Empagliflozin inhibits Na+/H+ exchanger activity in human atrial cardiomyocytes
Abstract
Aims
Recent clinical trials have proven gliflozins to be cardioprotective in diabetic and non-diabetic patients. However, the underlying mechanisms are incompletely understood. A potential inhibition of cardiac Na+/H+ exchanger 1 (NHE1) has been suggested in animal models. We investigated the effect of empagliflozin on NHE1 activity in human atrial cardiomyocytes.
Methods and results
Expression of NHE1 was assessed in human atrial and ventricular tissue via western blotting. NHE activity was measured as the maximal slope of pH recovery after NH4+ pulse in isolated carboxy-seminaphtarhodafluor 1 (SNARF1)-acetoxymethylester-loaded murine ventricular and human atrial cardiomyocytes. NHE1 is abundantly expressed in human atrial and ventricular tissue. Interestingly, compared with patients without heart failure (HF), atrial NHE1 expression was significantly increased in patients with HF with preserved ejection fraction and atrial fibrillation. The largest increase in atrial and ventricular NHE1 expression, however, was observed in patients with end-stage HF undergoing heart transplantation. Importantly, acute exposure to empagliflozin (1 μmol/L, 10 min) significantly inhibited NHE activity to a similar extent in human atrial myocytes and mouse ventricular myocytes. This inhibition was also achieved by incubation with the well-described selective NHE inhibitor cariporide (10 μmol/L, 10 min).
Conclusions
This is the first study systematically analysing NHE1 expression in human atrial and ventricular myocardium of HF patients. We show that empagliflozin inhibits NHE in human cardiomyocytes. The extent of NHE inhibition was comparable with cariporide and may potentially contribute to the improved outcome of patients in clinical trials.
Background
Gliflozins have been developed as oral antidiabetic drugs acting via increased urinary glucose excretion by selective inhibition of Na-glucose contransporter 2 (SGLT2) in the renal proximal tubules.1 Surprisingly, large clinical trials have shown a reduced incidence of hospitalization for heart failure (HF) and cardiovascular death in diabetic as well as non-diabetic patients treated with gliflozins. Numerous potential mechanisms were proposed4 including direct cardiac effects [inhibition of cardiac Ca/calmodulin-dependent protein kinase II (CaMKII)],5 effects on contractile and myofilament function,6, 7 up-regulation of cardiac glucose transporter 1 (GLUT1)8 as well as metabolic changes.9, 10 However, the cardiac target for these drug effects remains elusive, especially because cardiac SGLT2 expression is negligible.5 Recently, empagliflozin has been shown to reduce the activity of cardiac Na+/H+ exchanger 1 (NHE1) in mouse, rat, and rabbit hearts,11, 12 a mechanism of action, which has repeatedly been suggested to be cardioprotective.13, 14 However, to date, it was unclear if empagliflozin could also inhibit NHE in human myocardium.
Aims
We aimed at investigating the acute effect of empagliflozin on NHE activity in isolated human cardiomyocytes and characterizing NHE1 expression in human cardiac tissue of patients with HF.
Methods
Between April 2019 and November 2019, patients undergoing cardiac surgery at the University Hospital Regensburg were prospectively tested for eligibility. Right atrial appendage biopsies were collected during surgery. In addition, left ventricular (LV) tissue of patients with LV hypertrophy and normal systolic LV function [ejection fraction (EF) > 50%] was collected from septum resections obtained during surgical aortic valve implantation. Atrial and LV tissue was also collected from explanted hearts of heart transplant recipients. The study was approved by local ethics committees (University of Regensburg, Bavaria, Germany, as well as University of Göttingen, Lower Saxony, Germany) and performed in accordance with the Declaration of Helsinki (first released in 1964; most recent revision 2013). Patients provided written consent prior to tissue donation. Experiments with murine cardiomyocytes conformed to Directive 2010/63/EU of the European Parliament, to the ‘Guide for the Care and Use of Laboratory Animals’ (Eighth Edition, 2011) published by the US National Institutes of Health, and to local institutional guidelines.
