Enhancing Environmental DNA Sampling Efficiency for Cetacean Detection on Whale Watching Tours
Funding: This research was funded by Biodiversa+, the European Biodiversity Partnership under the 2021-2022 BiodivProtect joint call for research proposals, co-funded by the European Commission (GA No. 101052342) and with the funding organizations: Fundo Regional para a Ciência e Tecnologia (FRCT), Governo Regional dos Açores; M2.2/eWHALE/001/2023; Ministry of Universities and Research (MUR), from the FIRST and IGRUE special account current account relating to the European partnership Biodiversa+ Call 2021–eWHALE project, Italy; Rannís (Rannsóknamiðstöð Íslands), Iceland; and the Austrian Science Fund (FWF) [doi: 10.55776/I6389].
Lauren Kelly Rodriguez and Belén García Ovide contributed equally to this work.
ABSTRACT
Monitoring cetaceans is essential for evaluating ecosystem health and informing the establishment of marine protected areas. Conventional cetacean monitoring techniques, such as photo-identification, acoustic surveys, and satellite tagging, are often resource-intensive, costly, and sometimes intrusive. Environmental DNA (eDNA)-based methods have emerged as non-invasive, cost-efficient complements based on the analysis of genetic material shed into the environment. However, eDNA research is still evolving, with ongoing efforts to optimize field sampling and laboratory protocols. Building on the challenges of conventional monitoring methods, this study sought to refine eDNA sampling parameters to offer a more efficient and scalable approach for cetacean research, leveraging citizen science platforms. From June to October 2023, eDNA samples were collected across three regions in the Northeast Atlantic Ocean and Mediterranean Sea aboard whale-watching vessels or monitoring platforms engaging citizen scientists. Samples were analyzed for total DNA concentration using Qubit fluorometry and target DNA concentration with quantitative polymerase chain reactions (qPCR). Key variables tested in the field included water volume (2, 5, and 10 L), sampling timing (immediately after a whale was present and at 5-, 10-, and 20-min intervals), and three filter types (pore sizes of 1.2, 0.8, and 0.45 μm). Our results illustrate that larger water volumes (10 L), sampling immediately after a whale breach or fluking behavior, and Smith-Root eDNA filters (1.2 μm pore size) significantly increased eDNA detection probability and signal strength. However, the combination of certain filter types with different water volumes had a significant impact on detection probability, with smaller pore sizes more effectively yielding detections with a lower water volume. These findings provide guidance for future cetacean research initiatives and highlight the potential of eDNA methods in enhancing research and conservation efforts through scalable citizen science-based initiatives.
1 Introduction
Monitoring marine mammals is a vital component of assessing ecosystem health as well as providing the basis for establishing marine protected areas (MPAs; Hoyt 2004). Cetaceans can serve as biological indicators, offering insights into the health of marine ecosystems, for example, via their abundance, changes in population structure, and responses to anthropogenic influences such as hazardous chemicals and litter (Fossi et al. 2012, 2020; Motivarash et al. 2020; Guo et al. 2023). As apex predators, they play a critical role in maintaining the balance of ecosystems via a multitude of direct and indirect interactions in marine food webs (Kiszka et al. 2022; Rupil et al. 2022). At the same time, their conservation is essential for the provision of ecosystem services to coastal communities (Rupil et al. 2022). Despite their ecological importance, obtaining meaningful data on marine mammal abundance, distribution, and habitat use has historically been a challenge (Taylor et al. 2007; Kowarski et al. 2020). Cetaceans are elusive species to study, and there is no unique monitoring method capable of providing a comprehensive understanding of their ecology. Each approach offers, in fact, only a partial perspective, leaving gaps in our knowledge on their behavior, movements, and interactions within their environment (Cunningham and Lindenmayer 2005; Redfern et al. 2006). Moreover, conventional monitoring methods—such as photo-identification, acoustic surveys, and satellite tagging—can be labor-intensive, expensive, and occasionally invasive (Norman et al. 2023; Johnson et al. 2009), requiring highly trained personnel and costly equipment, making them difficult to scale across large geographic areas (McIntyre 2014; Raudino et al. 2019; Kowarski et al. 2020).
In this context, environmental DNA (eDNA)-based molecular analyses have emerged as powerful, non-invasive, and cost-effective complements and/or alternatives for studying marine mammals and local marine biodiversity. eDNA refers to genetic material that organisms shed into their environment via skin cells, mucus, feces, or urine (Ficetola et al. 2008; Barnes and Turner 2016). By collecting and analyzing water samples, researchers can detect the presence of species without physically encountering them. This approach not only minimizes disturbances to endangered species but also provides a broader snapshot of biodiversity in marine ecosystems (Bohmann et al. 2014; Bessey et al. 2020). Several studies have demonstrated the effectiveness of eDNA for detecting a wide range of marine species, including cetaceans and potential prey species present at a location (Suarez-Bregua et al. 2022; Székely et al. 2021; Boyse et al. 2024). For instance, Visser et al. (2021) combined eDNA with biologging data to reveal the prey of deep-sea cetacean predators, while Alter et al. (2022) used eDNA metabarcoding to detect dolphins and their prey in the waters around New York (USA). Additionally, eDNA has the potential to be used for obtaining genetic information at the population level, which has already been showcased for marine megafauna and a freshwater fish species (Adams et al. 2019; Jiménez-Mena et al. 2022; Andres et al. 2023). These examples highlight the potential of eDNA as a complementary tool for conventional marine mammal research.
