Capture—Incubate—Release: An Animal-Friendly Approach to Assess Local Aquatic Macroinvertebrate Species Diversity Through Environmental DNA Metabarcoding
Funding: M.S. was supported by the DBU scholarship program (20020/685). D.B., F.L., M.W., and A.J.B. are members of and supported by the Collaborative Research Center (CRC) RESIST funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) 1439/1—project number: 426547801.
ABSTRACT
Metabarcoding of environmental DNA (eDNA) from water samples has become an important tool for aquatic biodiversity assessment because it is minimally invasive, time-efficient, and generates comprehensive taxa lists. Nevertheless, species lists differ noticeably from those obtained via bulk metabarcoding of the local benthic community because of eDNA traces transported in the water column. However, it is important for several assessments to capture local biodiversity signals. Our goal was to test whether we can combine the advantages of both methods, that is, obtaining a local signal and being minimally invasive. Therefore, our developed method includes capturing local benthic invertebrates, incubating them in a water container for eDNA enrichment and analysis, and releasing them back to their habitat. We first quantified eDNA release over time for 10 invertebrate species in a laboratory setting using qPCR. We found that a 5-min incubation is sufficient to successfully detect 50% of the replicates for six of the 10 species. Three of the species showed a significant increase in eDNA molecules over time. However, the experiment showed a species-specific eDNA release pattern that was not directly linked to body sclerotization nor biomass. As a second experiment, we sampled bulk samples at three field sites and incubated the bulk samples for 0, 20, 40, and 80 min in containers filled with stream water to compare taxa lists obtained via metabarcoding of bulk and the enriched eDNA samples. Our results showed a much higher overlap between bulk and enriched eDNA metabarcoding (55%–60%) in comparison to reported overlaps between bulk and stream eDNA metabarcoding from other studies (often < 20%). This overlap did not change with incubation time. Thus, our study demonstrates that it is possible to detect most locally occurring species via eDNA metabarcoding after shortly incubating them in water. Therefore, this approach has great potential for point-sample eDNA analysis of macroinvertebrates without compromising animal welfare.
1 Introduction
Biodiversity data obtained for aquatic invertebrates with genetic methods are increasingly considered for biomonitoring and ecological quality assessments, especially DNA metabarcoding of bulk samples and environmental DNA (eDNA) metabarcoding (Altermatt et al. 2025; Brantschen et al. 2021; Elbrecht et al. 2017; Kuntke et al. 2020; Sander et al. 2024). A major advantage of eDNA-based metabarcoding is its potential to capture stream biodiversity with just a single water sample (Deiner et al. 2016; Stat et al. 2017). As a result, eDNA metabarcoding, with its noninvasive and efficient sampling method and its comprehensive taxonomic lists, has emerged as a key tool for assessing freshwater biodiversity and is considered in the development of future biodiversity monitoring programs (Kissling et al. 2024). However, when incorporating new techniques into existing regulatory biomonitoring programs like the European Water Framework Directive (WFD), cross-calibration of data obtained with existing and new techniques is essential. Bulk metabarcoding, which includes sampling a stream's local aquatic invertebrate community using kicknet sampling and subsequent analysis of the DNA from these bulk samples, generates results similar to those of traditional morphological assessments (Elbrecht et al. 2017) and ecological status class assessments (Elbrecht et al. 2017; Macher et al. 2025; Meyer et al. 2021; Zizka et al. 2020). In contrast, eDNA-based taxa lists for aquatic invertebrates are typically comparable to morphological assessments only at the genus or family level (Mächler et al. 2019), but differ substantially at the species level (Gleason et al. 2021; Hajibabaei et al. 2019; Macher et al. 2018; Múrria et al. 2024; Pereira-da-Conceicoa et al. 2021). These disparities between species lists generated by traditional biomonitoring techniques and eDNA metabarcoding are subject to a multitude of factors that influence the efficacy of eDNA-based detection.
In lotic environments, eDNA molecules can be transported over distances of > 12 km (Deiner and Altermatt 2014; Wacker et al. 2019) leading to the detection of species inhabiting stream sections further upstream of a study area. Furthermore, DNA can persist in the sediment for several weeks when bound to organic matter (Shogren et al. 2017). Both characteristics lead to the detection of not only the local but also the regional community (Dejean et al. 2011; Pilliod et al. 2014; Shogren et al. 2017; Thomsen, Kielgast, Iversen, Møller, et al. 2012; Thomsen, Kielgast, Iversen, Wiuf, et al. 2012). This impairs the comparability of eDNA metabarcoding to more point-based assessments like bulk metabarcoding. An innovative approach to be considered is to incubate locally collected organisms in water for a certain period of time in order to amplify the local eDNA and minimize the eDNA contribution from upstream regions. Using the extracted DNA from the incubated water would allow for the release of animals back to the ecosystem after incubation. Such a concept has been applied in marine contexts (see Nichols et al. 2022) and has yet to be considered for streams.
However, such potential solutions must regard differences in eDNA shedding and decay rates among and even within species (Holman et al. 2022; Sansom and Sassoubre 2017). Seasonal variations are a contributing factor to the amount of DNA released into the environment due to life cycle patterns and temperature changes (Schmidt et al. 2021). Moreover, eDNA shedding rates are influenced by an organism's life stage; younger, smaller individuals undergoing molting and developmental processes tend to shed more DNA (Tréguier et al. 2014). Another important factor influencing eDNA shedding is the habitus of a species. Several studies have shown that DNA from softer-bodied and mucous vertebrates, such as fish and amphibians (Evans et al. 2016; Pilliod et al. 2013) and freshwater invertebrates like mussels and snails (Goldberg et al. 2013; Sansom and Sassoubre 2017), can be detected in large mesocosms after several hours or days. In contrast, research conducted on both indoor settings and natural ponds on invertebrates with exoskeletons has found that the DNA amount they release into their environment is low, which is especially observed in heavily sclerotized crustaceans (Andruszkiewicz Allan et al. 2021; Crane et al. 2021; Tréguier et al. 2014). Nevertheless, a high abundance of a particular species can still result in successful eDNA detection (Goldberg et al. 2013). However, no previous study has investigated the shedding rates of several differently sclerotized lotic macroinvertebrates for a shorter period (only few minutes or hours) to predict their detection probabilities. The ability to detect most species following a brief incubation regardless of sclerotization could significantly expand the practical applications of incubation methods, enabling their use in monitoring natural stream ecosystems.
- Species detection success increases with incubation time but differs between species depending on their level of sclerotization. We predict that hard-bodied species like crustaceans release less DNA than medium hard-bodied species like many insect larvae and that soft-bodied species like planarians release the highest amount of DNA.
- The proportion of species detected by both bulk and enriched eDNA metabarcoding increases with time. This increase is predicted because the number of DNA molecules shed from the target taxa into the water continuously increases, thereby changing the ratio between regional DNA from the stream water and local DNA from the bulk sample in the water container.
A schematic overview of the underlying assumptions on which the two hypotheses are based on including an overview of each workflow, is presented in Figures 1 and 2.