The NHE1 expression and activity were analysed using western blotting and NH4+ pulse method, respectively. For detailed description of the experimental methodology, we kindly refer to Supporting Information, Data S1.
Experimental data are presented as means ± standard error of the mean and continuous clinical data as means ± standard deviation. Statistics were based on the number of patients or mice, respectively. Mixed effects analysis with Holm–Sidak's post hoc test, Wilcoxon matched-pairs signed-rank test, and Brown–Forsythe and Welch ANOVA test with two-stage step-up procedure of Benjamini, Krieger, and Yekutieli was applied to test for statistical significance as appropriate. Differences in patients' clinical baseline characteristics were analysed using Pearson's χ2 test and Fisher's exact test for categorical data and ANOVA, Student's t-test, or Kruskal–Wallis test for continuous data, respectively. Bonferroni correction was applied for multiple comparisons. All these tests were performed in GraphPad Prism 8 or Stata/MP 13.0. Two-sided P-values below 0.05 were considered statistically significant.
Results
Right atrial tissue of 52 patients with coronary and/or valvular heart disease undergoing elective cardiac surgery as well as of 4 patients with end-stage ischaemic heart disease undergoing cardiac transplantation was analysed for atrial NHE1 expression (Table 1). These patients represent a typical cohort of patients with heart disease. They were 66 ± 11 (mean ± standard deviation) years old and predominantly of male gender (91%). Approximately one-third of the patients suffered from diabetes mellitus, and 86% had arterial hypertension. Forty-one per cent were diagnosed with HF, requiring signs and symptoms. Seven per cent exhibited severely reduced left ventricular ejection fraction (LVEF) [heart failure with reduced ejection fraction (HFrEF), EF < 40%, transplanted patients not included]. Fifteen (27%) patients were diagnosed with HF with preserved ejection fraction (HFpEF) (LVEF > 50) additionally requiring elevated N-terminal pro-brain natriuretic peptide levels and either LV hypertrophy (LV mass index ≥ 115 g/m2 for male patients and ≥95 g/m2 for female patients), left atrial enlargement (left atrial volume index > 34 mL/m2), or diastolic dysfunction according to current guidelines. The majority of patients were in sinus rhythm (SR; 89%), while 11% were diagnosed with atrial fibrillation (AF).
| N = 69 | Analysis of NHE1 expression | Analysis of NHE function | P | ||||
|---|---|---|---|---|---|---|---|
| All patients (N = 56) | Non-failing (N = 33) | HFpEF (N = 15) | HFrEF (N = 4) | HTX (N = 4) | N = 13 | ||
| Age (years), mean ± SD | 66 ± 11 | 65 ± 9 | 75 ± 6* | 65 ± 4 | 47 ± 16†,Δ,ϕ | 68 ± 9 | <0.001A |