An enticing aspect of eDNA research is its capability to engage non-specialists in data collection through citizen science initiatives (Larson et al. 2020), thus offering a new gateway for citizen scientists to contribute to marine conservation. The potential simplicity of eDNA sampling protocols—collecting water samples and filtering them—makes them particularly well-suited for integration into ongoing volunteer-based monitoring programs (Clarke et al. 2023). This has led to a growing number of citizen science initiatives adopting eDNA sampling, with promising results for biodiversity surveys and species detection (Biggs et al. 2015; Couton et al. 2023). At the same time, citizen science has long played a role in marine mammal research, with community-based programs collecting valuable data on species sightings, behavior, and distribution (Harvey et al. 2018; Donnelly-Greenan et al. 2019; Matear et al. 2019; Rodriguez et al. 2021). However, large-scale sampling campaigns revolving around cetaceans and combining citizen science and marine biodiversity monitoring have not yet been implemented.
Despite its promise, eDNA research is still in its early stages, with ongoing efforts to optimize both field sampling and laboratory protocols. The choice of DNA extraction methods, PCR assay design, and sampling parameters—such as water volume, filter type, and collection timing—significantly influences success and comparability across studies (Calderón-Sanou et al. 2020; Klymus et al. 2020; Bruce et al. 2021; Thalinger, Deiner, et al. 2021; Rodriguez et al. 2025). While eDNA has been widely applied in freshwater and coastal marine systems, where transport and distribution patterns are fairly well understood (Bohmann et al. 2014; Hänfling et al. 2016; Mächler et al. 2019; Alam et al. 2020), open ocean settings introduce additional challenges due to complex water movement and dispersion patterns (Barnes and Turner 2016). Recent studies have explored techniques to enhance marine megafauna eDNA detection. Sampling whale fluke prints—calm patches of water left on the surface by diving whales (Levy et al. 2011)—can increase the likelihood of detecting whale DNA (Robinson et al. 2024). However, additional research is needed to refine the necessary sampling effort with regard to the volume of water collected and eDNA persistence in the water column. Székely et al. (2021) and Parsons et al. (2018) demonstrated that eDNA could be successfully detected from large marine mammals with a minimum water volume of 4–5 L, and Robinson et al. (2023) had successful detections from as little as 250 mL of water. These findings have yet to be tested across different species and environmental conditions, and to date, successful sampling protocols vary widely regarding the necessary time commitment and equipment.
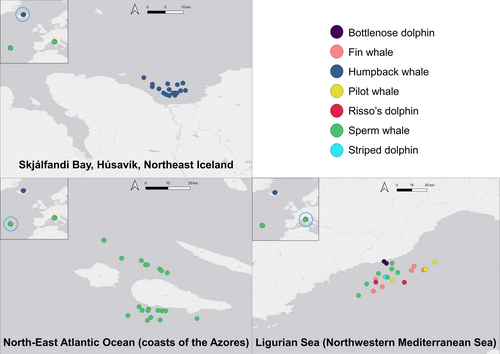
Opportunistic eDNA sampling using commercially operated vessels presents a unique opportunity to scale up eDNA sampling in a cost-effective manner. Whale-watching tours, in particular, are conducted regularly in areas of ecological significance, often providing daily access to areas frequented by marine mammals and specifically aiming to encounter them. Hence, partnerships with whale-watching operators allow researchers to gather data on marine mammals and local biodiversity at a frequency and scale that would be difficult to achieve using conventional research vessels alone (Rodriguez et al. 2021; Mazor et al. 2013). This study focuses on three regions with rich marine mammal biodiversity: the Northeast Atlantic Ocean (coasts of the Azores Islands), the Ligurian Sea (Northwestern Mediterranean Sea), and Skjálfandi Bay (Húsavík, Northeast Iceland). Each of these regions presents unique opportunities and challenges for eDNA-based research and has established whale-watching companies that are deeply invested in marine conservation and enthusiastic about contributing to scientific research. In the Azores, sperm whales (Physeter macrocephalus) are a focal species for ecotourism, with whale watching contributing 2.2% of the region's GDP (Queiroz et al. 2014; Ressurreição et al. 2022). Integrating eDNA sampling into these tours could provide high spatiotemporal resolution, long-term data on sperm whale distribution and feeding ecology. In Skjálfandi Bay, Iceland, where humpback whales (Megaptera novaeangliae) are frequently encountered, eDNA sampling can support ongoing photo-identification, biopsy sampling, and the deployment of data loggers (Klotz et al. 2017; Akiyama et al. 2019; Vatcher 2020) to further knowledge of humpback whale trophic ecology. In the Mediterranean, the Ligurian Sea is characterized by a rich diversity of marine mammals, yet populations in this region face a range of anthropogenic threats, including ship strikes, noise pollution, bycatch, chemical pollution, epizootics, and entanglement in fishing gear (Notarbartolo-di-Sciara et al. 2008). Thus, eDNA sampling offers a non-invasive method for collecting data on cetaceans to support the implementation of additional protected areas (Beng and Corlett 2020). In all three regions, opportunistic eDNA sampling has not been previously incorporated into monitoring efforts; yet whale-watching activities provide almost daily access to marine mammals, offering an ideal opportunity to integrate eDNA sampling.