2 Materials and Methods
2.1 Indoor Experiment
2.1.1 Sampling and Experimental Setup
For the indoor experiment, we used 10 different species varying in their degree of sclerotization to assess how much of their DNA is released in the water column at five different time points. The species used were Dugesia gonocephala (Duges, 1830) (Turbellaria), Chironomus salinarius Kieffer, 1915 (Diptera), Glossiphonia complanata (Linnaeus, 1758) (Hirudinea), Ephemera danica Müller, 1764 (Ephemeroptera), Perlodes sp. (Plecoptera), Gammarus fossarum Koch, 1836 (Amphipoda), Asellus aquaticus (Linnaeus, 1758) (Isopoda), Ancylus fluviatilis O.F. Müller, 1774 (Gastropoda), Potamopyrgus antipodarum (J.E. Gray, 1843) (Gastropoda), and Potamophylax rotundipennis (Brauer, 1857) (Trichoptera). Specimens were sampled from April 2021 to August 2022 in North Rhine-Westphalia (Germany) or, in the case of C. salinarius, ordered from a pet shop (Garnelio, Mannheim, Germany). Initially, we started with one individual per replicate for each species, but for four species, the number of individuals per replicate had to be increased to allow DNA detection. These species were A. aquaticus (10 individuals), G. fossarum (20 individuals), Potamophylax rotundipennis (20 individuals), and C. salinarius (200 individuals). For species Perlodes sp., A. aquaticus, and Potamopyrgus antipodarum, the sampled area did not provide enough specimens to repeat the experiment with more individuals per replicate. We classified each species into one of three categories: soft-bodied, medium hard-bodied, or hard-bodied (see Figure S1 for information on the classification of each species into one of the categories). The soft-bodied category contains species that do not exhibit pronounced sclerotization or casings, i.e., C. salinarius and D. gonocephala. As medium hard-bodied species, we classified species that either had some sclerotized parts mixed with soft body parts like E. danica and Perlodes sp. or had harder body surfaces like G. complanata. Hard-bodied species included species with a casing like A. fluviatilis, Potamopyrgus antipodarum, and Potamophylax rotundipennis, or species whose bodies were almost completely sclerotized like G. fossarum and A. aquaticus. After sampling, specimens were stored in bottles filled with stream water and immediately transported to the laboratory to reduce stress and to avoid animal death. The experiment was carried out at room temperature (20°C) on the same day as the sampling in a separate room where no laboratory work was conducted. The animals were not fed during the experiment. We did not observe cannibalism, but for some species, like Perlodes sp., Potamopyrgus antipodarum, and Potamophylax rotundipennis, we observed reduced mobility of specimens after 40 min and mortality after 80 min. For an overview of the different steps of the experiment, from sampling to laboratory work, see Figure 1. For each species and experiment, we used 10 replicates. The experimental vessels were bleached beforehand (3% sodium hypochlorite for 20 min), sterilized with UV-C for 30 min, and then rinsed thoroughly with distilled water. For the experiment, twelve 500 mL sterilized vessels were filled with 400 mL of tap water (predisinfected with chlorine and its compounds by the local waterworks) sterilized with UV-C, which was filtered through an aquarium pump (Aquaristikwelt24 OHG, Worms, Germany). Two vessels were used as negative controls to check for contamination. Specimens were handled with sterilized forceps and rinsed in sterilized tap water before transferring them to the vessels. Before each sampling, the water in the vessels was mixed. At five time points (5, 10, 20, 40, and 80 min), 50 mL of water were carefully sampled from the middle of each replicate vessel with a sterile 50 mL syringe (Diagonal, Münster, Germany) to not stress the animals, transferred to a 50 mL falcon tube, and immediately stored on dry ice until further processing. Enriched eDNA samples were filtered on the same day using a vacuum pump and 0.45 μm cellulose nitrate membrane filters (diameter: 47 mm; Nalgene, New York, USA), ripped into small pieces using bleached forceps (3%–4% sodium hypochlorite, rinsed with distilled water afterwards), and placed into a twist-top tube filled with one spoon of 1 and 2 mm zirconia beads. Samples were immediately transferred to liquid nitrogen and then stored at −80°C overnight. To check for air contamination, negative filter controls, for which only the surrounding air was filtered using a vacuum pump, were taken before the first sample and after the last sample was filtered. To work as sterile as possible, gloves were changed between samples, and the working space was cleaned with 80% ethanol. Specimens were separately stored in 96% technical ethanol at room temperature until further processing.
2.1.2 Extraction of Filters
Laboratory work for the filtered enriched eDNA samples was always conducted on the day after the experiment in a separate sterile eDNA laboratory. As we originally intended to assess DNA and RNA, we used a protocol with which both can be co-extracted. DNA and RNA were first lysed from the filter by adding 600 μL guanidinium isothiocyanate (GITC) lysis buffer (4 M guanidinium thiocyanate, 10 mM Tris) to each sample, which was then ground for 2 min at 2400 rpm (Mini BeadBeater 96, Biospec products). After centrifugation for 1 min, 240 μL of the lysate of each sample was transferred to a 96-well plate. Then, 320 μL isopropanol was added per sample and mixed for 5 min at 1000 rpm, followed by adding 40 μL silica beads (SeraSil-Mag 400 silica-coated beads, Sigma-Aldrich, St. Louis, USA) and 120 μL TE minimum buffer (pH 8.0) and mixing the samples for 5 min at 1000 rpm. Then, the beads were separated on a magnet for 4 min, and the supernatant was discarded. Samples were washed with 400 μL isopropanol each and mixed for 2 min at 1000 rpm. After 2 min on the magnet, the supernatant was discarded and 300 μL ethanol (80%) was added. The liquid was mixed for 2 min at 1000 rpm, and the supernatant was discarded after the beads had settled on the magnet for 2 min. This washing step was repeated, and afterward, the samples were dried for 5 min at 55°C and 600 rpm to remove the remaining ethanol. DNA was then eluted in 70 μL TE minimum buffer and mixed for 5 min at 1200 rpm. After 5 min on the magnet, 50 μL of the supernatant was transferred to a new 96-well plate. Extractions were frozen at −80°C until further processing.
2.2 Barcoding and Preparation of qPCR Standards
During sampling, specimens were determined morphologically to genus or species level. To verify the identification or to obtain species level, single specimen barcoding of the cytochrome c oxidase I (COI) gene was performed. Before DNA extraction, the dry weight of each specimen was calculated using a precision scale for later normalization of the data (see Appendix S1). The detailed laboratory steps performed for barcoding of the specimens are listed in the Appendix S1 and the primers used for barcoding in Table S1. The obtained sequences were further used to design two sets of species-specific COI qPCR (quantitative PCR) primers using Primer3Plus (https://www.primer3plus.com/index.html) (see Table S2 for the primers used). These primers were used to measure the amount of DNA released per specimen of a species to the water during the time interval. We then prepared two qPCR standard series with dilutions of 0.2 × 107, 0.2 × 106, 0.2 × 105, 0.2 × 104, 0.2 × 103, and 0.2 × 102 template molecules/μL with both primer pairs for each species, using the DNA of one specimen per species to obtain a standard curve of six points (for details on the preparation of standards, see Appendix S1).