| Male gender, N (%) | 51 (91%) | 30 (91%) | 14 (93%) | 3 (75%) | 4 (100%) | 12 (92%) | 0.77Chi |
| BMI (kg/m2), mean ± SD | 28 ± 4 | 29 ± 5 | 28 ± 3 | 28 ± 4 | 25 ± 4 | 27 ± 4 | 0.17A |
| Coronary artery disease, N (%) | 56 (100%) | 33 (100%) | 15 (100%) | 4 (100%) | 4 (100%) | 12 (92%) | 0.36Chi |
| Severe aortic stenosis, N (%) | 6 (11%) (47) | 1 (3%) (29) | 4 (27%) (14) | 1 (25%) (4) | — | 1 (8%) | 0.098Chi |
| LVEF, mean ± SD | 55 ± 13 | 61 ± 6 | 59 ± 5 | 35 ± 1#,⁋ | 20 ± 7†,Δ,Ω,ϕ | 61 ± 7π | <0.001A |
| Heart failure, N (%) | 23 (41%) | 0 (0%) | 15 (100%)* | 4 (100%)# | 4 (100%)† | 3 (23%)‡,∂ | <0.001Chi |
| HFpEF, N (%) | 15 (27%) | 0 (0%) | 15 (100%)* | 0 (0%)⁋ | 0 (0%)Δ | 1 (8%)∂ | <0.001Chi |
| HFmrEF, N (%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (15%) | 0.064Chi |
| HFrEF, N (%) | 4 (7%) | 0 (0%) | 0 (0%) | 4 (100%)#,⁋ | 0 (0%)†,Δ | 0 (0%)π,ϕ | <0.001Chi |
| Heart transplant, N (%) | 4 (7.1%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (100%) | 0 (0%) | <0.001Chi |
| IVSd (mm), mean ± SD (N) | 11.9 ± 2.1 (42) | 12.5 ± 2.0 (24) | 11.6 ± 1.4 (12) | 12.3 ± 2.1 (3) | 8 ± 1 (3)†,Δ | 11.9 ± 2.1 (10) | 0.008A |
| LAVI (mL/m2), mean ± SD (N) | 34.7 ± 10.2 (18) | 30.9 ± 11.1 (10) | 41.0 ± 7.3 (6) | 35.1 ± 2.3 (2) | — | 24.7 ± 3.8 (3) | 0.087A |
| LA dilation, N (%) (N) | 21 (37.5%) (48) | 7 (21%) (29) | 10 (71%) (14)* | 1 (25%) (2) | 3 (75%) (3) | 4 (31%) (12) | 0.01Chi |
| E/A, mean ± SD (N) | 0.94 ± 0.39 (23) | 0.91 ± 0.31 (17) | 1.14 ± 0.60 (5) | 0.5 (1) | — | 0.7 ± 0.3 (3) | 0.29A |
| E/e′, mean ± SD (N) | 11.9 ± 5.1 (25) | 10.2 ± 3.5 (18) | 16.6 ± 6.5 (6)* | 11 (1) | — | 10.5 ± 5.3 (4) | 0.031A |
| Diastolic dysfunction, N (%) (N) | 30 (54%) (39) | 16 (48%) (25) | 12 (80%) (12) | 2 (50%) (2) | — | 1 (8%) (5) | 0.077Chi |
| NT-proBNP (pg/mL), median (IQR) (N) | 373 (138, 1601) (51) | 161 (80, 249) (28) | 1075 (538, 2046) (15)* | 2977 (2624, 3708) (4)# | 2963 (1274, 4885)† | 491 (201, 1695)‡,π | 0.001KW |
| Hypercholesterolaemia, N (%) | 33 (59%) | 22 (67%) | 6 (40%) | 2 (50%) | 3 (75%) | 5 (28%) | 0.26Chi |
| Arterial hypertension, N (%) | 48 (86%) | 30 (91%) | 13 (87%) | 3 (75%) | 2 (50%) | 9 (69%) | 0.17Chi |
| Sinus rhythm, N (%) | 50 (89%) | 33 (100%) | 10 (67%)* | 4 (100%) | 3 (75%)† | 11 (85%) | 0.013Chi |
| Atrial fibrillation, N (%) | 6 (11%) | 0 (0%) | 5 (33%)* | 0 (0%) | 1 (25%)† | 2 (15%) | 0.013Chi |
| Paroxysmal | 3 (5%) | 0 (0%) | 2 (13%) | 0 (0%) | 1 (25%) | 2 (15%) | 0.14Chi |
| Persistent/permanent | 3 (5%) | 0 (0%) | 3 (20%)* | 0 (0%) | 0 (0%) | 0 (0%) | 0.023Chi |
| GFR (mL/min/1.73 m2), mean ± SD | 70 ± 21 | 73 ± 22 | 65 ± 19 | 76 ± 21 | 57 ± 18 | 64 ± 25 | 0.46A |
| Creatinine (mg/dL), mean ± SD | 1.3 ± 1.3 | 1.3 ± 1.7 | 1.2 ± 0.3 | 1.0 ± 0.3 | 1.5 ± 0.3 | 1.5 ± 1.3 | 0.95A |
| Diabetes mellitus, N (%) | 20 (36%) | 12 (36%) | 5 (33%) | 3 (75%) | 0 (0%) | 4 (31%) | 0.27Chi |