While the integration of large-scale eDNA sampling campaigns into citizen science initiatives holds immense promise for marine mammal monitoring with regard to cetacean abundance, trophic ecology, and population genetics, it is essential that the underlying protocols are fully optimized and practicable in collaboration with non-experts and volunteers before such efforts can be effectively implemented. In this study, we systematically modified three components of the eDNA sampling process—(i) timing of eDNA sample collection following the target species' fluke-up or breaching behavior, (ii) water volume collected, and (iii) eDNA filter type—while considering the practical constraints of conducting fieldwork aboard whale-watching vessels and under the premise that sampling should be manageable by citizen scientists alone in the future. We used the detectability and concentration of cetacean eDNA via a novel, order-specific (i.e., cetacean-specific) qPCR assay as a proxy for evaluating the influence of these parameters. In addition, total eDNA concentrations were also evaluated for all samples to ensure correct sample handling and detect differences in overall eDNA concentration between locations. We hypothesize that larger water volumes and immediate sampling will result in higher detection rates and cetacean DNA concentration and that a filter type with a larger mesh width will outperform other commercially available filter types. The results of this study will serve as the basis for future large-scale eDNA and citizen science initiatives to study marine mammal ecology and population genetics.
2 Materials and Methods
2.1 eDNA Sampling
From June to October 2023, environmental DNA samples were collected throughout three regions across the Northeast Atlantic Ocean (Azores, Iceland) and Northwestern Mediterranean Sea (Italy) on board whale-watching vessels and citizen science platforms (Figure 1, Table 1). Water was collected from the surface near a whale or dolphins' flukeprint or breaching site (Figure 2) using pre-sterilized buckets (rinsed 3× with household bleach and water diluted 1:10) then filtered through a commercially available eDNA filter using a peristaltic pump (Italy and Azores) or a citizen science diaphragm air pump (Iceland; Table 1). Scientists onboard the vessels identified target species and noted other metadata such as sampling coordinates, weather, and animal behavior. Each sampling team modified one variable of the sampling: water samples in the Azores were collected either from the fluke or breach site of a whale immediately after the whale was present and either 5, 10, or 20 min after the whale left the area in which they breached or fluked. Environmental conditions during these delayed sampling events were calm, with sea states ranging between Beaufort scale 0 and 3, reflecting minimal to moderate surface disturbance, and were typical for the region during whale-watching activities. Boat operators attempted to maintain the vessel's position using GPS coordinates and idle engines to minimize movement during the 5, 10, and 20-min time delays. Nevertheless, some drift and current-driven dispersion or vertical mixing of eDNA is expected under open-water conditions, potentially influencing detection success over time. Water samples in Italy were of different volumes (2, 5, 10 L) but filtered from the same bucket during a sampling event. Per cetacean encounter, one of two filter types was used for the three eDNA samples: either self-preserving Smith-Root filters with polyethersulfone (PES) filter membrane and a 1.2 μm pore size or Sylphium filters with a PES membrane and a 0.8 μm pore size enclosed in a hard-case housing. Water samples in Iceland were filtered through three commercially available and widely used eDNA filters: the self-preserving Smith-Root filters and Sylphium filters as described above, and Sterivex filters with a polyvinylidene fluoride (PVDF) membrane, 0.45 μm pore size and a hard-case housing as a third option. The Icelandic eDNA sampling aimed to filter a volume of 10 L across each of the three filter types using water collected from a fluke print and filtered from the same 40 L container. Control samples were collected throughout the study by each partner. Each control sample consisted of 7–10 L of purified water collected from the same bucket and tubes used in the field, with two types of controls: (1) “from the sea” in which sampling equipment was not cleaned prior to filtration, and (2) “bleached”, in which sampling equipment was thoroughly cleaned before the filtration of purified water. Additional location-specific sampling information can be found in Table 1.
| Region | Ligurian Sea (North western Mediterranean Sea) | Northeast Atlantic Ocean (coasts of the Azores) | Skjálfandi Bay, Húsavík, Northeast Iceland |
|---|---|---|---|
| Sampling parameter tested | Water volume | Distance (location + time) from target animal | Filter type |
| Target species | Bottlenose dolphin (Tursiops truncatus), fin whale (Balaenoptera physalus), Risso's dolphin (Grampus griseus), long-finned pilot whale (Globicephala melas), sperm whale (Physeter macrocephalus), and striped dolphin (Stenella coeruleoalba) | Sperm whale (Physeter macrocephalus) | Humpback whale (Megaptera novaeangliae) |
| Field sampling partner | Tethys Research Institute (Italy) | CW Azores (Pico Island, Azores, Portugal) | North Sailing and Ocean Missions (Húsavík, Iceland) |
| Sampling period | July–October 2023 | July 2023 | July–October 2023 |
| Vessel | Two-masts motorsailer (21 m) | Semirigid inflatable motor boat (10.5 m) | Oak motor boat, wooden sailing boat (24 m) |
| Tour type | Week-long monitoring with 6–11 participants | Daily 3 h tours with up to 24 customers | Daily 3 h tours with up to 48 customers, weekly “Whales, Sails, & Science” tours (3.5 h), with up to 20 customers |
| Type and number of filter |
Smith-Root (1.2 μm filter pore size; Smith-Root SKU: 10996-25; n = 33 field samples, n = 3 controls) Sylphium (0.8 μm filter pore size; Sylphium Molecular Ecology ID: SYL010-08-20; n = 29 field samples, n = 3 controls) |
Sylphium (n = 42 field samples, n = 6 controls) |
Smith-Root (n = 18 field samples, n = 3 controls) Sylphium (n = 15 field samples, n = 3 controls) Sterivex (0.45 μm pore size; Merck Millipore ID: SVHV010RS; n = 2 field samples, n = 0 controls) |
| Type of pump | Solinst; Model 410 (Solinst Canada Ltd.) | ID: 12.34.SB (Eijkelkamp) | eDNA citizen scientist peristaltic pump SKU: 12099 (Smith-Root) |
| Total number of samples | 68 | 42 | 32 |

After the water was filtered, the filters were pumped with air for 30 s to 1 min to dry out the filter pores. All Sylphium and Sterivex filters received 1.5 mL of TES buffer (0.1 M TRIS, 10 mM EDTA, 2% sodium dodecyl sulfate; pH 8) and proteinase K (20 mg/mL) in a ratio of 190:1, whereas Smith-Root filters did not require the addition of preservation buffer. Smith-Root filters were stored immediately in a refrigerator (4°C) in Italy and at ambient temperature (< 25°C) in Iceland, while Sylphium and Sterivex filters were stored in freezers (−18°C). At the end of the sampling season, all samples were shipped to the University of Innsbruck (Austria) for subsequent analysis using express shipping (between 2 and 5 business days for Italy, between 7 and 10 business days for Iceland).