2.3 qPCR—Quantifying Released DNA
During the qPCR measurements, samples and standards were run in triplicates for each primer to reduce errors resulting from inaccurate measurements of the qPCR machine. Triplicate samples were randomized so that each sample was placed on three different plates to account for plate variability. We used the Quant Studio 3 System with the following settings: Exp. Type: Comparative CT, Chemistry: SYBR Green Reagents, Run Mode: Fast. qPCR reactions for each assay consisted of 1x qPCR PerfeCTa SYBR Green FastMix Low ROX (VWR, Darmstadt, Germany), 100 nM of each primer, and 2.5 μL DNA. Cycling conditions were as follows: 30 s at 95°C, 40 cycles for 5 s at 95°C, and 20 s at 60°C, followed by a melt curve program of increasing temperature from 60°C to 95°C. For each qPCR run, we included three qPCR negative controls for each primer containing only the mastermix and primers. Per species, we analyzed 300 samples (10 replicates × 5 time points × 2 primer pairs × 3 qPCR replicates) with 24 qPCR negative controls and 24 water and air filter controls. Where qPCR assays did not quantify any DNA, we repeated the experiment with a higher number of specimens used (see Section 2.1.1). We repeated the qPCR three times for samples that did not exhibit amplification success in all triplicates. If amplification was still unsuccessful, the samples were excluded from the analysis. To gain reliable copy number estimations, it is necessary to have three different Ct values for each sample because such low amounts of input DNA could lead to large differences based on pipetting errors.
We used LinRegPCR version 2021.1 (Ruijter et al. 2009) to calculate the starting concentrations of the samples from the qPCR measurements. The program uses baseline fluorescence with baseline subtraction and adjusts a Window-of-Linearity. Based on that, PCR efficiencies for each sample and the mean qPCR efficiency per amplicon were calculated and used to determine the starting concentration of the samples using the fluorescence threshold and the individual Ct value of the samples (Ramakers et al. 2003). To remove between-run variations, we used the software Factor-qPCR version 2020.0 (Ruijter et al. 2015).
2.4 Statistical Analysis
Standard curves for each species were calculated using the serial dilutions and were used to calculate DNA copy numbers for the samples. The number of DNA copies detected per time point was used as a proxy for the eDNA shedding rate, defined as the DNA shed from organisms into the environment. Copy numbers were normalized using copy numbers divided by the dry weight of the individuals per replicate and adapted to the remaining water volume at the time of sampling to account for the reduction of water volume after each sampling. For this, we multiplied the copy numbers for each replicate with the remaining water volume (×1 at timepoint 5 min, ×0.875 at timepoint 10 min, ×0.75 at timepoint 20 min, ×0.625 at timepoint 40 min, and ×0.5 at timepoint 80 min). For biomass analysis, we used the dry weights of the individuals and divided the weight and copy number by the number of individuals per replicate.
Statistical analyses were performed in R version 4.0.5 (R Core Team 2021), and all plots were generated using the ggplot2 package (Wickham 2009). We used linear regression to assess the effect of increasing biomass on DNA copy number release. The effect of incubation time on DNA copy number was tested using linear mixed effect models (LMM) fit by restricted maximum likelihood (REML) with the function lmer() implemented in the lme4 package (Bates et al. 2015). Incubation time was defined as a continuous variable that was log-transformed to improve model fit. Replicates were implemented as a random factor to account for differences between individuals. The model was structured to implement different slopes per replicate to account for specimen-specific differences in activity. Model diagnostics were checked by plotting residuals versus fitted values.
2.5 Field Experiment
2.5.1 Sampling and Experimental Setup
For our field experiment, we used kicknet samples instead of single species for the incubation approach. For this, we sampled three streams in the Emscher catchment (North Rhine-Westphalia, Germany): Boye (51.5612, 6.9330; catchment size = 74.36 km2; length = 13.8 km), Borbecker Mühlenbach (51.4379, 6.9676; catchment size = 62.12 km2; length = 11.1 km), and Wittringer Mühlenbach (51.5642, 6.9851; catchment size = 6.83 km2; length = 3.1 km). The workflow from sampling to laboratory work is shown in Figure 2. We took one multihabitat kicknet sample per stream according to WRRL (Meier et al. 2006) but with 10 subsamples, because the streams were not sufficiently diverse and relatively small (see Figure S2). Before transferring the kicknet sample to a sterilized 20 L bucket filled with 7 L stream water, we added three spike-ins (artificial DNA oligonucleotides, see Appendix S1 for more information on the sequences used) to the bucket with a concentration of 10,000 copies per 100 mL stream water. The spike-ins included the primer sequences of the fwhF2/fwhR2n (Vamos et al. 2017) and the fwhF2/EPTDr2n (Leese et al. 2021) primer pairs, which we later used in our metabarcoding workflow with random bases inserted between the primer regions. The objective was to use the change in the read proportions of the spike-ins during the incubation process as a reference to the hypothesized increase of the DNA proportion of the living animals from the bulk sample (see Figure 2). The kicknet sample was then transferred to the bucket. The water temperature was 8.5°C with a pH of 7.5 for the Boye, 11.5°C and a pH of 8.48 for the Borbecker Mühlenbach, and 9.5°C and a pH of 8.14 for the Wittringer Mühlenbach. Immediately after transferring the kicknet sample, we sampled 100 mL water from the bucket (t0) five times with a sterile syringe (Diagonal, Münster, Germany). Based on the results of the first experiment, we also sampled five times 100 mL after 20 (t20), 40 (t40), and 80 min (t80). Before each sampling, we mixed the water inside the bucket with the living animals inside for 20 s to stir up the DNA and waited then another 20 s to let the suspended matter settle down again to minimize clogging of the filters. Mixing the water before sampling improved detection success in another study using incubation (Trimbos et al. 2021). The enriched eDNA samples were stored on ice in the field and, after they were filtered, immediately stored on ice again. Each 100 mL enriched eDNA sample was filtered separately, and the filter was folded and stored in a 2 mL twist-top tube filled with one spoon of 1 and 2 mm zirconia beads. Filters were stored in the laboratory at −80°C and processed the next day in a separate laboratory. Kicknet samples containing the animals and debris were stored in 96% ethanol at room temperature.
