SmartScreen-AIS: A High-Throughput qPCR Chip for Nationwide Surveillance of Aquatic Invasive Species
Funding: This work was supported by the U.S. Forest Service's Rocky Mountain Research Station and the Department of Defense's Environmental Security Technology Certification Program (ESTCP RC21-5121).
ABSTRACT
Effective wildlife conservation requires frequent and widespread data on species occurrence. With the maturation of eDNA-based monitoring—now widely recognized as sensitive, cost effective, and legally defensible—nationally coordinated eDNA strategies are beginning to take shape. Such ambitious initiatives will require eDNA analytics with the throughput and sensitivity required for surveillance of many protected, pathogenic, and invasive species across broad geographic scales. Here, we help meet this need with SmartScreen-AIS: a high-throughput qPCR (HT-qPCR) chip with 46 assays targeting aquatic invasive species of widespread concern. SmartScreen-AIS was validated for use throughout the continental United States and can be subdivided into smaller chip formats as desired for use in specific regions or biomes. Assay performance in HT-qPCR was strong relative to conventional qPCR, with slightly lower specificity in some cases (due to pre-amplification) but significantly higher sensitivity. Contamination was rare, PCR inhibition was minimal to nonexistent, and demonstration at three military installations detected eDNA from all species on the chip that were known to be present and one species that was previously undocumented. Cost savings will depend on the number of assays used and samples tested, but in this study we estimate that eDNA analyses were 75% cheaper using HT-qPCR than they would be with our conventional qPCR protocol. To facilitate use, we provide appendices with assay details, bench protocols, a script for processing results, and an online app with state-level assay specificity information. SmartScreen-AIS has the potential to advance early detection of invasive species in the United States, and we hope our HT-qPCR workflow inspires chip development and use globally.
1 Introduction
Environmental laws often mandate wildlife monitoring to inform human land use and species protections. Still, management agencies continually lack data sufficient for actionable decisions (Davis et al. 2024; Roper et al. 2018). This reality is changing with the maturation of environmental DNA (eDNA)-based monitoring. Organismal DNA shed into environment can now be collected at broad scales by technicians, citizen scientists, or automated samplers and analyzed using proven, legally defensible methods to determine species presence (Laschever et al. 2023; Sepulveda et al. 2020). Recognition that eDNA technology satisfies the need for efficient and widespread wildlife surveys has sparked calls for national coordination (Kelly et al. 2024; Lodge 2022). Indeed, the United States government recently released their National Aquatic Environmental DNA Strategy, a “call to action to harness the power of eDNA to explore, map, monitor, and better understand aquatic life to sustain and restore freshwater and marine ecosystems and their biological resources into the future” (National Science and Technology Council 2024). Aside from non-trivial tasks of coordinating across sectors, building expertise and operational capacity, and integrating eDNA data into decision-making, implementing the National Aquatic Environmental DNA Strategy will require that “standards, best practices, and technical readiness of approaches” be identified and recommended.
Which eDNA analysis methods are best-suited for national implementation? The answer will likely depend on project-specific goals, expertise, and funding. Fortunately, wildlife managers have no shortage of options—from targeted PCR on quantitative and digital platforms to assessment of entire communities via metabarcoding (Schenekar 2023). Targeted approaches tend to be more sensitive than metabarcoding (Bylemans et al. 2019; McCarthy et al. 2023; McColl-Gausden et al. 2023; Wood et al. 2019) at the expense of throughput. Recently, high-throughput quantitative PCR (HT-qPCR) has emerged as an intermediate approach that retains the sensitivity of targeted methods while enabling simultaneous detection of dozens of taxa (Elmore et al. 2025; Miller et al. 2016; Sleeting et al. 2025; Sørensen et al. 2024; Wilcox et al. 2020; Wittwer et al. 2024). In HT-qPCR, pairwise combinations of samples and targeted assays are robotically or microfluidically dispensed into nanoliter-scale wells on a chip, then amplified and fluoresced as in conventional qPCR. This miniaturization greatly increases analytical efficiency, decreases costs, and limits the quantity of sample consumed per analysis. It is most effective when a moderate number of specific taxa are of interest and high sensitivity is required, as when screening for protected, pathogenic, and invasive species.