2.2 Molecular Analysis
2.2.1 Lysis
For lysis, Sylphium and Sterivex filters (within their housing units) were incubated for 3 h at 56°C. Smith-Root filter membranes were removed from their housing (using 3× singed forceps), placed into a 2 mL tube, then soaked with 300 μL of buffer consisting of 285 μL TES buffer and 15 μL of Proteinase K. All tubes were then incubated for 3 h at 56°C. Following incubation, Smith-Root filter membranes were transferred (using a DNA-free toothpick) into a plastic inlet, which was then placed back into the original 2 mL tube. The tubes were centrifuged at 18,626 g for 10 min, resulting in approximately 300 μL lysate at the bottom of the tubes. Lysate from the Sylphium and Sterivex filters was removed with single-use 3 mL plastic syringes, resulting in 1–2 mL of lysate per sample.
2.2.2 DNA Extraction
Extraction was carried out on all lysates (n = 138) and four extraction controls (consisting of TES buffer) with the BioSprint 96 instrument (QIAGEN; Venlo, The Netherlands) using the BioSprint 96 DNA Blood Kit (ID: 940057; QIAGEN) in accordance with the manufacturer's instructions, except for using 100 μL of TE-buffer instead of AE-buffer for elution (detailed protocol: Rodriguez and Thalinger 2024). All extractions were performed in dedicated laboratory spaces with proper ventilation and cleaning procedures adhering to the processing of eDNA samples (e.g., surface cleaning with bleach, sterilized DNA-free gloves, and protective wear; Thalinger, Rieder, et al. 2021, Thalinger, Deiner, et al. 2021; Hymus 2016).
2.2.3 Total DNA Quantification
Total DNA concentrations (ng/μL) per extract were measured in triplicate via a Qubit fluorometer using the Qubit dsDNA High Sensitivity (HS) Assay Kit (Life Technologies, Carlsbad, California, US; ID: Q32851). Qubit standards and DNA sample tubes were prepared according to the manufacturer's protocol (ID: Q32856; Thermo Fisher Scientific, Waltham, MA, USA) and using 1 μL of extract.
2.2.4 qPCR
A targeted qPCR assay (named Cet-CSS, corresponding to Cetacean Citizen Science Samples) was designed to target all relevant species across the sampling locations (Table 2). Target cetacean species were bottlenose dolphin (Tursiops truncatus), fin whale (Balaenoptera physalus), humpback whale (Megaptera novaeangliae), Risso's dolphin (Grampus griseus), long-finned pilot whale (Globicephala melas), sperm whale (Physeter macrocephalus), and striped dolphin (Stenella coeruleoalba). These species, along with 27 other non-target mammals (including Felis silvestris, Ovis orientalis, Rattus norvegicus, Canis lupus, Homo sapiens, Bos taurus, and Sus scrofa), 32 fish species, 38 shark species, and 20 invertebrate species which can be found throughout the Northeast Atlantic and the Mediterranean Sea or in close association with humans, were the basis of a sequence alignment created in BioEdit 7 (Hall 2004) to manually select the best mitochondrial region for the qPCR assay (File S1).
| Target gene | Name | Forward (5′–3′) | Optimal annealing temp. (°C) | Fragment length (bp) | Assay LODa |
|---|---|---|---|---|---|
| Reverse (5′–3′) | |||||
| MGB Probe (5′–3′) | |||||
|
tRNA-Trp tRNA-Asn tRNA-Asn |
Cet-CSS-F Cet-CSS-R Cet-CSS-P |
AAGGAYTGCAAGACTATATCTTACATCA GCTGTTAACTAAAARTTCGTGGGA TGTATCCCTCCAATCTAGTGAG |
60 | 115 | 0.0001 |
- Note: The assay was designed to target the following species: bottlenose dolphin (Tursiops truncatus), fin whale (Balaenoptera physalus), humpback whale (Megaptera novaeangliae), Risso's dolphin (Grampus griseus), long-finned pilot whale (Globicephala melas), sperm whale (Physeter macrocephalus), and striped dolphin (Stenella coeruleoalba). Bold letters indicate degenerate bases in each forward and reverse primer.
- a Calculated in accordance with the discrete method presented in Klymus et al. (2020) across dilution series of all target species.
