A rare case of severe right heart failure due to pulmonary artery lymphomatoid granulomatosis
Hongling Su and Rong Wei contributed equally to this work.
Abstract
Lymphomatoid granulomatosis is a rare, vascular-centric, and vessel-destroying lymphoproliferative disease that hardly involves the pulmonary arteries. Herein, we report a case with severe right heart failure and pulmonary arterial stenosis caused by pulmonary artery lymphomatoid granulomatosis. This case was diagnosed by percutaneous transluminal pulmonary artery biopsy and was effectively treated with stent implantation and steroid administration.
Introduction
Lymphomatoid granulomatosis (LYG) is a rare, vascular-centric, and vascular-destructive lymphoproliferative disease involving the lungs, kidneys, liver, skin, and central nervous system.1 Pulmonary artery involvement is extremely rare in this disease. Epstein–Barr virus (EBV) infection has been reported to be crucial in the pathogenesis and pathology of the LYG.2
Case report
A 46-year-old man was admitted with progressive exertional dyspnoea for 6 months. The patient had no history of smoking, asthma, tuberculosis, lung disease, heart disease, immune disorders, and other chronic diseases or drug usage except one episode of low fever (body temperature 37.8°C) at 6 months prior to admission. Computed tomography pulmonary angiography (CTPA) taken at 2 months before admission showed filling defects in the main pulmonary artery trunk and bilateral main pulmonary arteries, and the patient was then diagnosed with suspected pulmonary embolism or tumour. Subsequent exploratory thoracotomy revealed that the pulmonary arteries were involved, and the involved arteries became stiff and extensively adhered to surrounding tissues. The malignant tumour originated from the pulmonary artery was therefore highly suspected. The ‘tumour’ was inoperable, and the patient declined operation owing to the high risk incurred by the anatomical position of the ‘tumour’. However, biopsy showed that the lump was composed of non-specific inflammatory cells, not supporting the initial diagnosis of malignant tumour. In the meanwhile, the patient suffered from severe right ventricular failure (New York Heart Association functional class IV) according to echocardiography and clinical signs. Therefore, diuretics were administered but did not alleviate the right heart failure. The patient then had been afflicted with progressive exertional dyspnoea till admission to our hospital.
Physical examinations upon admission found distention of bilateral jugular veins and accentuation of the second heart sound at pulmonary valve auscultation area, and the apex displaced laterally. Systolic murmur was detected at scapular region. Both lungs were clear. Neither abdominal mass nor organomegaly was detected. Arterial blood gas test demonstrated that PO2 was 49.2 mmHg, PCO2 25 mmHg, and SO2 82.6% with room air. Other laboratory test results showed that the patient's D-dimer was 0.2 μg/mL (<0.5 μg/mL), N-terminal pro b-type natriuretic peptide 3666.0 pg/mL (<125 pg/mL), white blood cell count 4.7 × 109/L (3.5–9.5 × 109/L), lymphocyte count 1.47 × 109/L (1.20–3.50 × 109/L), eosinophil count 0.05 × 109/L (0.05–0.50 × 109/L), red blood cell count 5.25 × 1012/L (4.00–5.50 × 1012/L), haemoglobin 142 g/L (120–160 g/L), platelet count 191 × 109/L (100–300 × 109/L), serum total bilirubin 22.7 μmol/L (5.1–29.6 μmol/L), direct bilirubin 9.1 μmol/L (0.0–6.8 μmol/L), indirect bilirubin 13.6 μmol/L (5.0–24.3 μmol/L), alanine aminotransferase 14 U/L (9–50 U/L), serum albumin 39.9 g/L (40.0–55.0 g/L), serum creatinine 68.5 μmol/L (53.0–106.0 μmol/L), blood urea 4.3 mmol/L (2.0–7.5 mmol/L), C-reactive protein 33.3 mg/L (0.0–5.0 mg/L), erythrocyte sedimentation rate 25 mm/h (0–15 mm/h), serum α-fetoprotein 4.92 ng/mL (<8.78 ng/mL), and carcinoembryonic antigen 0.83 ng/mL (<5.00 ng/mL). Moreover, the antibody tests revealed negative for tuberculosis antibodies (IgM and IgG), negative for T-SPOT.TB, negative for human immunodeficiency virus, negative for auto-antibodies, positive for EBV-CA IgG, negative for EBV-CA IgM, and negative for EBV-EA IgA, and that IgG4 was 1.150 g/L (0.03–2.01 g/L).