Here, we present a general workflow for building HT-qPCR chips from targeted assays while ensuring continued specificity and sensitivity. In the process, we introduce SmartScreen-AIS: a 46-assay chip targeting aquatic invasive species of widespread concern. Invasive species can have enormous impacts on imperiled native species, ecological services, and infrastructure (Gallardo et al. 2016; Mayfield et al. 2021; Pyšek et al. 2020), costing an estimated US$200 billion annually in the United States alone (Pimentel et al. 2005, adjusted for inflation) and increasing over time (Diagne et al. 2021). Early detection is crucial and greatly facilitated by eDNA sampling (Larson et al. 2020; Morisette et al. 2021; van Rees et al. 2022), as articulated by the Early Detection Rapid Response (EDRR) framework for invasive species management (Reaser et al. 2020). SmartScreen-AIS was validated for use throughout the continental United States and can be subdivided into smaller chip formats based on interest (e.g., geographically tailored) to support the mission of the National Aquatic Environmental DNA Strategy and other ambitious eDNA monitoring initiatives across the globe.
2 Methods
2.1 General HT-qPCR Workflow
2.1.1 Laboratory Procedures
Our HT-qPCR workflow utilized the SmartChip Real-Time PCR System (Takara Bio) and involved loading TaqMan probe-based assays and samples onto 384-well “source plates” along with mastermix. Source plate contents were then robotically transferred to a 5184-well aluminum chip for amplification and fluorescent imaging. All steps mirrored those of Elmore et al. (2025) and Sleeting et al. (2025), but with source plate modifications to accommodate the 48-assay SmartChip format. Molecular work was conducted at the National Genomics Center for Wildlife and Fish Conservation in dedicated pre- and post-PCR hoods that were irradiated with ultraviolet light for 1 h prior to use.
First, 46 taxon-specific assays (Kronenberger et al. 2024 and herein) and two internal positive control assays (IPCs; Deer et al. 2010, with modifications described in Elmore et al. 2025) were mixed to 5×; see Appendix 1: Assay Summary for details. For each chip, a 384-well assay source plate was then assembled using the layout recommended in the SmartChip MyDesign Kit User Manual. Wells were 21.4 μL total and contained 2× assay, 1× SmartChip Probe qPCR Master Mix (Takara Bio), and 1× ROX reference dye. The plate was sealed with Microseal B PCR Plate Sealing Film (Bio-Rad), lightly vortexed, centrifuged at high speed for 1 min, then frozen at −20°C until shipping for analysis. Next, prior to loading the sample source plate, samples were “pre-amplified” to enrich for target taxa in 45-μL reactions containing 12 μL eDNA template, 100 copies IPC template, 1× TaqMan Environmental Mastermix 2.0 (Applied Biosystems), and 44 nM each primer. On the pre-amplification plate, two IPC templates (one for each IPC assay) were alternately pipetted in a checkerboard pattern to evaluate contamination. Together the IPCs also indicate PCR inhibition through delayed or absent amplification. Also included were no-template and positive controls, the latter consisting of pooled target templates from either conventional qPCR products or gBlocks gene fragments (IDT). Thermal cycling conditions were 95°C/10 min, [95°C/15 s, 60°C/3 min] × 12 cycles. Pre-amplification products were cleaned by adding 4.5 μL ExoSAP-IT (Applied Biosystems) to each well and incubating at 37°C/30 min followed by 80°C/15 min. A 384-well sample source plate was then assembled according to the recommended layout with four replicates per sample along with pre-amplified no-template and positive controls. Wells were 16.7 μL total and contained 6.7 μL cleaned pre-amplification product and 1× SmartChip Probe qPCR Master Mix. The plate was sealed with Microseal B PCR Plate Sealing Film, lightly vortexed, centrifuged at high speed for 1 min, then frozen at −20°C until shipping for analysis. Assay and sample source plates were sent on dry ice to the Molecular Biology and Genomics Core at Washington State University for dispensing and amplification on a SmartChip with 150-nL wells. Thermal cycling conditions were 95°C/9 min 45 s, [95°C/10 s, 60°C/53 s] × 45 cycles. Resulting data were analyzed as described below.
The 48-assay SmartChip format described here can accommodate 24 samples in quadruplicate reactions along with control wells. If instead using the smaller available formats—24 assays × 48 samples and 12 assays × 88 samples—all steps are the same except for lower total volume in the sample source plate (14.9 and 14 μL for 24- and 12-assay chips, respectively). See Appendix 2: HT-qPCR Protocols for step-by-step bench protocols and example source plate layouts.