Sequences were aligned using Clustal Omega (Sievers et al. 2011). Multiple candidates of forward and reverse primer, as well as a TaqMan probe, were selected manually and then tested in silico using the following software to identify potential dimers and hairpins as well as assess melting temperatures and GC content: Primer Express v3.0.1 (Applied Biosystems), Primer3 (Untergasser et al. 2012), and Primer Premiere (PREMIER Biosoft). One ambiguous base was selected within each primer to optimize amplification of all target species samples in this study and minimize the potential of human and terrestrial mammal non-target amplification (Zheng et al. 2008). The probe was labeled with 6-FAM and an MGB-Q530 quencher (5′ and 3′ respectively, Table 2). It is important to note that several cetacean species which were not targeted in this study and either do not occur in the study region or were not present in proximity to the obtained water samples can also be amplified with this assay (see File S1).
PCR preparation was conducted in a separate room with appropriate ventilation, and workbenches were treated by UV light at least once per working day in addition to the previously described laboratory conditions. Extracts of target species DNA were shared by project partners at University College Cork, obtained from strandings and biopsies for other projects. qPCRs were conducted using the QTower 3G platform (Qiagen; Venlo, the Netherlands), always with two negative controls (molecular-grade H2O) on each plate. A gradient qPCR was performed to determine the optimum annealing temperature from 57.5°C to 62°C. Dilution series with DNA extracts from target species tissue were used for these gradient qPCRs, in which 10 μL reactions consisted of 5 μL 2× TaqMan Environmental Master Mix (ID: 4396838, EMM, Life Technologies), 1 μL primer/probe mix (0.4 μM per primer and 0.2 μM probe), 1 μL nuclease-free water, and 3 μL DNA extract or +/− control. Due to expected low quantities of target DNA (Rodriguez et al. 2025; Beng and Corlett 2020; Klymus et al. 2020), the cycle number for all qPCRs was optimized to 45 cycles. Optimized cycling parameters were as follows: initial enzyme activation at 95°C for 10 min, denaturation (45 cycles) at 95°C for 15 s, and a combined annealing and extension step (45 cycles) at 60°C (optimized temperature). The assay was further tested in vitro for specificity with DNA extracts from non-target species such as fish (Clupea harengus) and terrestrial mammals (Homo sapiens, Felis catus, Canis lupus)—species most likely to generate non-target amplifications based on the generated alignment. The limit of detection (LOD) was calculated following the discrete threshold method defined by Klymus et al. (2020) using the rate of positive amplifications from triplicate standard curves of serial dilutions of target DNA from known concentrations. Hence, separate dilution series were created for all target species (dilution step: 1:10, starting concentration: 10 ng/μL, 8 points). Extracts of eDNA samples were screened in triplicate and separately for each target species, including triplicate dilution series of target DNA per plate in reactions and thermocycling conditions as described above. None of the PCR and extraction negative controls tested positive.
2.2.5 Sanger Sequencing
A subset of positive amplifications for each species (n = 48), as well as two control samples that yielded positive amplifications (control samples that were purposely collected and filtered with unsterilized sampling equipment to test for potential contamination between sampling events during the same whale-watching trip), were purified using the ExoSAP-IT Express PCR Product Cleanup Reagent for enzymatic treatment (Affymetrix-USB Corporation, Santa Clara, California, USA), then sent to Eurofins Genomics Germany GmbH (Ebersberg, Germany) for sequencing. Once sequence data was received from Eurofins Genomics, sequences were trimmed using Bioedit 7.7 (Informer Technologies) and then BLASTed (NCBI) and matched to the custom alignment to verify target species taxonomy.
2.3 Statistical Analysis
2.3.1 Total DNA Concentrations
DNA concentrations per eDNA sample (ng/μL) were averaged across three technical replicates. The average DNA concentrations per country and sampling modification were assessed using a one-way ANOVA to test between the independent groups using the aov function in base R (Faraway 2002).
2.3.2 Modeling eDNA Detections and Signal Strength
To analyze the binary outcomes of target species detections (Yes/No) for all samples collected, as well as the signal strength of these detections per sample (i.e., the mean amount of target-specific genetic material that was amplified via qPCR triplicates per sample), a series of generalized linear mixed-effects models (GLMMs) and linear mixed-effects models (LMMs) were constructed using the lme4 (v1.1-35.5) package in R (Bates et al. 2003). The full dataset was pre-filtered to remove parameters with insufficient sample size (Azores: samples collected 20 min after the target animal was present; Iceland: samples filtered with Sterivex eDNA filter capsules). The detection model was developed using a binomial error structure, and the signal strength model was developed using a Gaussian distribution of errors. The response variable for the detection model was binary (Yes/No detection of target DNA per water sample; at least one positive amplification out of three was required to render a sample positive), whereas the response variable for the signal strength model was continuous—representing the mean Ct value of each eDNA sample (as a proxy for target species eDNA concentration; higher Ct values indicate lower levels of DNA, whereas lower Ct values indicate higher concentration of target species DNA in the sample). Fixed effects considered in the models included species (the target cetacean species), water volume (the volume of water sampled), filter (the type of eDNA filter used), timing (the timing of sample collection relative to the presence of the target animal), and species and country as well as water volume and filter as interaction terms to describe context-dependent detections between the different species analyzed across regions as well as the filter pore size and capacity to optimally filter water (Table S1). For all models implemented in this study, reference levels were defined by base parameters which had the most observations within the dataset (species = sperm whale, water volume = 10 L, filter = Sylphium, timing = immediate, country = Italy; Pierce and Schafer 1986). A random effect of PairedID was included in two models to account for non-independence of samples collected during the same sampling event (Barr 2013).
The second GLM assessed cetacean eDNA detection probability as a function of the timing of sampling after the target species was present using the data subset obtained from the Azores.