| HbA1c (%), mean ± SD (N) | 6.3 ± 1.3 (52) | 6.2 ± 0.8 (31) | 6.1 ± 1.6 (14) | 8.0 ± 2.7# (4) | 5.6 ± 0.3 (3) | 5.6 ± 0.8 (11) | 0.016A |
| Antidiabetic treatment, N (%) | 20 (36%) | 12 (36%) | 5 (33%) | 3 (75%) | 0 (0%) | 4 (31%) | 0.27Chi |
| Dietetic treatment, N (%) | 2 (3.6%) | 2 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7.7%) | 0.80Chi |
| Medical treatment, N (%) | 10 (17.9%) | 4 (12.1%) | 3 (20%) | 3 (75%)# | 0 (0%) | 2 (15.4%) | 0.029Chi |
| Metformin, N (%) | 7 (12.5%) | 4 (12.1%) | 2 (13.3%) | 2 (50%) | 0 (0%) | 2 (15.4%) | 0.30Chi |
| Gliptin, N (%) | 5 (8.9%) | 1 (3.0%) | 3 (20%) | 1 (25%) | 0 (0%) | 0 (0%) | 0.10Chi |
| Dapagliflozin, N (%) | 1 (1.8%) | 1 (3.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.89Chi |
| Insulin ± medical treatment, N (%) | 8 (14.3%) | 6 (18.2%) | 2 (13.3%) | 0 (0%) | 0 (0%) | 1 (7.7%) | 0.68Chi |
- BMI, body mass index; GFR, glomerular filtration rate; HFmrEF, patients with heart failure with mid-range ejection fraction; HbA1c, haemoglobin A1c; HFpEF, patients with heart failure with preserved ejection fraction; HFrEF, patients with heart failure with reduced ejection fraction; HTX, patients with end-stage heart failure undergoing heart transplantation; IQR, interquartile range; IVSd, interventricular septum thickness in diastole; LA, left atrium; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; NHE, Na+/H+ exchanger; NT-proBNP, N-terminal pro-brain natriuretic peptide; SD, standard deviation.
- Values are either the total number of patients (relative proportion in parentheses), mean ± SD or median with IQR, as indicated. If data were not available for all patients studied, the number of patients used for analysis is given in parentheses. Relative proportions always refer to the total number of patients of the respective subgroup. P-values are calculated using Pearson's χ2 test for categorical data (Chi), ANOVA (A), or Kruskal–Wallis test (KW) for continuous data, respectively. Bonferroni correction was applied for multiple comparisons. The bold emphasis is applied to highlight statistically significant p-values < 0.05.
- * P < 0.05 for HFpEF vs. Non-failing.
- # P < 0.05 for HFrEF vs. Non-failing.
- † P < 0.05 for HTX vs. Non-failing.
- ‡ P < 0.05 for NHE function vs. Non-failing.
- ⁋ P < 0.05 for HFrEF vs. HFpEF.
- Δ P < 0.05 for HTX vs. HFpEF.
- ∂ P < 0.05 for NHE function vs. HFpEF.
- Ω P < 0.05 for HTX vs. HFrEF.
- π P < 0.05 for NHE function vs. HFrEF.
- ϕ P < 0.05 for NHE function vs. HTX.
In addition, LV tissue was obtained from patients with LV hypertrophy and normal systolic LV function (EF > 50%) undergoing aortic valve replacement surgery as well as from end-stage failing hearts explanted during cardiac transplantation (Table 2).