2.1.2 Fluorescence Data Processing
Raw fluorescence data were exported from SmartChip qPCR software (WaferGen Biosystems) and processed using the flrpwr package (Hutchins in prep) and a custom R Markdown script produced with the rmarkdown package (Allaire et al. 2024) in R (version 4.0.4; R Core Team 2023). The script baseline-corrects and ROX-normalizes fluorescence data and then runs an affine transformation, as described by Patrone et al. (2020). In essence, this collapses each amplification curve into a reference positive control curve with a certain degree of error depending on signal noise and curve morphology. Amplification curves with over 10% transformation error are disregarded, and those that remain have significantly lower detection thresholds. We found this to produce more accurate calls than the usual thresholding approach based on change in normalized fluorescence (ΔRn). The script plots all curves for each assay-sample combination with at least one replicate amplifying. Amplification calls are manually evaluated by the user for quality assurance and quality control (QA/QC), with the primary goal of ensuring non-amplifications and amplifications were correctly classified by the software. Incorrect classifications can be corrected as necessary within the script. Then, the amplification status of control wells and IPCs is plotted in chip-view to visualize potential sensitivity or contamination issues. Final calls and quantification cycle (Cq) values are exported as CSV files. See Appendix 3: HT-qPCR Analysis to access the R Markdown script. We considered any assay-sample combination with at least two of four replicates amplifying to be positive for the target taxon. Any results that remained ambiguous after QA/QC were cross-checked using conventional qPCR.
2.2 Invasive Species HT-qPCR Chip Validation
2.2.1 Primer Compatibility Testing
We built SmartScreen-AIS by re-validating the 46 invasive species assays described by Kronenberger et al. (2024) to ensure continued performance of the high-throughput platform (Figure 1). This was required because our HT-qPCR protocol involves a “pre-amplification” step in which eDNA samples are enriched for target DNA in a highly multiplexed primer pool. This is intended to retain assay sensitivity when samples are partitioned into miniaturized wells but can sometimes instead reduce sensitivity via dimer formation. We therefore screened for inter-assay primer dimers using MFEprimer-4.0 (Wang et al. 2019) with default settings, except returning all dimers and not just those with bound 3′ ends. A strong dimer of −17.0 ΔG was produced between the reverse primer of the marbled crayfish (Procambarus virginalis ) assay and the forward primer of the red swamp crayfish (Procambarus clarkii ) assay, which reduced the sensitivity of the red swamp crayfish assay during initial testing. To ameliorate this, we designed a new marbled crayfish reverse primer (5′-GATGCACCTGCATGAGCAATA-3′) and reassessed assay performance using the eDNAssay classifier of Kronenberger et al. (2022) and the validation framework of Kronenberger et al. (2024). See Appendix 5: Revised Marbled Crayfish Assay for comprehensive testing information.

2.2.2 Assay Specificity Testing
In addition to potentiating dimer formation, pre-amplification can reduce assay specificity if the additional thermal cycles allow for cross-amplification that did not occur to detectible levels when assays were initially validated. We screened for this issue in 151 assay-nontarget taxon combinations, 111 (73.5%) of which we deemed “high risk” because they had moderate-to-high likelihoods of cross-amplifying (eDNAssay assignment probabilities > 0.3) but did not cross-amplify via conventional qPCR when tested by Kronenberger et al. (2024). Nontarget templates were pre-amplified as describe above, diluted 1:100 to approximate per-reaction copy numbers on a chip, then run in singlicate using the conventional qPCR protocol of Kronenberger et al. (2024). This is a low-cost approach to assess the impact of additional thermal cycles on assay specificity in a targeted way. We confirmed the validity of this approach by running 84 (75.7%) of the 111 high risk combinations directly on a chip and calculating raw concordance as the proportion of results in agreement across platforms.