Figures were created in R (v 4.3.1; R Core Team 2023) using ggplot2 (v 3.4.4; Wickham et al. 2024), dplyr (v 1.1.3, Wickham et al. 2023), reshape2 (v 1.4.4; Wickham 2020) and viridis (v 0.6.5; Garnier et al. 2024).
3 Results
3.1 Total DNA Concentrations
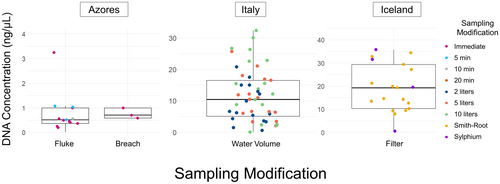
Total DNA measurements for all samples varied across the three countries, with eDNA extracts from Iceland having the highest concentrations on average (19.23 ng/μL; ANOVA p < 0.05; Figure 3). Sylphium filters showed a high variance of total DNA concentration, ranging from an average of 0.53 ng/μL (SE = 0.06, range = 0.00–3.25 ng/μL) in the Azores to an average of 26.61 ng/μL (SE = 2.31, range = 0.00–35.73 μL) in Iceland. Total DNA concentrations were higher when larger water volumes were filtered, as showcased by measurements from Italian samples: there was an average concentration of 8.36 ng/μL for 2 L samples, 9.38 ng/μL for 5 L samples, and 17.16 ng/μL for 10 L samples.
3.2 eDNA Detections
The final binomial GLMM for eDNA detection showed that the random effect had a variance of 0.92 and a standard deviation of 0.96, indicating relatively low between-group variability in detection probabilities across paired sample events. Compared to 10 L, both 2 and 5 L were associated with marginal negative effects, with estimated changes in the log-odds of detection probability being −0.80 and −1.44 (respectively). The use of 1.2 μm pore size (i.e., Smith-Root filters) showed a strong positive effect on detection probability, with a positive estimate of 3.61 units from the intercept (p < 0.05; Table S2, Figure S1). Sampling timing also showed negative estimates for both 5-min (−1.06) and 10-min (−0.81) delays after the target animal's presence, but these effects were not statistically significant. Compared to the reference level (Italy), samples collected in Iceland showed a significant decrease in detection probability (−2.48), while the effect for samples collected in the Azores was negative but not statistically significant (−2.40). The interaction between water volume and filter type resulted in significantly fewer detections of target species eDNA with 2 L samples filtered through Smith-Root filters (−3.89). A similar interaction was observed for 5 L samples, though it was not statistically significant (−2.87). The model converged with an AIC of 145.6; the scaled residuals were centered around zero.
The binomial GLM that was fit to data from Italy and Iceland to further investigate the effects of filter type and water volume converged with an AIC of 95.69 with a reduction in deviance from 94.63 (null model) to 83.69 (residual deviance), suggesting an improved model fit when accounting for the subset of covariates. Lower water volumes (2 L and 5 L) were associated with reduced detection probability compared to 10 L samples, though these effects were not statistically significant (2 L = −2.23; 5 L = −1.83). The use of Sylphium filters (0.8 μm pore size) had a significant negative effect on detection probability overall, with an estimated decrease of −2.77 compared to filters with a greater pore size (Smit-Root 1.2 μm, p < 0.05). However, a strong positive effect, i.e., increased likelihood of target DNA detection, was observed for Sylphium filters in combination with lower water volumes. In particular, the combination of 2 L filtered through Sylphium filters showed a significant increase in detection probability (estimate = 3.06, p < 0.05). Although 5 L filtered through Sylphium filters also indicated a positive effect (2.12), it was not statistically significant. The scaled residuals were well-distributed, with no evidence of overdispersion or underdispersion, confirming that model assumptions were met (Table S3, Figure S2).
The GLM modeling the effect of timing on eDNA detectability for the Azores sample subset converged with an AIC of 53.17. There were no significant effects of timing with this subset of data, though estimates in detection probability show that waiting for 5 or 10 min decreased the chance of detecting cetacean DNA (5 min = −0.49, 10 min = −0.78). There was a small reduction in deviance of 0.67 (residual deviance = 49.121) when compared to the null mode (deviance = 49.795), thus suggesting that timing alone was not a strong predictor of cetacean eDNA detection in this dataset (Table S4, Figure S3).
3.3 Signal Strength of eDNA Detections
By using the Cet-CSS assay, we were able to detect eDNA of all target species at a Limit of Detection of 0.0001 ng/μL (Figure 4).
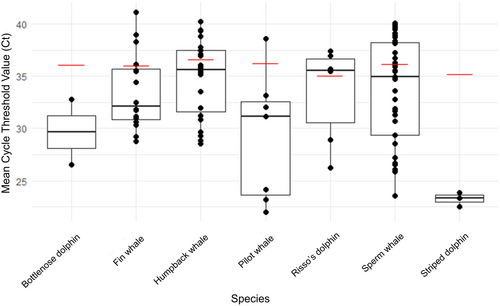
Out of 403 reactions (not including standards, NCs, or field controls), 243 yielded positive amplifications with the Cet-CSS assay based on the presence of a Ct value < 40, as determined by the QTower 3G's real-time detection software (Figure 5). Forty out of 123 reactions amplified sperm whale DNA in samples taken in the Azores (33%), 56 out of 96 samples from Iceland amplified humpback whale DNA (58%), 147 out of 183 reactions from Italian samples amplified either bottlenose dolphin, fin whale, Risso's dolphin, long-finned pilot whale, sperm whale, or striped dolphin DNA (80%). Three out of 42 reactions led to positive amplifications in two of the 14 field control samples (control type: “from the sea”; filter type: Sylphium; location: the Azores and Italy). Of the 48 PCR products sent for Sanger sequencing, 35 were confirmed as the target cetacean species that was observed during sampling (File S1). All other PCR products did not produce any usable sequencing results, likely caused by too low concentrations of target DNA or an artifact of the short fragment length that was being sequenced.