2.5.2 Laboratory Work
The filters were ripped into smaller pieces, and bulk samples were homogenized following the protocol used in Buchner, Beermann, et al. (2021). For homogenization, a kitchen blender (Mini Blender & Blender Smoothie, Homgeek, China) was used, and samples were mixed for 30 s before transferring 5 × 1 mL to a twist-top tube with one spoon of each 1 and 2 mm zirconia beads. For each bulk sample, we took five replicates to obtain an equal comparison with the enriched eDNA samples with five 100 mL replicates from the buckets. Bulk samples were centrifuged for 3 min at maximum speed, and the supernatant (ethanol) was discarded. Bulk samples and filters were processed together in the following steps. The following lysis and extraction steps were identical for the bulk and enriched eDNA samples. Lysis was performed following the steps of the lysis of the enriched eDNA samples of the first experiment for single specimen barcoding (Appendix S1), but with 900 μL TNES and 100 μL Proteinase K for each sample. For extraction of the bulk and enriched eDNA samples, we used a spin column protocol (vacuum manifold; Buchner 2022a) with 500 μL binding buffer and 250 μL lysate. Extraction was performed in replicates, and we included nine extraction negative controls per plate, which were positioned on the 96-well plate as described in Elbrecht and Steinke (2019). Samples were then mixed at 900 rpm for 5 min, and 750 μL of the mixture of each sample was transferred to a 96-well filter plate placed in a vacuum manifold. The samples were washed twice with 500 μL wash buffer. All following steps were performed according to the protocol from Buchner 2022a. After DNA extraction, samples were purified using 80 μL Sera Speed beads (carboxylated 1:50 in SPRI buffer) and 40 μL of the extract per sample. Samples were incubated for 5 min at 1000 rpm on a thermomixer and then placed on a magnet for 2 min, after which the supernatant was discarded. Samples were washed twice with 100 μL wash buffer and dried for 5 min to remove excess ethanol. The magnet was removed, and samples were eluted with 40 μL elution buffer and mixed for 5 min at 1000 rpm. As a last step, the samples were again placed on the magnet for 2 min to settle the beads, and 35 μL of the supernatant were transferred to a new 96-well plate.
To increase the reliability of the results, two different primers were employed. For metabarcoding, a two-step PCR approach using tags in both steps was performed (Bohmann et al. 2022). In the first step, we used tagged versions of the fwhF2/fwhR2n and the more insect-specific fwhF2/EPTDr2n primer pairs with varying lengths and a universal tail (Leese et al. 2021). Each reaction contained 1× Multiplex PCR Master Mix (Qiagen Multiplex PCR Plus Kit), 0.2 μM of each primer, 0.5× CoralLoad Dye and 1 μL purified DNA extract, which was filled up to a total of 10 μL with PCR-grade water. The cycling conditions were as follows: 95°C for 5 min, 20 cycles of 95°C for 30 s, 58°C (fwhF2/fwhR2n)/54°C (fwhF2/EPTDr2n) for 90 s, 72°C for 30 s, and a final elongation at 68°C for 10 min. After the first step, the samples were purified following the PCR cleanup and size selection with a magnetic beads protocol (Buchner 2022b). For the second step, we used primers matching the universal tail which included one of 96 i5/i7 indices and the P5/P7 Illumina adapter (Buchner, Beermann, et al. 2021; Buchner, Haase, et al. 2021). The reactions contained 1× Multiplex PCR Master Mix (Qiagen Multiplex PCR Plus Kit), 0.5 μM of each primer, 0.5× CoralLoad Dye and 2 μL of purified PCR product. Cycling conditions were as follows: 95°C for 5 min, 25 cycles of 95°C for 30 s, 61°C for 90 s, 72°C for 30 s, and a final elongation of 68°C for 10 min. PCR products were normalized with magnetic beads (Buchner 2022c). Afterward, samples were pooled, purified, and measured on a Fragment Analyzer Automated CE System (Advanced Analytical Technologies GmbH) using the NGS Standard Sensitivity Kit. The library was sent to Macrogen Europe B.V. for sequencing on a single flow cell using an Illumina HiSeq X sequencer with a 300-cycle kit (150 bp paired-end reads) and 5% Phi-X spike-in.
2.6 Data Analysis
Demultiplexed sequence reads were processed using the APSCALE pipeline version 1.4.0 (Buchner et al. 2022), which is based on VSEARCH and cutadapt (Rognes et al. 2016; Martin 2011). Standard settings were used, and the OTUs were clustered with a 97% similarity threshold. Taxonomic assignment was performed via BOLDigger version 2.1.0 (Buchner and Leese 2020) using the following thresholds: ≥ 98% for species level, ≥ 95% for genus level, ≥ 90% for family level, ≥ 85% for order level, < 85% for class level, which are the default settings in BOLDigger where the species threshold is based on the study of Hebert et al. (2003). Subsequently, the extraction replicates were merged, retaining all OTUs from both replicates. Furthermore, the maximum number of reads among negative controls per OTU was subtracted from each OTU in the samples to account for contamination and tag switching. Only OTUs with a similarity of 90% and an assignment of at least to family level were retained for further analysis. Additionally, we filtered the data set for macroinvertebrates and removed all terrestrial taxa after comparing our taxa list to the entries deposited in freshwaterecology.info (Schmidt-Kloiber and Hering 2015, 2024).
2.7 Statistical Analysis
The following statistical analyses were performed in R version 4.0.5, and all plots were generated using the ggplot2 package. We used LMMs fit by REML to assess the effects of the two predictors, incubation time and number of replicates, on the proportion of the number of (I) species and (II) OTUs shared between bulk and enriched eDNA metabarcoding. Incubation time was used as a continuous variable and number of replicates as a categorical variable ranging from 1 to 5. For both enriched eDNA and bulk metabarcoding samples, we used five replicates which were randomly shuffled to obtain permuted data for each number and combination of replicates. In the LMMs, streams were used as a random effect per time point to enable different patterns per stream and per time point for each stream. Model diagnostics were checked by plotting residuals versus fitted values. An indicator species analysis using presence–absence data and using the function multipatt() in the package indicspecies (De Cáceres et al. 2010) was applied to identify species associated with either sample type and time point or a combination of both. We used the indicator value index (Dufrêne and Legendre 1997) rather than a correlation-based index, as it is preferred for biomonitoring aspects (see Sander et al. 2024). In the Results and Discussion sections, the two data sets are referred to by their reverse primer, fwhR2n (fwhF2/fwhR2n) and EPTDr2n (fwhF2/EPTDr2n) respectively, as both primer pairs share their forward primer.
3 Results
3.1 eDNA-Based Detection of Individual Species—Indoor Incubation Experiment
In the qPCR assays, we did not detect any contamination in all negative controls. While in general nine of 10 qPCR assays (one for each of the 10 species) successfully amplified template DNA from the water samples, only seven qPCR assays resulted in sufficient amplification for data analysis. Overall, primer efficiency was consistent for all tested species, ranging from 1.70 to 2.05 depending on the primer used (Data S2). For the chironomid C. salinarius, the inferred copy numbers differed by an order of magnitude between the two primer pairs despite having similar efficiencies (primer pair A: 1.88, primer pair B: 1.86) (Figure 3C). For other species, the difference in inferred copy numbers between primer pairs was less pronounced (Figure 3).

3.2 Influence of Biomass on eDNA Shedding
Inferred DNA copy numbers did not increase with increasing biomass for any of the species tested (R2 and p-values displayed in Figure S3). However, the detection success for several species improved when the number of individuals used per replicate and consequently their biomass was increased.