In some cases, reduced specificity may not be evident until eDNA samples are tested. On our first three chips for field demonstration (see below), the Chinese mystery snail assay produced slight amplifications in HT-qPCR but not in conventional qPCR. This may have been due to cross-amplification of flies and lacewings (orders Diptera and Neuroptera), which were commonly flagged by Primer-BLAST (Ye et al. 2012) according to Kronenberger et al. (2024). We therefore redesigned the Chinese mystery snail assay, with two each of forward and reverse primers to accommodate discrete target haplotypes (forward: 5′-TCACTGCATTTAGCTGGTGCA-3′ and 5′-CATTACACTTGGCTGGTGCG-3′; reverse: 5′-CAAATTGTATACCACATCAACGTATATTAATT-3′, and 5′-GCATACCACATCAACGCATATTAATT-3′; probe: 5′-TTAGGGGCTGTTAATT-MGB-3′). We assessed assay performance using the eDNAssay classifier of Kronenberger et al. (2022) and validation framework of Kronenberger et al. (2024). See Appendix 6: Revised Chinese Mystery Snail Assay for comprehensive testing information.
2.2.3 Assay Sensitivity Testing
We tested sensitivity in HT-qPCR for all assays using a master standard curve. To obtain the most accurate copy number estimates, we first used droplet digital PCR for absolute quantification of synthetic DNA (conventional qPCR products or gBlocks gene fragments) corresponding to each target taxon. Reactions were 22 μL total and contained 2 μL template DNA, 1× EvaGreen Supermix (Bio-Rad), and 1 mM each primer, run on a QX200 Droplet Digital PCR System (Bio-Rad). Thermal cycling conditions were 95°C/5 min, [95°C/30 s, 60°C/1 min] × 45 cycles, 4°C/5 min, 90°C/5 min—the last two steps included for dye stabilization as in McDermott et al. (2013). Once quantified, templates were normalized, pooled, and serially diluted five-fold from 250 to 0.4 copies per pre-amplification reaction, then run on a chip as described above with 20 replicates (or 14 for the revised Chinese mystery snail assay, which was tested later). We estimated the limit of detection (LOD; lowest copy number that amplifies in at least 95% of replicates) and limit of quantification (LOQ; the lowest copy number that is quantified with a coefficient of variation below 35%) using the discrete-threshold and curve-fitting methods of Klymus et al. (2020).
To evaluate sensitivity of HT-qPCR relative to conventional qPCR, we re-diluted standard curves using the same stocks and curve parameters described above. Standard concentrations were increased threefold to match total copies per reaction in qPCR (4 μL template) with HT-qPCR pre-amplification (12 μL template). We tested eight replicates of each standard across 24 assays and estimated LOD and LOQ values for conventional qPCR using the discrete threshold and curve-fitting methods described above. The number of replicates varied across platforms, so we randomly selected eight of 20 total HT-qPCR replicates per assay and recalculated LOD and LOQ. Median differences were tested for significance using paired Wilcoxon signed-rank tests in the R package coin (Hothorn et al. 2008).
2.2.4 Field Demonstration at Military Installations
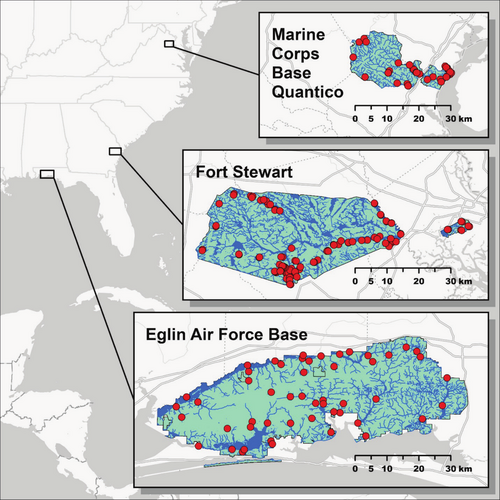
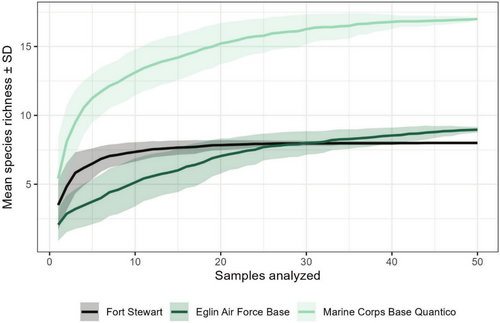