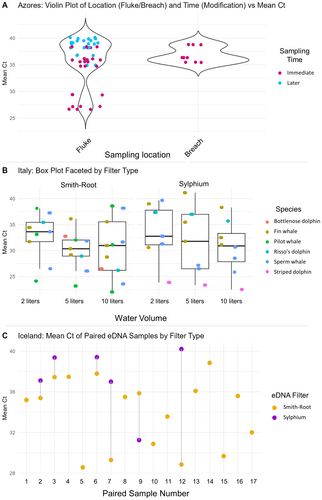
The final LMM (Table S5, Figure S4) best representing the effects of sampling parameters on mean Ct values per sample included a random intercept to account for grouping effects of samples collected during the same event (Figure 6). The random effect had a variance of 12.76 and a residual variance of 4.90, indicating moderate between-group variability and lower within-group variability. Lower water volumes (2 and 5 L) showed positive effects on the average Ct values, i.e., target eDNA signals were weaker (2.02 and 0.52 higher than the reference Ct, respectively). The use of Smith-Root eDNA filters significantly decreased Ct values by an average of 3.34 (p < 0.05). The interaction terms between water volume and filter type were not significant. The timing of sampling showed a significantly positive effect on mean Ct values, with sampling delays of 5 min increasing the mean Ct by 4.40 (p < 0.05). Delays of 10 min increased the mean Ct by 6.52, though this effect was not significant. Of all target species, striped dolphin showed a significant negative effect (−11.15 Ct, p < 0.05), while humpback whale showed a significant positive effect (3.79, p < 0.05) on Ct values. The model had a log-likelihood of −213.95, with an AIC of 456.58.
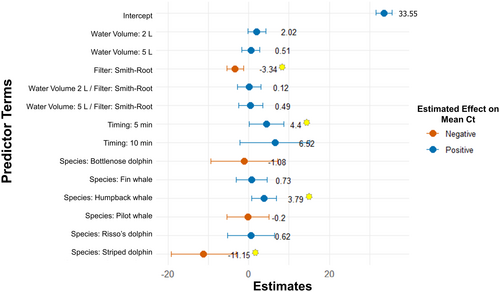
Due to the variable effects of sampling timing on detection in the Azores (detection probability was not significantly affected, yet mean Ct values in the full LMM differed significantly), we implemented an additional linear model (LM) to further investigate this relationship (Equation 5). The model showed that Ct values were significantly higher when samples were collected not immediately after the animal's presence, with a mean increase of 4.49 Ct units (p < 0.05). The model had a residual standard error of 3.69 and explained approximately 25.8% of the variation in mean Ct values (adjusted R2 = 0.21; Table S6; Figure S5).
4 Discussion
This study provides valuable insights into identifying effective protocols for collecting eDNA samples to monitor cetacean presence, trophic ecology, and potentially population structure. By modifying key variables of the eDNA sampling process onboard whale-watching vessels and citizen science platforms operating in three ecologically diverse marine regions—the Northeast Atlantic Ocean around the Azores, the Ligurian Sea (Northwestern Mediterranean Sea) off Italy, and Skjálfandi Bay off of Iceland—we successfully demonstrated the feasibility of this approach for future large-scale monitoring efforts. Our findings build on existing research by providing additional insights into how various field sampling parameters influence the efficiency of cetacean eDNA collection, particularly in the context of whale-watching platforms. Using a novel, order-specific qPCR assay, our findings indicate that collecting larger volumes of water (10 L) and filtering the water collected immediately after target species presence through Smith-Root self-preserving eDNA filters significantly enhanced the amount of cetacean eDNA collected as well as the general cetacean detection probability. Albeit total eDNA concentrations were found to be significantly different between sampling locations (independent of filter type and filtered water volume), cetacean eDNA could be reliably detected at low concentrations and independent of the target species, substantiating the reliability of the Cet-CSS TaqMan assay for the detection of the seven cetacean species. This assay, though not species-specific, provides a useful tool for whenever direct comparisons in detectability and signal strength between cetacean species are warranted.
High total DNA concentrations (ng/μL) were observed in Iceland compared to the Azores and Italy. These higher concentrations were likely due to the influence of nutrient and biomass runoff from nearby rivers flowing into Skjálfandi Bay, high primary productivity, and seasonal plankton blooms, which attract large filter-feeding species such as humpback, fin, and blue whales (Klotz et al. 2017; Moroz 1996). Such high overall eDNA levels offer a unique advantage for future biodiversity studies, particularly those focusing on planktonic taxa, via the implementation of eDNA metabarcoding to assess the local species communities (Deiner et al. 2017; Boyse et al. 2024). However, nutrient-rich and sediment-loaded waters may impede the filtration process, as the clogging of filter pores can reduce the efficiency of eDNA sampling (Majaneva et al. 2018). Under these conditions, Sterivex filters with 0.45 μm pore size, Sylphium filters with 0.8 μm pore sizes, and Smith-Root filters with 1.2 μm pores were directly compared during sampling events in Iceland. Only two Sterivex filters were used due to the handling and filtration process being too time intensive, especially with regard to future potential sampling by citizen scientists. Additionally, Sterivex filters were incapable of reliably processing water volumes beyond 2 L, thus rendering the independent investigation of the effect of filters on cetacean eDNA capture impossible. Ultimately, the Sterivex filters were removed from statistical modeling due to low sample size, yet they both yielded positive detections of the target species with a relatively low average Ct (31.27).