3.3 Influence of Incubation Time on eDNA Shedding
A longer incubation time linearly increased the inferred eDNA copy numbers of A. fluviatilis, C. salinarius, and G. fossarum (see Figure 3, Table 1, and Data S5). In contrast, copy numbers for E. danica, D. gonocephala, G. complanata, and Potamophylax rotundipennis did not increase over time (Figure 3).
| Species | Estimate | SE | F-value | p | ndf | ddf | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primer A | Primer B | Primer A | Primer B | Primer A | Primer B | Primer A | Primer B | Primer A | Primer B | Primer A | Primer B | |
| Af | 0.04 | 0.03 | 0.003 | 0.003 | 119.94 | 135.71 | < 0.001*** | < 0.001*** | 1 | 1 | 9.13 | 9.33 |
| Cs | 0.04 | 0.04 | 0.005 | 0.005 | 48.79 | 44.26 | < 0.001*** | < 0.001*** | 1 | 1 | 9.0 | 9.0 |
| Dg | 0.003 | 0.007 | 0.003 | 0.005 | 0.837 | 2.37 | 0.385 | 0.169 | 1 | 1 | 8.59 | 6.79 |
| Ed | 0.008 | 0.008 | 0.005 | 0.004 | 3.32 | 3.65 | 0.102 | 0.089 | 1 | 1 | 9.0 | 9.0 |
| Gc | 0.007 | −0.003 | 0.007 | 0.01 | 1.03 | 0.08 | 0.341 | 0.784 | 1 | 1 | 7.78 | 6.66 |
| Gf | 0.04 | 0.04 | 0.01 | 0.01 | 16.43 | 12.63 | 0.003** | 0.007** | 1 | 1 | 8.88 | 8.0 |
| Pr | 0.008 | 0.009 | 0.005 | 0.004 | 2.30 | 5.05 | 0.169 | 0.055 | 1 | 1 | 7.62 | 8.01 |
- Note: Presented are the species tested, REML (Restricted Maximum Likelihood) Estimate, Standard error (SE), F-value, p-value, numerator degrees of freedom (ndf), and the denominator degrees of freedom (ddf). The response variable was the number of DNA molecules measured, and the predictor variable was incubation time. Asterisks indicate significance. Af, Ancylus fluviatilis; Cs, Chironomus salinarius; Dg, Dugesia gonocephala; Ed, Ephemera danica; Gc, Glossiphonia complanata; Gf, Gammarus fossarum; Pr, Potamophylax rotundipennis. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
3.4 Influence of Sclerotization on eDNA Shedding
We assigned the 10 species to three different sclerotization categories: soft-, medium hard-, and hard-bodied to assess if species assigned to the same category exhibit similar detection success and copy numbers. Species detection and eDNA accumulation with increasing incubation time were not higher for soft-bodied species (Figure 3 and Table S3). The soft-bodied D. gonocephala showed a moderately high detection success (> 50% for all time points) with one individual per replicate but no increase in copy numbers with time, whereas the soft-bodied species C. salinarius was only quantifiable after increasing the individual size to 200, which led to 100% detection success and high DNA copy numbers (Figure 3 and Table S3). The copy numbers for the two medium hard-bodied species, G. complanata and E. danica, did not increase over time and G. complanata was the only species with a detection frequency below 50% throughout the time points (Figure 3 and Table S3). In contrast, E. danica had a 100% detection rate. The qPCR assay for the medium hard-bodied stonefly Perlodes sp. only amplified DNA in 13 out of 300 samples. For G. fossarum and Potamophylax rotundipennis, both classified as hard-bodied, detection success reached high values only after increasing abundance to 20 and was higher for the former, for which we also detected higher DNA copy numbers (Figure 3 and Table S3). The hard-bodied A. fluviatilis was an exception here, as the species reached a high detection success rate of at least 80% among time points and primer pairs, and high DNA copy numbers with only one individual used (Figure 3 and Table S3). Detection success for the hard-bodied snail Potamopyrgus antipodarum was limited to longer incubation times (40 and 80 min) and to only one primer pair (Data S2) and for the hard-bodied crustacean A. aquaticus, detection success was inconsistent throughout time points even after increasing specimen numbers to 10 (Table S3).
3.5 eDNA-Based Detection of the Local Invertebrate Bulk Community—Field Experiment
Metabarcoding analysis yielded 9448 (fwhR2n) and 583 (EPTDr2n) OTUs based on 274,147,988 (fwhR2n) and 286,576,549 (EPTDr2n) reads after quality filtering (Data S3). Reads per sample ranged from 2,643,806 to 5,146,703 (fwhR2n) and from 1,476,075 to 5,244,817 (EPTDr2n), with mean read numbers of 3,655,307 (fwhR2n) and 3,821,020 (EPTDr2n) (Data S3). The negative controls contained 56,767 (fwhR2n) and 7801 (EPTDr2n) reads, accounting for 0.021% (fwhR2n) and 0.002% (EPTDr2n), respectively. Of the three spike-ins we added to the stream water within the incubation container, only one was detected in a single sample with a low read abundance. Consequently, further analysis of the spike-in results was not feasible. After filtering the OTU lists, 52% of the OTUs in the fwhR2n data set were lost due to non-target amplification compared to only 1% in the EPTDr2n data set. In the fwhR2n data set, we detected 100 more species, and also more exclusive species, than in the EPTDr2n data set, especially from the taxonomic groups Trichoptera, Gastropoda, Bivalvia, Copepoda, Annelida, and Amphipoda, with the last being only detected with the fwhR2n primer pair (Figure S4).
3.6 Comparison of Bulk and Enriched eDNA Metabarcoding
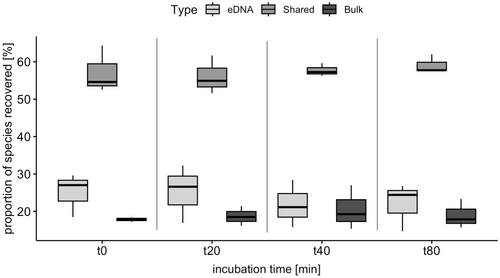
In general, 60% of species and OTUs were detected in the bulk metabarcoding samples and the enriched eDNA isolated from the water, whereas 25% more species and OTUs were detected in the enriched eDNA than in the bulk metabarcoding samples (Table 2 and Data S4). The proportion of species shared between enriched eDNA and bulk metabarcoding samples ranged from 50% to 60% for all time points and was always higher than the proportion of exclusively detected species (Figure 4 and Figure S5; Figure S6 for results on OTUs and S7, S8 and S9 for the EPTDr2n data set). For the fwhR2n data set, the proportion of shared species (55%) was slightly lower than for the EPTDr2n data set (60%).
| Primer pair | Level | Shared | eDNA-exclusive | Bulk-exclusive | Total |
|---|---|---|---|---|---|
| fwhF2/fwhR2n | Species | 196 | 108 | 18 | 322 |
| OTUs | 360 | 172 | 47 | 579 | |
| fwhF2/EPTDr2n | Species | 136 | 65 | 11 | 212 |
| OTUs | 239 | 110 | 23 | 372 |
Species composition between time points was similar with more than half of the species (56.25% for fwhR2n and 60.70% for EPTDr2n) found at all four time points, whereas most of the exclusive species were found at one of the four time points, respectively (23.68% for fwhR2n and 15.42% for EPTDr2n) (Figures S10 and S11 for the EPTDr2n data set). The composition between shared species and bulk metabarcoding- and enriched eDNA-exclusive species did not show remarkable divergences in terms of the proportion of orders, and none of the orders was exclusively found in one sample type, except for Bivalvia, which was only detected in the enriched eDNA samples of the EPTDr2n data set (Figures S12 and S13 for the EPTDr2n data set). Diptera and Annelida accounted for most of the species detected in both sample types, followed by Trichoptera. The indicator species analysis revealed that most indicator species belonged to the aforementioned orders. While 42 species were associated with the bulk metabarcoding samples, with 12 of them being exclusively found, only three species were associated with the enriched eDNA samples from all four time points (see Data S5 for more information on indicator species classification).