Performance of our invasive species chip was tested using 201 eDNA samples collected at three military installations overseen by the U.S. Department of Defense: Fort Stewart in Georgia (n = 100), Eglin Air Force Base in Florida (n = 51), and Marine Corps Base Quantico in Virginia (n = 50) (Figure 3). Samples were collected in areas deemed most likely to harbor invasive species. We used the protocol of Carim et al. (2016) to filter 5 L of water or, in cases of clogging, as much water as could be passed through three filters. For lentic waterbodies, subsamples were typically collected at up to 10 locations and pooled in a sterile bag prior to filtering to increase detection probability. We evaluated the contamination risk posed by this subsampling protocol by collecting eight field controls at Fort Stewart immediately after eDNA sample collection, placing 1 L of tap water in a sterile bag and filtering as usual. Filters were stored in silica desiccant and transferred to a −20°C freezer as soon as possible. Environmental DNA was extracted using the modified DNeasy Blood and Tissue Kit (QIAGEN) protocol of Franklin et al. (2019). Samples were run on nine total HT-qPCR chips and raw fluorescence data were processed as described above. We compared invasive species eDNA detections with installation-level occurrences from local biologists, the Global Biodiversity Information Facility (https://www.gbif.org), and the Nonindigenous Aquatic Species database (https://nas.er.usgs.gov). Any ambiguous results or surprising detections were cross-checked in triplicate using the conventional qPCR protocol of Kronenberger et al. (2024). To assess the power of our sampling efforts to characterize installation-scale occurrence of species on the chip, we generated species accumulation curves in the R package vegan (Oksanen et al. 2025) by randomly subsampling the data without replacement 100 times for each sample size up to 50 samples.
3 Results
3.1 General HT-qPCR Workflow
3.1.1 Laboratory Procedures
Our protocol proved to be robust across the nine 48-assay SmartScreen-AIS chips analyzed to test eDNA samples. Of 2484 total no-template control wells, six (0.2%) showed evidence of contamination; two were replicates of the same assay-sample combination and the rest were singletons. All 2484 positive control wells amplified. Our two IPC assays—afforded their own wells along with the 46 invasive species assays to complete the 48-assay chip, with templates dispensed in a checkerboard pattern to further assess contamination—provided an added level of assurance. Of 864 negative IPC wells, two (0.2%) showed evidence of contamination. All 864 positive IPC wells amplified but one (99.9%), indicating a lack of substantial PCR inhibition.
3.1.2 Fluorescence Data Processing
The R Markdown script designated 3054 (8.3%) of 36,984 total sample wells across nine chips as amplifying. Upon QA/QC we changed 126 (4.1%) of the amplifications to non-amplifications and 21 (0.1%) of the non-amplifications to amplifications, ultimately considering 2949 (7.9%) of the 36,984 total sample wells to have amplified. See the Appendix 3: HT-qPCR Analysis for HTML documents containing QA/QC decisions.
Not all amplifications were characterized as detections given our requirement that amplification occur in ≥ 2 wells. Of 9246 total assay-sample combinations, 750 (8.1%) yielded detections. Of these, 667 (88.9%) amplified in all four replicate wells. We used conventional qPCR to cross-check 76 (0.8%) of the 9246 total assay-sample combinations that had ambiguous or surprising results—excluding 23 tests to confirm the initial (pre-revision) Chinese mystery snail assay detections as spurious. Based on this, we changed 17 (48.6%) of 35 ambiguous or surprising amplifications to non-amplifications and 3 (7.3%) of 41 non-amplifications to amplifications, ultimately considering 716 (7.7%) of the 9246 total assay-sample combinations to yield detections.
3.2 Invasive Species HT-qPCR Chip Validation
3.2.1 Primer Compatibility Testing
Pairwise dimer analysis in MFEprimer-4.0 identified 105 potential primer dimers as strong as −11.4 ΔG. We judged dimers weak enough to not necessitate in vitro investigation into sensitivity impacts aside from running the master standard curve (see below) and proceeded with validation.