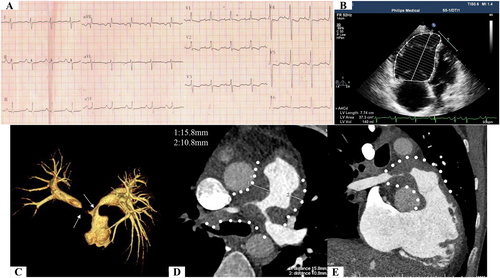
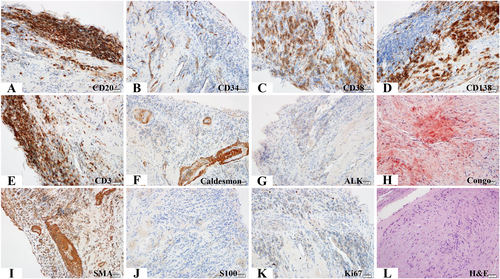
Electrocardiogram (ECG) displayed right axis deviation; inverted T waves in lead V1, V2, V3, V4, and V5; deep S wave in lead I; and significant R wave in lead aVR (Figure 1). Echocardiography demonstrated complete occlusion of the right pulmonary artery, severe stenosis of the proximal left pulmonary artery, and enlarged right atrium (21.2 cm2/1.67 m2 in end-systolic period) and ventricle (37.3 cm2/1.67 m2 in end-diastolic period) (Figure 1). Tricuspid annular plane systolic excursion (TAPSE) was 9 mm, and peak tricuspid regurgitation velocity (Vmax) was 4.3 m/s. CTPA revealed a massive filling defect with irregular boundaries in the main pulmonary artery and the proximal left and right pulmonary arteries. The lesion had a broad base on the wall of the pulmonary artery and protruded toward the lumen, leading to severe vascular stenosis (Figure 1). The lesion did not expand significantly within the 2 month period. Computerized tomography (CT) value was 40–60 Hounsfield units. Fluorodeoxyglucose-positron emission tomography (FDG-PET) showed multiple masses or nodules with abnormally high uptake of FDG in the main pulmonary trunk and bilateral main pulmonary arteries (standardized uptake valuemax was 4.82) and no extrapulmonary abnormal uptake (Figure S1). Pulmonary functional test was normal. CT and PET did not identify any pulmonary parenchymal disease (Figure S1). Furthermore, right cardiac catheterization showed that pulmonary artery pressure was 113/27/55.6 mmHg. After evaluation by a multidisciplinary team composed of cardiologists, respiratory physicians, oncologists, a radiologist, and a pathologist, we decided to preform percutaneous transluminal pulmonary artery biopsy (PTPB) and ascertain the pathogenesis. The collected samples by the PTPB contained fibres and smooth muscles with mucinous degeneration, indicating local amyloidosis. Furthermore, haematoxylin and eosin (H&E) staining of the biopsy samples revealed aggregation of small vessels, infiltration of inflammatory cells, and necrotic foci around fibrous tissue. Immunohistochemistry further identified that the samples were positive for CD34 (+), CD38 (+), CD138 (+), and smooth muscle actin (+) staining; focally positive for CD20 (+, focal), CD3 (+, focal), caldesmon (+, focal), and Ki67 (1–2%) staining; and negative for ALK (−), S-100 (−), and Congo (+/−) staining (Figure 2).