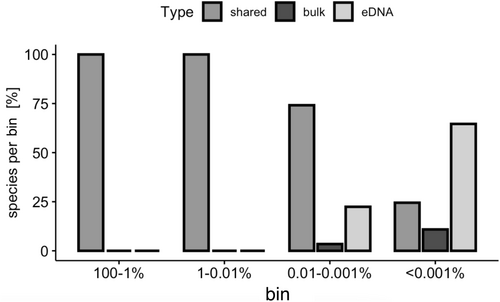
Additionally, most reads (> 90%) were assigned to species detected in both sample types (Figures S14 and S15), and the read proportions of shared OTUs and species were higher than those of the exclusively found OTUs and species, which mostly had a read proportion of < 0.001% (Figure 5, Figures S16–S18 for the EPTDr2n data set). When not considering OTUs and species with read proportions < 0.001%, 80% of the detected OTUs and species were shared between bulk and enriched eDNA metabarcoding, whereas 20% of the previously shared OTUs and species were filtered out. The proportion of eDNA-exclusive species and OTUs was reduced to 7%.
3.7 Effect of Incubation Time and Number of Replicates
Incubation time did neither affect the proportion of species (LMM: F = 4.05, p-value = 0.17, ndf = 1, ddf = 2.27) nor the OTUs (LMM: F = 6.61, p-value = 0.12, ndf = 1, ddf = 2) shared between bulk and enriched eDNA metabarcoding for the fwhR2n data set, although an increase in shared OTUs and species was observed, especially for the Boye (Figure 6, Figure S19, and Data S5). For the EPTDr2n data set, incubation time did not affect the proportion of shared OTUs (LMM: F = 14.07, p-value = 0.06, ndf = 1, ddf = 2), but a small, yet significant increase in the proportion of shared species was observed with increasing incubation time (0.05% increase per min, (LMM: F = 19.49, p-value = < 0.001, ndf = 1, ddf = 45.16) Figures S20 and S21 and Data S5).
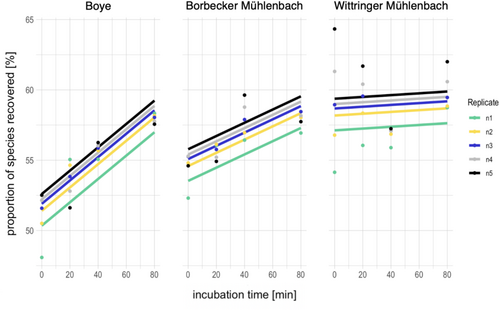
The number of used replicates significantly increased the proportion of shared OTUs and species by 5% for both data sets (LMM: fwhR2n OTUs: F = 10.33, p-value = < 0.001, ndf = 4, ddf = 50 (Figure S19); fwhR2n species: F = 3.53, p-value = 0.013, ndf = 4, ddf = 51.91 (Figure 6); EPTDr2n OTUs: F = 3.02, p-value = 0.026, ndf = 4, ddf = 50 (Figure S21); EPTDr2n species: F = 3.7, p-value = 0.01, ndf = 4, ddf = 52 (Figure S20)) (Data S5). The proportion of eDNA-exclusive OTUs/species increased (~10%), whereas the proportion of bulk metabarcoding-exclusive species/OTUs decreased by approximately 10% (Figures S5 and S8).
4 Discussion
Our findings showed that incubating specimens in water for a short period prior to eDNA sampling resulted in a high detection success (laboratory single-species experiments) and a high degree of overlap between bulk from locally collected specimens and enriched eDNA metabarcoding samples (field bulk metabarcoding experiment). Thus, our approach, which integrates the advantages of both bulk and eDNA metabarcoding, provides a valuable strategy to the challenge of capturing local community signals by using eDNA collected from water. However, the study also revealed some unexpected patterns of eDNA accumulation, which are discussed below.
4.1 eDNA-Based Detection of Individual Species—Indoor Incubation Experiment
4.1.1 Influence of Incubation Time on DNA Enrichment of Target Molecules
We hypothesized that the detection of DNA target molecules will increase with time. Contrary to our expectation, DNA molecules of our target species only increased with time for three of the seven tested species (i.e., excluding species Potamopyrgus antipodarum, A. aquaticus, and Perlodes sp. that did not show consistent amplification results), namely for A. fluviatilis, C. salinarius, and G. fossarum. For these species, the target molecules increased linearly over the 80-min sampling period, indicating that the rate of DNA molecule release was constant and steady after transferring the animals into the experimental vessel. The gradual increase in target molecules with time may be characteristic for the species that exhibited increased activity levels during the experiment. This was true for G. fossarum, where individuals were observed actively swimming, and C. salinarius, which exhibited twitching movements throughout the experiment. In contrast, the snail A. fluviatilis displayed a lower activity level. Other species-specific factors, in particular body hardness and body attachments like shells or gills, may influence the shedding rate and will be discussed in the next paragraphs. Furthermore, the three species that showed an increase in their DNA release over time potentially exhibited greater vitality levels during the experiment than the other seven species. After 40 min of incubation in the experimental vessel filled with tap water, the vitality of most animals began to decline, with individuals of orders Plecoptera and Trichoptera exhibiting immobility due to their greater sensitivity to reduced flow velocity, while individuals of the snail Potamopyrgus antipodarum retreated into their shells. The number of DNA copies released by individuals of Potamophylax rotundipennis declined between 40 and 80 min of incubation (Figure 3), possibly due to the reduced activity of the individuals. DNA decay may have resulted in the reduction of the present DNA, which was not compensated by freshly shed DNA. For some species, there was a delay of 2 h between sampling the animals and starting the experiment, which may have had a negative impact on the animals' vitality. Furthermore, the relocation of the animals from the sampling vessel filled with stream water to the experimental vessel filled with filtered tap water may have further impacted their vitality, and this factor should be taken into consideration when interpreting the study's results. Contrary to other studies that used long incubation times of hours or even days (24 h–5 days, Andruszkiewicz Allan et al. 2021; 12 h–4 weeks, Sansom and Sassoubre 2017), we detected at least half of the specimens immediately after transferring the animals to the experimental vessel and 80% of them after an 80-min incubation (Figure 3). The transfer of the animals to the experimental vessels was potentially the moment when the animals released the highest amount of DNA due to the immediate change in ambient water, likely resulting in strong physiological responses in the organisms.