3.2.2 Assay Specificity Testing
Assay specificity in HT-qPCR was tested for 151 assay-nontarget taxon combinations, 111 (73.5%) of which were high risk as defined in the methods. Following pre-amplification, dilution, and conventional qPCR, 14 (9.8%) of the 151 total assay-nontarget taxon combinations did cross-amplify, all being high risk. Cross-amplifications included confamilials for six assays: virile crayfish, rusty crayfish, revised marbled crayfish, smallmouth and spotted bass, Mozambique tilapia, and brook trout; see https://nationalgenomicscenter.shinyapps.io/state-level-specificity for details. Running 84 (75.7%) of the 111 high risk combinations directly on a chip produced similar results, with seven (8.3%) cross-amplifying. Raw concordance between specificity testing methods was 92.9% and discordant results occurred an equal number of times (n = 3) in each. On both conventional and high-throughput qPCR platforms, specificity is not complete for some assays in some regions of the United States (primarily crayfishes in the Southeast); we strongly recommend users consider the testing gaps and known lapses in specificity described by Kronenberger et al. (2024) in their appendix and state-level assay specificity as presented in our R Shiny app (https://nationalgenomicscenter.shinyapps.io/state-level-specificity).
3.2.3 Assay Sensitivity Testing
Discrete-threshold LOD values were 0.4–10 DNA copies per reaction (median: 2) and discrete LOQ values were 2–50 copies per reaction (median: 10). Limit of detection could not be modeled using the curve-fitting method of Klymus et al. (2020) in four cases where all or nearly all replicates of the highest dilution (0.4 copies per reaction) amplified, potentially due to high stochasticity in amplification when templates are very dilute. Otherwise, modeled LOD values were 0.7–79.8 copies per reaction (median: 1.1) and modeled LOQ values were 3.0–117.0 copies per reaction (median: 12.0). Assay sensitivity in HT-qPCR was significantly higher than in conventional qPCR (Figure 2), as reflected in median LOD (discrete: z = −3.15, p = 0.002; modeled: z = −3.24, p = 0.001). Median LOQ did not differ significantly between platforms (discrete: z = −0.49, p = 0.623; modeled: z = −1.49, p = 0.137). See Appendix 1: Assay Summary for per assay results.

3.2.4 Field Demonstration at Military Installations
SmartScreen-AIS performed as intended in tests of eDNA samples from three military installations (Figure 3). Field controls were negative for all taxa. All installations produced eDNA detections of American bullfrog, common carp or koi, channel catfish, mosquitofish, feral swine, and Asian clam (Figure 4 and Latin names therein). Additional eDNA detections included grass carp and hydrilla at Fort Stewart, marbled or slough crayfish, grass carp, and Brazilian waterweed at Eglin Air Force Base, and virile crayfish species complex (two assays), red swamp crayfish, green sunfish, smallmouth or spotted bass, snakehead, goldfish or Prussian carp, sea lamprey, rainbow trout, brown trout, and hydrilla at Marine Corps Base Quantico. Records of QA/QC decisions made are available as HTML documents in Appendix 3: HT-qPCR Analysis, and eDNA sample metadata including final results are in Appendix 4: Sample Metadata. All species detected were previously documented in or around the installations except for virile crayfish and feral swine at Marine Corps Base Quantico. No species known to be present were missed. Species accumulation curves reached asymptotes in species richness by 50 samples, indicating that additional sampling would be unlikely to yield detections of new species (Figure 5).

4 Discussion
Ambitious eDNA monitoring initiatives such as the National Aquatic Environmental DNA Strategy in the United States will require analytics validated for use at broad geographic scales and capable of throughput sufficient to meet diverse monitoring objectives. To the extent that targeted assays are favored over metabarcoding (e.g., for higher sensitivity), HT-qPCR has clear advantages (Elmore et al. 2025; Miller et al. 2016; Sleeting et al. 2025; Sørensen et al. 2024; Wilcox et al. 2020; Wittwer et al. 2024). For example, with our eDNA extraction and conventional qPCR protocols we can analyze a single eDNA sample in 16 triplicate reactions before it is exhausted, whereas using 48-assay HT-qPCR chips we can analyze the same sample in 768 quadruplicate reactions. SmartChip formats currently exist for up to 384 assays per chip; this would enable 1536 quadruplicate reactions per sample—likely more than enough to cover all species of interest. Furthermore, assays from a fully validated panel can be subdivided into smaller chip formats as needed to most efficiently accomplish monitoring goals.