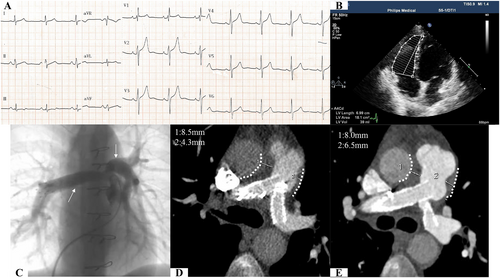
After consultation with another multidisciplinary team including pathologists, an oncologist, and a haematologist, the diagnosis was made that these findings were consistent with pulmonary arterial LYG (Grade 1), which involved the main pulmonary artery trunk and bilateral main pulmonary arteries but no other organ. Based on the results of CT, PET, and the pathological grading, haematologists thought that steroids should be preferred because no other organ was involved and the low-grade lesions did not exhibit significant progression. Then pulmonary artery stent implantation was performed (Figure 3) as a palliative strategy to relieve the patient's severe symptoms. Meanwhile, the patient was treated with 60 mg prednisone (1 mg/kg). Also, aspirin and clopidogrel were administered for antiplatelet after stent implantation. At 2 months post stent implantation, the right cardiac catheterization showed that pulmonary artery pressure decreased to 58/15/30 mmHg. At 13 months after stenting, ECG displayed upright T waves (Figure 3), and the echocardiography showed nearly normal-sized right ventricle (18.1 cm2/1.75 m2 in end-diastolic period) and right atrium (16.2 cm2/1.75 m2 in end-systolic period) (Figure 3) with 17 mm of TAPSE and 2.5 m/s of peak tricuspid regurgitation velocity (Vmax). In addition, CTPA at 3 and 13 month follow-ups (Figures 3D and E) showed that the stents of the pulmonary arteries were patent and that the lesions not progressive. Arterial blood gas analysis showed that PO2 was 94.9 mmHg, PCO2 35.4 mmHg, and SO2 94% with room air. Conventional laboratory test results, such as N-terminal pro b-type natriuretic peptide, C-reactive protein, and erythrocyte sedimentation rate, were all within normal ranges at the 13 month follow-up. Prednisone was tapered by 10 mg, and 100 mg aspirin was maintained for lifetime.
Discussion
LYG originating from pulmonary artery wall is rarely reported and can present as severe pulmonary artery stenosis and refractory right heart failure. It is difficult to distinguish the pulmonary artery stenosis caused by LYG from that caused by embolism,3 pulmonary vasculitis,4 sarcoma,5 fibrosing mediastinitis,6 and lung parenchymal tumours.7 In this case, the LYG-caused pulmonary artery stenosis was predominantly located at the trunk of the main pulmonary artery and the proximity of left and right main pulmonary arteries. Importantly, the involvement of other sites such as lung parenchyma and extrapulmonary organs and the abnormalities in immune and haematological systems were not found through physical examinations, imaging examinations, and laboratory tests. Moreover, pathological examinations by PTPB were crucial to pinpoint the pathogenesis as the LGY-caused pulmonary artery stenosis. Therefore, after comprehensive consultation with multidisciplinary medical teams including cardiologists, oncologists, haematologists, radiologists, and pathologists, a palliative therapeutic modality of percutaneous pulmonary artery stenting combined with an appropriate steroid were determined. The strategy turned out effective, as the percutaneous pulmonary artery stenting dramatically relieved the patient's symptoms and signs, the enlarged right heart returned nearly normal, and the serial CTPA follow-ups demonstrated inactive lesions.
Percutaneous pulmonary artery stenting has been proved effective and safe for chronic stenotic pulmonary artery disease. The procedure-related complications include lung reperfusion injury, stent migration, haemothorax, and rapid pulmonary oedema,7, 8 which should be avoided to the maximum extent. In this case, no complication occurred. Moreover, balloon-expanding stent with greater tension is preferred because the LYG-caused lesions and surrounding tissues are stiff and rigid. Additionally, subsequent tapering and maintaining administration of glucocorticoids are an indispensable modality.
Taken together, we for the first time report a case with pulmonary arterial LYG causing severe pulmonary artery stenosis and right heart failure as well as the palliative but effective therapeutics to this case, which might have referral value for physicians.
Conflict of interest
None declared.
Funding
This work was supported by the National Natural Science Foundation of China (81770300, 81860059, and 81460072); CAS “Light of West China” Program; International Joint Research Program of Gansu Province (18YF1WA046); Innovation and Entrepreneurship Program of Lanzhou City (2018-RC-78); the Science Foundation of Gansu Provincial Hospital (18GSSY5-4).