4.1.2 Influence of Biomass on eDNA Shedding
For none of the tested species, we found a correlation between target molecule release and increasing biomass for the time period studied (Figure S3). Some studies on invertebrates have reported such a correlation (e.g., Sansom and Sassoubre 2017) while others did not (Goldberg et al. 2013; Tréguier et al. 2014). Therefore, differences in observed eDNA shedding rates may be attributed to other factors such as sclerotization or species-specific characteristics, which will be discussed in the following sections.
4.1.3 Influence of Sclerotization on eDNA Shedding
We hypothesized that detection success and DNA target molecule release differ between hard-, medium-, and soft-bodied species and that the former will release the lowest number of target molecules, followed by medium- and then soft-bodied species. However, in discordance with the hypothesis, we did not find a clear correlation between the release of target molecules and the sclerotization of the body because detection success was not always higher for soft-bodied species in comparison to hard-bodied. In the following paragraphs, we discuss the results for each of the three categories.
Soft-bodied species: While the soft-bodied planarian species D. gonocephala released constantly detectable amounts of DNA with only one individual present, the smaller but also soft-bodied chironomid C. salinarius was only detectable after increasing abundance to 200 individuals, after which it was detected in all replicates immediately (Figure 3). In addition to the differences in individual size, and therefore surface area, planarians release mucous, which is a potential source for a higher DNA release in comparison to chironomids.
Medium hard-bodied species: As expected, the medium-hard bodied classified leech species G. complanata showed an intermediate detection success. G. complanata was detected less frequently than D. gonocephala (Figure 3), which was expected because the latter has a comparably softer skin, which probably caused that DNA from epidermal cells was more easily shed into the water. In contrast, although invertebrates with pronounced exoskeletons are known to release low amounts of DNA into the surrounding water (Mauvisseau et al. 2019; Schmidt et al. 2021), the mayfly E. danica was detectable with a constantly moderate DNA copy number throughout all replicates and time points and showed a higher detection success than the soft-bodied D. gonocephala (Figure 3). Because E. danica possesses large gills, the body surface of the individuals is enlarged. Coupled with the constant movement of the gills (Eastham 1939) these traits support a high DNA shedding despite their exoskeleton. The only species that could not be detected using qPCR was the plecopteran Perlodes sp., from which only one individual per replicate could be sampled due to its low abundance in the study region. Additionally, the individuals died after the 40- and 80-min intervals of the experiment, probably due to their sensitivity to low flow velocities (Dewson et al. 2007; Pastuchová et al. 2008) and hence, they experienced stress under the experimental conditions. Plecopterans also showed low detection probabilities in other studies that suggested low shedding rates as a cause (Mauvisseau et al. 2019).
Hard-bodied species: In contrast to Hypothesis 1, the snail A. fluviatilis was detectable in 80% of the samples after a 5-min incubation (Figure 3). However, the snail Potamopyrgus antipodarum was detected only after 40 and 80 min at low detection frequencies (Table S3). These differences might be explained by the 4.5× lower biomass of Potamopyrgus antipodarum in comparison to A. fluviatilis. However, we did not find a positive effect of increased biomass on eDNA shedding rate for A. fluviatilis. Another trait that might explain differences in detections is contact of soft body parts with the water. Individuals of Potamopyrgus antipodarum can retract completely into their shells because they possess an operculum, which has been shown to reduce DNA release (Carew et al. 2018), whereas A. fluviatilis' softer foot is constantly exposed to the surface of the experimental vessel, potentially leading to the shedding of more DNA. To add to this, members of the snail family Ancylidae possess so-called “lobe-gills,” which are highly developed secondarily derived gills that enable a completely efferent and afferent circulation, likely increasing DNA shedding through their respiratory processes (Hunter 1954). In contrast to A. fluviatilis, the shedding rate of the other species classified as hard-bodied was more in accordance with the hypothesis (Figure 3). The caddisfly Potamophylax rotundipennis, whose soft body is mostly covered by a self-built case, reducing the exposed body area, was only detected via eDNA when abundances in experimental vessels were increased to 20 individuals. Similarly, the highly sclerotized crustacean G. fossarum was detected reliably from eDNA only at abundances of 20 individuals per experimental vessel, confirming the low DNA shedding rates of crustaceans (Andruszkiewicz Allan et al. 2021; Crane et al. 2021; Tréguier et al. 2014). Unfortunately, we could not sample more individuals of A. aquaticus because of their rare occurrence in the sampled area.
Overall, our study neither found a clear correlation of species detection success with body sclerotization nor with biomass. Thus, other factors, such as specimen size and species characteristic features like body shells and gills, seem to have a greater influence on the detection success in water samples. However, if the biomass or abundance of a species is high enough, even hard-bodied species with a pronounced exoskeleton, or a case covering most of their body, can be detected after a 5-min incubation. Therefore, to estimate the probable detection success, it is important to determine not only how much DNA a single individual of a species sheds into the environment but also how abundant a species is at a certain site.
4.2 eDNA-Based Detection of the Local Invertebrate Bulk Community—Field Experiment
4.2.1 Influence of Incubation Time on Shared Detection
With Hypothesis 2 we predicted the proportion of species detected by both bulk and enriched eDNA metabarcoding to increase with time. In agreement with our hypothesis, we detected a significant, albeit modest, increase in the shared species for the EPTDr2n data set. Contrarily, using the fwhR2n data set, we did not detect a statistically significant increase in shared species or OTUs over time, although for one stream (Boye) at least an increasing trend was observed. We predicted an increase in the proportion of shared species because we expected that eDNA shed from specimens into the water over time would dilute the background signal from the stream water and thereby lead to a higher overlap between bulk and enriched eDNA metabarcoding. Previous studies have shown that DNA accumulates over time, which is supported by our results from the single-species indoor experiments. It is known that DNA accumulation can come to a halt after several hours (Seymour et al. 2018) or days (Strickler et al. 2015) due to DNA decay, but not when analyzing short-time periods of only minutes. Our results suggest that the animals already released sufficient DNA into the water immediately after the start of the experiment (time point “0 min”), explaining the highly constant number of species detected by both approaches (Figure 6). The process of transferring the animals from the kicknet to the bucket was likely the moment when stress was highest and DNA abrasion from epidermal body walls, such as animal skins, was amplified, leading to a sudden and high release of DNA molecules. For fish it is known that introducing animals to a new environment can cause an immediate and high release of DNA molecules (Klymus et al. 2015). However, we did not take a water sample directly from the stream to verify whether the incubation process indeed resulted in an enrichment of the DNA of the locally sampled animals or if the stream water was already enriched with the DNA of the locally occurring species before the incubation process. Unfortunately, our spike-in approach did not produce results that would resolve this question. Therefore, it should be considered for future applications of the proposed method to use a direct stream sample as a control sample to more directly assess the added value of kicknet sampling incubation in comparison to a normal water sample to obtain a more localized signal. However, in comparison to other studies reporting an overlap between stream water eDNA and bulk metabarcoding of often < 20% (e.g., Gleason et al. 2021; Hajibabaei et al. 2019; Pereira-da-Conceicoa et al. 2021), the proportion of species detected using enriched eDNA and bulk metabarcoding in our study was substantially higher with 60% overlap (Figure 4). A high proportion of 60% of shared taxa was also achieved with stream samples before, but only when looking at family or genus level (Deiner et al. 2016; Macher et al. 2018; Mächler et al. 2019). When samples were individually evaluated, as we did in this study, the proportion of shared OTUs reported decreased remarkably in other studies (Gleason et al. 2021; Hajibabaei et al. 2019). In addition, > 90% of the reads for both OTUs and species were shared between the methods and most OTUs and species that were exclusively found with either of the approaches had a read proportion of < 0.001% (Figure 5), which further strengthens the assumption that exclusively detected OTUs and species are actually rare taxa. This is supported by the infrequent detection of exclusive OTUs and species between the four time points. When species and OTUs with low read proportions (< 0.001%) were excluded, the proportion of shared species and OTUs increased to 80%, whereas eDNA-exclusive taxa were reduced to only 7%. This increase in shared species and OTUs was accompanied by a loss of 20% of the taxa that were shared before filtering.