| LVH (N = 5) | HTX (N = 5) | P | |
|---|---|---|---|
| Age (years), mean ± SD | 65 ± 8 | 49 ± 15 | 0.07S |
| Male gender, N (%) | 3 (60%) | 5 (100%) | 0.44F |
| LVEF, mean ± SD | 65 ± 9 | 20 ± 6 | <0.0001S |
| IVSd (mm), mean ± SD (N) | 13.5 ± 2.1 (2) | 8.3 ± 0.9 (4) | 0.01S |
| Hypercholesterolaemia, N (%) | 1 (20%) (4) | 3 (60%) | 0.52F |
| Arterial hypertension, N (%) | 3 (60%) | 3 (60%) | 1.0F |
| Sinus rhythm, N (%) | 5 (100%) | 4 (80%) | 1.0F |
| Atrial fibrillation, N (%) | 0 (0%) | 1 (20%) | 1.0F |
| Paroxysmal | 0 (0%) | 1 (20%) | 1.0F |
| Persistent/permanent | 0 (0%) | 0 (0%) | 1.0F |
| Unspecified | 0 (0%) | 0 (0%) | 1.0F |
| Diabetes mellitus, N (%) | 1 (20%) | 0 (0%) | 1.0F |
| HbA1c (%), mean ± SD (N) | 5.7 ± 1.37 (5) | 5.49 ± 0.33 (4) | 0.78S |
| Antidiabetic treatment, N (%) | 1 (20%) | 0 (0%) | 1.0F |
| Dietetic treatment, N (%) | 1 (20%) | 0 (0%) | 1.0F |
| Medical treatment, N (%) | 0 (0%) | 0 (0%) | 1.0F |
| Insulin ± medical treatment, N (%) | 0 (0%) | 0 (0%) | 1.0F |
- HbA1c, haemoglobin A1c; HTX, patients with end-stage heart failure undergoing heart transplantation; IVSd, interventricular septum thickness in diastole; LVEF, left ventricular ejection fraction; LVH, patients with left ventricular hypertrophy and normal systolic left ventricular function; SD, standard deviation.
- Values are either the total number of patients (relative proportion in parentheses), mean ± SD or median with interquartile range, as indicated. If data were not available for all patients studied, the number of patients used for analysis is given in parentheses. Relative proportions always refer to the total number of patients of the respective subgroup. P-values are calculated using two-tailed Student's t-test (S) for continuous data or Fisher's exact test (F) for categorical data, respectively. The bold emphasis is applied to highlight statistically significant p-values < 0.05.
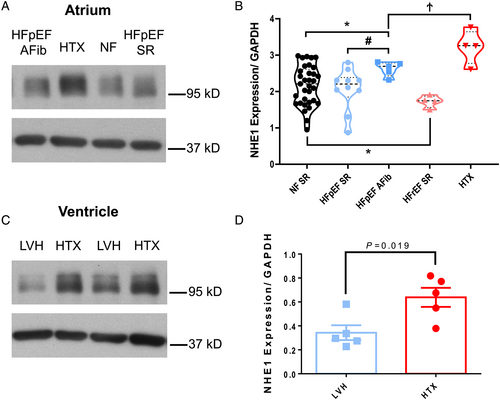
We first aimed at systematically analysing NHE1 expression in human atrial appendage biopsies of 56 patients. Figure 1 shows original western blots and mean data. A strong expression was observed at ~95 kD (predicted molecular weight: 90 kD15) (Figure 1). Interestingly, there was no difference in atrial NHE1 expression in HFpEF patients with SR compared with non-failing control hearts [mean densitometric values normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH): 2.17 ± 0.09 vs. 2.07 ± 0.18; NF SR vs. HFpEF SR, N = 33 vs. 10; P = 0.11]. In contrast, HFpEF patients suffering from AF exhibited a significantly increased atrial NHE1 expression (mean densitometric values normalized to GAPDH: 2.63 ± 0.09; N = 5, P vs. HFpEF SR = 0.004), which was only surpassed by dramatically increased atrial NHE1 expression in patients with end-stage HF (mean densitometric values normalized to GAPDH: 3.23 ± 0.24; N = 4, P vs. HFpEF SR = 0.002) (Figure 1). On the other hand, atrial NHE1 expression was slightly decreased in less sick HFrEF patients with SR (mean densitometric values normalized to GAPDH: 1.73 ± 0.08; N = 4; P vs. NF SR = 0.002) (Figure 1). In accordance with atrial data, ventricular NHE1 expression was significantly increased in patients with end-stage HF compared with patients with LV hypertrophy and normal LV function (mean densitometric values normalized to GAPDH: 0.64 ± 0.08 vs. 0.34 ± 0.06; N = 5 vs. 5; P = 0.019) (Figure 1).
