscore the oversimplification held by relying on LVEF only for classification of HF as a complex clinical syndrome. This is further supported by our finding that the relationship between LVEF and mortality was not linear, and a time-dependent effect could be present. A recent article by Mele et al. suggested to focus on HF aetiology as a guiding factor for disease categorization, prognostic assessment and the conception of therapeutic measures.34
As to comorbidities within our HF population, we showed higher mortality with increasing comorbidity burden, and their prevalence aligns with prior research investigating their distribution.35 While holding significance in HF across LVEF categories, they have been shown to be of both higher prevalence and clinical relevance in HFpEF.31 Accordingly, Fu et al. underscored their role not only as key drivers of adverse outcomes but also as predisposing factors for HFpEF.36 This suggests that cardiac dysfunction itself may not be the primary determinant of adverse outcomes in HFpEF, highlighting the therapeutic importance of targeting comorbidities within this category instead. Although T2DM is typically not the most prevalent comorbidity compared with, for example, ASCVD and hypertension in HF, its importance with respect to HF prognosis has been established for decades, as evidenced by Bauters et al.37 Our finding of lower overall 30 day mortality for HFpEF was no longer significant in T2DM patients upon stratification by T2DM status, which is in line with established evidence that the presence of T2DM is commonly associated with a worsened HF prognosis.38 This further underlines the role of early initiation of antidiabetic medication in improving HF prognosis, as supported by recent evidence displaying a lower risk of death from cardiovascular causes in patients receiving a sodium–glucose cotransporter-2 (SGLT-2) inhibitor, regardless of T2DM status.39-42 Antidiabetic medication has thus become a central pillar of HF therapy.
To build on the previously discussed sex differences in relation to comorbidities, it is important to emphasize the potential interplay between older age and a possibly more severe set of comorbidities experienced by women. This is reflected in the fact that women were significantly more likely to have HFpEF and be classified with NYHA IV, as shown by our results and the supporting evidence mentioned earlier. These factors together may significantly contribute to the higher mortality rates among women with HF that we observed.
Among the strengths of our study are the substantial sample size as well as the controlled environment provided by the University Hospital of Bern, being the largest tertiary cardiovascular centre in Switzerland. The study design and methodology, as well as our statistical approach, were clearly defined and performed, contributing to robust findings. Additionally, we considered and emphasized sex differences in HF, addressing a crucial aspect that is often not taken into consideration even in current cardiovascular research.
However, some limitations must be acknowledged with our findings. Date of HF diagnosis was not registered in our study population, which limited us from understanding how timing of LVEF measurements was related to the documentation of HF. In patients with multiple LVEF entries, we opted for the average value, recognizing that this might not ideally represent the overall disease trajectory and could potentially result in the misclassification of some participants. We tried to mitigate this limitation through our sensitivity analyses. Another limitation is that our study focused on all-cause mortality and not death from HF itself. Therefore, observed mortality rates cannot be attributed to cardiac dysfunction only in the patients we investigated. We did not adjust our models for common confounders such as smoking or the use of HF medications like mineralocorticoid receptor antagonists (MRAs) and SGLT-2 inhibitors, and including diagnosing HF based on ICD codes only may have been an oversimplification.
In conclusion, our study suggests that LVEF alone might not be sufficient to predict the prognosis of HF patients. Future studies should aim at understanding the role of novel imaging techniques in supporting LVEF as a prognostic factor in HF. In addition, other factors such as acute versus chronic HF, inpatient versus outpatient settings, sex and gender differences, and objective HF measures beyond LVEF should be incorporated into future studies aiming at HF characterization.
Acknowledgements
We are indebted to the patients who willingly consented to the use of their health-related data.
Conflict of interest statement
Taulant Muka works at Epistudia GmbH and acts as an unpaid advisor for the Academic Parity Movement, a non-profit organization uprooting academic bullying and discrimination. The other authors declare that they have no conflicts of interest.
Funding
This project was supported by AstraZeneca Schweiz as a project grant for LH.
Appendix A
| EF category | HR | 95% CI | |
|---|---|---|---|
| HFrEF (reference) | Reference | ||
| HFmrEF | 0.839 | 0.670 | 1.052 |
| HFpEF | 0.744 | 0.653 | 0.846 |
- Abbreviations: CI, confidence interval; EF, ejection fraction; HFmrEF, HF with mid-range ejection fraction; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; HR, hazard ratio.
| Period 1 | Period 2 | Period 3 | Period 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |||||
| Overall population | ||||||||||||
| HFrEF | Reference | Reference | Reference | Reference | ||||||||
| HFmrEF | 1.380 | 0.812 | 2.346 | 0.824 | 0.543 | 1.253 | 1.487 | 0.820 | 2.698 | 0.928 | 0.624 | 1.378 |
| HFpEF | 0.736 | 0.523 | 1.036 | 0.875 | 0.688 | 1.112 | 1.082 | 0.790 | 1.484 | 0.893 | 0.707 | 1.128 |
| Females | ||||||||||||
| HFrEF | Reference | Reference | Reference | Reference | ||||||||
| HFmrEF | 1.472 | 0.597 | 3.626 | 1.579 | 0.734 | 3.397 | 1.614 | 0.587 | 4.434 | 1.420 | 0.763 | 2.642 |
| HFpEF | 0.711 | 0.343 | 1.472 | 0.630 | 0.393 | 1.008 | 1.630 | 0.954 | 2.786 | 0.831 | 0.551 | 1.251 |
| Males | ||||||||||||
| HFrEF | Reference | Reference | Reference | Reference | ||||||||
| HFmrEF | 1.319 | 0.669 | 2.602 | 0.682 | 0.407 | 1.144 | 1.378 | 0.639 | 2.974 | 0.740 | 0.437 | 1.254 |
| HFpEF | 0.710 | 0.470 | 1.074 | 0.947 | 0.711 | 1.262 | 0.766 | 0.500 | 1.172 | 0.929 | 0.699 | 1.235 |
| T2DM | ||||||||||||
| HFrEF | Reference | Reference | Reference | Reference | ||||||||
| HFmrEF | 1.210 | 0.401 | 3.652 | 1.347 | 0.551 | 3.296 | 2.637 | 0.950 | 7.318 | 0.799 | 0.387 | 1.646 |
| HFpEF | 0.870 | 0.481 | 1.572 | 1.069 | 0.704 | 1.622 | 0.915 | 0.495 | 1.692 | 0.803 | 0.536 | 1.204 |
| No diabetes | ||||||||||||
| HFrEF | Reference | Reference | Reference | Reference | ||||||||
| HFmrEF | 1.390 | 0.742 | 2.604 | 0.675 | 0.402 | 1.135 | 1.223 | 0.566 | 2.646 | 1.015 | 0.631 | 1.631 |
| HFpEF | 0.618 | 0.396 | 0.967 | 0.804 | 0.594 | 1.090 | 1.264 | 0.862 | 1.854 | 0.924 | 0.693 | 1.233 |
- Abbreviations: CI, confidence interval; HFmrEF, HF with mid-range ejection fraction; HFpEF, HF with preserved ejection fraction; HFrEF, HF with reduced ejection fraction; HR, hazard ratio; T2DM, type 2 diabetes mellitus.