4.2.2 Differences in Community Composition Between Bulk and Enriched eDNA Metabarcoding
The inferred community composition from bulk and enriched eDNA samples was mostly consistent at the order level. Bivalvia was the only group, despite some planktonic taxa, that was exclusively found in the enriched eDNA samples analyzed with the EPTDr2n data set, with also some exclusively detected species in the enriched eDNA samples analyzed with the fwhR2n data set but not in the bulk metabarcoding samples. This is not unexpected given that mussels are filter feeders, so their DNA is released into the water at a constant rate, especially in conjunction with spawning, which can increase detection rates even at low abundances (Sansom and Sassoubre 2017). Vice versa, we expected to find more coleopteran species in the bulk metabarcoding sample due to their highly sclerotized bodies releasing less DNA into the water, as reported by a previous study comparing bulk samples to DNA extracted from fixative liquid (Zizka et al. 2019). However, we found more coleopteran species in the enriched eDNA samples than in the bulk metabarcoding samples. Although we detected more species in the enriched eDNA samples, these species were detected less frequently, that is, more stochastically detected than the exclusively detected species in the bulk metabarcoding samples. This explains why more indicator species were associated with bulk metabarcoding samples than enriched eDNA samples. The only species associated with the enriched eDNA samples from all four time points were the trichopterans Glyphotaelius pellucidus (Retzius, 1783), Lype phaeopa (Stephens, 1836), and Hydropsyche angustipennis (Curtis, 1834). Of these, only H. angustipennis was found exclusively in the enriched eDNA samples, with a moderate frequency (32%) among samples. Interestingly, this species builds no case, leaving its soft body directly exposed to the water, which may facilitate the shedding of DNA molecules into the surrounding water. This also applies to L. phaeopa, which was identified as an indicator for enriched eDNA samples. G. pellucidus, however, builds a case from leaves but prefers stream sections with low currents, such as stream shorelines and banks (Graf 2020; Schmidt-Kloiber and Hering 2015, 2024), which would potentially hamper detection using kicknets with a random distribution of samples in the stream. This stands in contrast to direct water sampling from the stream, capturing the macroinvertebrate community more holistically along the stream. Consequently, DNA detected from G. pellucidus in the incubation containers may be an artifact of the stream water or collected debris used for incubation. Ultimately, the remaining variation in detected species between bulk and enriched eDNA metabarcoding after incubation suggests that we either sampled living animals that released their DNA during incubation but could not be detected by bulk metabarcoding or that their DNA was carried over from the stream water or remaining particles therein. Using water from the laboratory (target DNA-free) instead of stream water for incubation and removing debris and particle-bound DNA by rinsing samples prior to incubation would likely minimize the problem of detecting taxa that are not present in bulk samples. However, in that case, DNA-free water must be transported to each sampling site, which inevitably leads to fewer sampling sites being visited per sampling day. Nevertheless, it would be interesting to compare the results obtained using laboratory water as the incubation medium with those obtained using stream water in the future.
4.2.3 Application for Future Biomonitoring
Our results demonstrate that collecting and shortly incubating invertebrates in water for subsequent eDNA analysis allows for a more localized assessment of the stream macroinvertebrate community, especially when removing OTUs with low read abundance. Although the handling time of the samples increased in contrast to direct eDNA sampling from the stream, it was still faster than pre-sorting coupled with morphological identification and has the key advantage of being much more animal-friendly than classical approaches where organisms are preserved in ethanol for determination. Although direct sampling of the water after transferring the animals was sufficient for detections, it is not possible to ascertain the exact time point at which the eDNA sampling was done as it included the handling and sampling time, which typically was between 2 and 5 min. Therefore, we suggest incubating the animals for 20 min to ensure a comparable design while still making it feasible to visit multiple sampling sites per day. Future validation studies should involve comparisons against direct stream water sampling employing more diverse stream types and evaluating the effectiveness of the method in determining ecological status classes.
5 Conclusion
This study highlights that local community composition can be obtained from eDNA by briefly incubating the collected organisms in water followed by the analysis of the enriched eDNA filtered from samples. In our study, species identity was the most important determinant of success, and body sclerotization was less important. Applying the approach to a bulk invertebrate sample of unknown composition, more than half of the recovered species were shared between bulk and enriched eDNA metabarcoding. Excluding taxa with low read abundances increased the proportion of shared species to up to 80% and thus greatly amplifies the local signature of the eDNA assessment. Hence, our study presents a new approach toward a more localized bioassessment while maintaining the minimal invasiveness of eDNA metabarcoding. This is an important step when aiming to apply eDNA metabarcoding as a complementary tool for local biodiversity assessments and biomonitoring.
Author Contributions
All authors: conception and design of the study. M.S. and M.-T.W.: acquisition of field data and performance of the experiments. M.S. and D.B.: laboratory and data analysis. M.S.: statistical analysis. All authors: interpretation of the data. M.S.: writing of the manuscript with the help of all authors.
Acknowledgments
We thank Armin Lorenz for supporting the selection of target species and sampling locations for the indoor experiment. We also thank Iris Madge Pimentel for statistical support and Till-Hendrik Macher for writing a script for data permutation used for the field experiment. We thank Jenni Raitoharju and Leonie Siebert for assisting with conducting indoor experiments. M.S. was supported by the DBU scholarship program (20020/685). D.B., F.L., M.W., and A.J.B. are members of and supported by the Collaborative Research Center (CRC) RESIST funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) 1439/1—project number: 426547801. Open Access funding enabled and organized by Projekt DEAL.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
All raw reads are accessible via the European Nucleotide Archive (ENA) under accession number PRJEB80904.