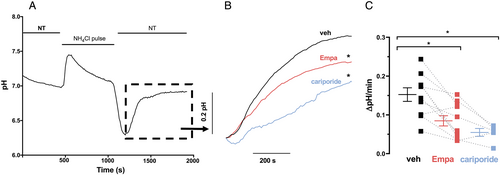
Next, we evaluated the effect of empagliflozin on NHE activity. We first investigated isolated mouse ventricular myocytes under normoglycemic conditions. After calibration of SNARF1 fluorescence (F580/F640) using the ‘nigericin–high K+ approach’ (see Supporting Information, Data S1 and Figure 2), NHE function was assessed as the maximal slope of pH recovery following a 10 min NH4+ pulse (Figure 3). Interestingly, exposure to empagliflozin (1 μmol/L) significantly slowed the pH recovery kinetics after NH4+ pulse consistent with inhibition of NHE function. The slope of pH recovery was (0.152 ± 0.017 pH/min vs. 0.085 ± 0.013 pH/min veh vs. Empa, N = 10 vs. 11, P < 0.05) comparable with the effect of the selective NHE inhibitor cariporide (10 μmol/L) (0.054 ± 0.010 pH/min, N = 5, P < 0.05 vs. veh) (Figure 3). This is in accordance to previously published data in mice, rats, and rabbits.11, 12

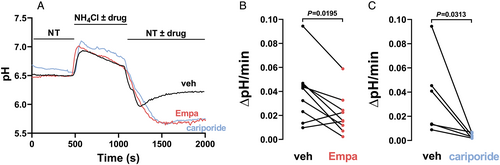
In a second step, we applied this experimental protocol to our human atrial myocytes and were able to induce a pH recovery following a 10 min NH4+ pulse. Figure 4 shows the original registration of SNARF1 fluorescence in a human atrial cardiomyocyte. The pH recovery after the NH4+ pulse was slow but still readily detectable (Figure 4). Importantly, here, we show for the first time that exposure to empagliflozin (1 μmol/L) almost completely abolished the pH recovery consistent with inhibition of NHE function in isolated human atrial myocytes (0.039 ± 0.008 pH/min vs. 0.021 ± 0.006 pH/min, N = 9 vs. 9, P = 0.0195) (Figure 4). Intriguingly, the inhibition was of similar magnitude as in the presence of the selective NHE inhibitor cariporide (10 μmol/L) (0.036 ± 0.013 pH/min, N = 6, P = 0.0313) (Figure 4).
Conclusions
We show here that atrial NHE1 expression was significantly increased in HFpEF patients with AF (but not SR) and even more so in patients with end-stage HF undergoing heart transplantation (even in SR) but not in less sick HFrEF patients. Importantly, we are the first to show that empagliflozin inhibits human NHE suggesting a mechanistic link to the clinical benefits observed in clinical trials.
At first glance, it seems counter-intuitive that NHE1 expression was not increased in HFpEF with SR and was even modestly reduced in HFrEF patients. However, it is unclear if atrial myocytes of HFpEF patients with SR or HFrEF patients had indeed abnormal contractile function, which may be a prerequisite for increased NHE1 expression. Although both patient cohorts showed markedly enlarged atrial dimensions (Table 1), which are usually accompanied by impaired contractile function, we did not directly measure atrial contractility. Moreover, the extent of contractile dysfunction may also be important. We show here that atrial NHE1 expression was significantly increased in HFpEF patients with AF in contrast to HFpEF patients with SR, which may be a consequence of the larger impairment of atrial contractile function in AF. In addition, the four patients who fulfilled the criteria for HFrEF had less impaired LVEF compared with patients with end-stage HF (mean LVEF 35 ± 1% vs. 20 ± 7%; Table 1), also indicating that the severity of contractile dysfunction may be important for regulation of NHE1 expression. This notion is further supported by our observation that NHE1 expression in ventricular myocardium of patients with end-stage HF is markedly up-regulated compared with ventricular myocardium of patients with LV hypertrophy and normal LV function.