We have provided a general workflow for HT-qPCR chip validation (Figure 1) and use (see appendices) while demonstrating SmartScreen-AIS: a chip with 46 assays targeting aquatic invasive species of widespread concern. While reaction volumes on the high-throughput platform were 150 times smaller than in our conventional qPCR protocol (100 nL versus 15 μL), pre-amplifying samples before dispensing on the chip yielded significantly higher sensitivity (Figure 2). However, the additional thermal cycles during pre-amplification can potentiate cross-amplification of some nontarget templates to levels not otherwise detectable. Indeed, we documented a lapse of specificity in HT-qPCR alone in nine (8.1%) of 111 in vitro specificity tests. In vitro testing focused on high-risk scenarios—nontarget taxa that had moderate-to-high likelihoods of cross-amplifying according to an in silico PCR model (eDNAssay; Kronenberger et al. 2022), but that did not cross-amplify when tested via conventional qPCR by Kronenberger et al. (2024). In that study, eDNAssay predicted assay specificity across 649 unique tests with 98–100% accuracy (depending on the classification threshold used) when predictions were empirically verified. However, there is an important caveat: the classifier was trained on conventional qPCR results, and predictions may be less accurate on the high-throughput platform. We nonetheless felt comfortable using it here because the consequences of any lapse in accuracy are buffered by the fact that cross-amplifications are very likely to be depressed (with low fluorescence) and can be confirmed by non-amplification in conventional qPCR. Another consideration is in vitro specificity testing method; we here employed two: (1) pre-amplifying nontarget templates, diluting 1:100, then running conventional qPCR, and (2) running nontarget templates directly on the chip. The first method is meant to increase efficiency and reduce costs when only one or two assays are relevant to (i.e., potentially cross-amplify) the nontarget taxa being tested, whereas the second method is useful for testing templates en masse against several relevant assays. Concordance between methods was high (92.9%) across 84 paired tests, and discordant results were evenly split, potentially reflecting stochasticity due to low-efficiency amplification. This suggests that pre-amplifying, diluting, and then running conventional qPCR is appropriate when direct testing on a chip is impractical.
Sensitivity of HT-qPCR in this study—with a median modeled LOD of 1.1 DNA copies per pre-amplification reaction—met or exceeded our expectations from prior studies. For example, Elmore et al. (2025) and Sleeting et al. (2025) also used 12 pre-amplification cycles and a SmartChip system and, while they did not estimate LOD, noted amplification in over 99% of positive control wells at 30 copies per pre-amplification reaction across 10 assays. Wilcox et al. (2020) used a different robotically loaded HT-qPCR system, OpenArray (Applied Biosystems), with 15 pre-amplification cycles and LOD for five of six assays was the lowest standard curve dilution level tested at 10 copies per reaction. Other studies have opted for the microfluidic Biomark system (Standard Biotools), with varying sensitivity. For example, using Biomark and 14 pre-amplification cycles, Miller et al. (2016) estimated LOD values across 47 assays to be 1.1–39.2 copies per pre-amplification reaction (median: 8). Wittwer et al. (2024) did not estimate LOD in their Biomark study but noted significantly lower Cq values in HT-qPCR than conventional qPCR following 28 pre-amplification cycles. However, Sørensen et al. (2024) noted lower sensitivity with Biomark after 18 pre-amplification cycles, estimating median LOD across 25 assays to be 45.6–122.7 copies per pre-amplification reaction (median: 74.1). All studies employed a mosaic of previously published conventional qPCR assays. It is therefore likely that differences in sensitivity stem not from assay-level effects but from pre-amplification conditions and choice of the HT-qPCR system. For example, although we used fewer pre-amplification cycles than Miller et al. (2016) and Sørensen et al. (2024), we used a larger pre-amplification volume (45 μL versus 5 μL and 25 μL, respectively) with the same or relatively more template (26% of total volume versus 26% and 12%, respectively), used different pre-amplification reagents, did not dilute pre-amplification product (they diluted 1:5), used more HT-qPCR cycles (45 versus 40), and used a SmartChip rather than a Biomark system; these attributes may partially explain our lower LOD values.
Here and in studies by Elmore et al. (2025) and Sleeting et al. (2025), which followed the same basic HT-qPCR workflow, contamination was rare despite increased likelihood from pre-amplification; over 99% of no-template control wells and negative checkerboard IPC wells were clean. Inhibition of PCR was minimal to nonexistent, as evidenced by amplification in 96–100% of positive IPC wells. Anecdotally, we have noticed, along with the aforementioned authors (personal communication), that inhibition seems lower than in conventional qPCR, presumably because inhibitors are diluted when amplifying pre-amplification product rather than eDNA samples directly. While not formally evaluated in this study, Elmore et al. (2025) and Sleeting et al. (2025) cross-checked over 1000 HT-qPCR results (n = 696 and 323, respectively) using conventional qPCR and found high concordance between the two platforms using our protocols (92% in both studies). Discordant results were relatively evenly split in amplification rate across platforms, as expected given high stochasticity from rare templates.
Field demonstration of SmartScreen-AIS at three military installations (Figure 3) produced detections for 20 assays, including two species not previously known to be present: virile crayfish and feral swine at Marine Corps Base Quantico (Figure 4). The virile crayfish detections (n = 2 across two assays) likely represent a new or previously unknown introduction as they are confined to a single stream reach and the species becomes common ~50 km north of the installation (Appendix 4: Sample Metadata). The feral swine detections (n = 5), however, likely represent field contamination as swine is a ubiquitous food species and not known to occur in the area. Wildlife managers may choose to corroborate unexpected detections from this assay and others targeting food species (e.g., trout) with temporally repeated sampling or traditional surveys. Species accumulation curves suggest that our level of sampling was sufficient to detect all species on the chip that were present at each installation (Figure 5). In fact, sampling could have been pared down—to ~10 representative samples at Fort Stewart and~30 at Eglin Air Force Base and Marine Corps Base Quantico—and likely detected all species that were ultimately detected. However, this does not mean that additional sampling was not valuable for delimiting distributions of known invasive species and increasing detection probabilities for new invaders.
We hope this study has illustrated the power of HT-qPCR for detecting eDNA from a moderate number of species with high specificity and sensitivity. An important consideration that remains for any monitoring program is cost. It is difficult to enumerate savings relative to conventional qPCR, but they are likely to be substantial and increase with the number of analyses per sample. Elmore et al. (2025) estimated that running 2690 assay-sample combinations on their 12-assay chip cost 60% less than conventional qPCR. We here estimate that running 9246 assay-sample combinations on our 48-assay chip saved even more, costing 75% less than conventional qPCR. This is likely an underestimate, as an equivalent number of analyses using conventional qPCR would require multiple samples per site given our protocols. With the scale of biodiversity loss far outpacing the capacity afforded by most monitoring budgets, HT-qPCR may provide the greatest return on investment in many cases. This technology deserves strong consideration for parallel detection of priority species going forward.
Author Contributions
J.A.K.: methodology, data acquisition, data analysis, results interpretation, writing (original draft), and writing (reviewing and editing). T.M.W.: study conception, funding acquisition, methodology, results interpretation, and writing (reviewing and editing). M.K.S.: study conception, methodology, results interpretation, and writing (reviewing and editing).
Acknowledgments
This work was funded by the U.S. Forest Service's Rocky Mountain Research Station and the Department of Defense's Environmental Security Technology Certification Program (ESTCP RC21-5121). It was much improved through conversations with Joanna Elmore and Michael Sleeting at the National Genomics Center for Wildlife and Fish Conservation. We thank Takara Bio technical staff for advice while troubleshooting our laboratory protocol, Patrick Hutchins at the U.S. Geological Survey's Northern Rocky Mountain Science Center for help establishing our results processing methods, Megan Shaffer and the Kelly Lab at the University of Washington for use of their ddPCR machine, and Derek Pouchnik at Washington State University for running our chips. Finally, many individuals assisted with eDNA sampling and reviewing the results of field testing at military installations, including Larry Carlile, Roy King, Rachael Rourke at Fort Stewart, Jeremy Preston at Eglin Air Force Base, and Alex Antram, Jena Nierman, and John Rohm at Marine Corps Base Quantico; we are immensely grateful for their contributions.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Assay details are available in Appendix 1: Assay Summary, bench protocols are in Appendix 2: HT-qPCR Protocols, an R Markdown script for processing raw fluorescence data along with output from the invasive species chips are in Appendix 3: HT-qPCR Analysis, sample metadata including final in situ testing results is in Appendix 4: Sample Metadata, detailed testing information for the new assays presented herein is in Appendix 5: Revised Marbled Crayfish Assay and Appendix 6: Revised Chinese Mystery Snail Assay, and an R Shiny app with state-level assay specificity information is available at https://nationalgenomicscenter.shinyapps.io/state-level-specificity.