Besides these considerations, NHE1 expression may be not even directly correlated to activity. Previous studies that have made use of smaller patient cohorts showed a discrepancy between NHE expression and activity in HF.16 They suggested that post-translational modifications, such as kinase-dependent phosphorylation (e.g. by CaMKII),17-19 might be responsible for increased NHE activity in HF, which was reported by numerous studies.16, 20 Irrespective of expression levels, increased NHE activity appears to play an important role in the pathophysiology of hypertrophy and HF. Studies using cardiac cells, for instance, have consistently demonstrated that NHE inhibitors block hypertrophic responses to various stimuli.21 Moreover, inhibition of NHE has been shown to reduce cardiac hypertrophy, fibrosis, remodelling, and systolic dysfunction in experimental models of HF.13, 14 The precise mechanism for NHE1 involvement in hypertrophy or HF remains to be determined. Interestingly, NHE1 activation has been shown to result in accumulation of intracellular Na, which reduces/reverses the driving force for the Na/Ca exchanger-mediated Ca efflux and leads to intracellular Ca overload and contractile dysfunction.14, 20, 22 In addition, intracellular alkalosis, which may also be a consequence of NHE1 activation, has been shown to increase myofilament Ca responsiveness and thus impair diastolic function.23 Indeed, we have shown recently that empagliflozin reduced myofilament Ca sensitivity and improved diastolic dysfunction in human myocardium.6 Accordingly, either the reduction of Na entry or normalization of intracellular pH by empagliflozin-dependent NHE1 inhibition may both ameliorate contractile dysfunction in HF. Interestingly, a recent study addressing the cardioprotective mechanisms of empagliflozin using deep learning in silico analyses with in vivo validation reported NHE1 blockade as the most robust possible underlying mechanism of action.24 However, if empagliflozin directly inhibits NHE1 or if inhibition of NHE activity is rather a consequence of upstream alterations in cellular Na load, intracellular pH or interference with post-translational modifications (e.g. by inhibition of CaMKII5) remains elusive. In conclusion, our data add important novel insights into the potential mechanisms mediating the cardioprotective effect of empagliflozin observed in large-scale clinical trials.2, 3
Acknowledgements
We acknowledge the expert technical assistance of G. Pietrzyk and T. Sowa (Department of Internal Medicine II, University Hospital Regensburg, Germany).
Conflict of interest
M.A., L.S.M., and S.S. receive compensation for talks for Boehringer Ingelheim, the company that sells empagliflozin. The other authors declare to have no duality of interest associated with this manuscript.
Funding
S.W. is funded by DFG grants WA 2539/4-1, WA 2539/5-1, WA 2539/7-1, and WA 2539/8-1. L.S.M is funded by DFG grants MA 1982/5-1 and MA 1982/7-1. S.W. and L.S.M. are also funded by the DFG SFB 1350 grant (project number 387509280, TPA6). S.W., S.S., and L.S.M. are supported by the ReForM C programme of the faculty. S.S. and S.P. are funded by the Else Kröner Fresenius Stiftung through a research grant. S.P. is supported by the German Society of Internal Medicine.
Author contributions
M.T., J.R., S.L., S.P., S.S., S.H., M.A., L.S.M., and S.W. contributed to analysis and interpretation of data, critically revised the article, and approved the final study. M.T. and S.W. designed the experiments and drafted the article. M.T. and J.R. performed data acquisition. M.T. and S.W. are both responsible for the integrity of the work as a whole.
Data availability
The data sets generated and/or analysed during the current study are available from the corresponding author on reasonable request. In order to exclude the possibility of unintentionally sharing private patient information, patient data can only be made available after informed consent about a specific request has been given by each patient.