While no significant differences were found in total DNA concentration between Smith-Root and Sylphium filters employed in Iceland, detection rates and average Ct values for the target species (humpback whale) differed. It is possible that the smaller pores of the Sylphium filter impeded the capture of humpback whale DNA, albeit 10 L were successfully processed. Additionally, if macroorganismal target eDNA is mostly present in the form of intact cells or cell conglomerates (Mauvisseau et al. 2021; Kirtane et al. 2023) immediately after a whale's fluking event, larger pore sizes may be more effective at detecting those eDNA particles before they degrade into extracellular fragments (as shown by the GLMs including filter type). While higher target eDNA concentrations may result from increased eDNA release, the role of disruptive environmental processes is equally important. Degradation and dispersal are influenced by several interacting factors, including ocean currents, turbulence, and bacterial activity (Collins et al. 2018; Lamb et al. 2022; Allan et al. 2021). High turbidity and water column mixing can hinder eDNA amplification due to either inhibition induced by suspended particles (Hunter et al. 2019; Lance and Guan 2020) or redistribution of eDNA, affecting its local concentrations and detectability. Samples collected in Skjálfandi Bay were specifically tested due to the regional characteristics of cold, nutrient-rich waters and showed no signs of PCR inhibition (Rodriguez et al. 2025).
In contrast to Icelandic waters, environmental conditions in the Ligurian Sea, such as higher water temperatures, increased UV exposure, and fewer sediments or nutrients in the water, may lead to smaller eDNA fragments being more abundant or being generated more rapidly (Jo et al. 2020; Mauvisseau et al. 2021). This suggests that smaller pore sizes, such as those used in Sylphium filters, are likely more effective for capturing these eDNA fragments in lower sampling volume, as shown by the GLM. Whereas Smith-Root filters, with their larger pore size, potentially led to small, extracellular eDNA traces not being deposited on the membrane. Across the entire generated dataset, though, larger pore size filters outperformed smaller pore sizes during direct comparisons, especially when filtering larger volumes. There is a possibility that increasing the number of biological replicates filtered through a smaller pore size may also enhance the amount of detectable cetacean DNA (Robinson et al. 2023; Liu et al. 2024). For the purposes of whale-watching boats and citizen science engagement, however, the number of replicates must be feasible enough to be completed during one tour.
Overall, the obtained Ct values decreased as filtered water volumes increased, indicating an accumulation of cetacean eDNA. eDNA filtration in seawater typically requires volumes ranging from 1 to 5 L to detect vertebrates such as marine mammals, both in inshore and offshore waters (Mächler et al. 2015; Valsecchi et al. 2020, 2021). The present work demonstrated that 10 L samples significantly optimized both the detection probability and the yield of target-specific eDNA compared to 2 and 5 L when evaluated across filter types, sampling locations, and target species—though smaller volumes were shown to be effective in one study region when filtered through a smaller pore size. In some instances, collecting 2 L of water did not result in any detection of a target species such as the bottlenose dolphin. For capturing high-quality cetacean eDNA, particularly for potential population genetic studies, higher volumes with larger pore sizes may be advantageous, as they increase the likelihood of capturing sufficient target-specific DNA (Minamoto et al. 2015; Li et al. 2018; Majaneva et al. 2018). For broader biodiversity studies aiming to capture both vertebrate and invertebrate prey species, higher volumes may also be more effective. In these cases, the eDNA would likely be a mix of larger and smaller fragments, including fecal matter, skin cells, and even entire organisms (Bohmann et al. 2014). Filtration of 10 L of seawater was feasible even on short-duration whale-watching tours with the aid of a peristaltic pump. This approach proved to be both practical and effective, allowing for the collection of higher-quality samples without compromising the customer experience and logistical constraints during whale-watching tours. Thus, there seems to be no strong deterrent to filtering large volumes as long as it is feasible within the constraints of the sampling environment.
Additionally, the effects of DNA preservation and transport should be considered when comparing the performance of different filter types. The use of preservation buffers is widely accepted in the eDNA field and has been shown to maintain eDNA quality effectively during storage and transportation (Wegleitner et al. 2015; Mauvisseau et al. 2021). These buffers can stabilize eDNA at ambient temperatures, reducing degradation and improving overall sample quality (Kumar et al. 2020; Lopez et al. 2024). In this study, Sylphium and Sterivex filters required a preservation buffer, whereas Smith-Root filters were self-preserving via a desiccating membrane (Thomas et al. 2019), enabling storage at room temperature for up to three months, facilitating handling, and reducing the risk of contamination (Thomas et al. 2019). Considering these factors is essential when samples are collected potentially by non-experts far from the laboratory and need to be shipped over long distances (e.g., between Iceland and Austria, where the samples were analyzed for this study). An internal positive control (IPC) is recommended by the European Standards for sampling, capture, and preservation of environmental DNA from water (SIST EN 17805:2023) to assess the negative impacts of eDNA sample transport and storage (Bruce et al. 2021). As such, IPCs are commonly added to the preservation buffer, as has been the case for a subset of the processed samples (Rodriguez et al. 2025). However, the use of different filter types hindered the addition of IPC to the entire sample set, thus making a direct investigation of transport and storage effects on different filter types in this study impossible. While both Sylphium and Smith-Root filters have enclosed filter housings, which are recommended for reducing contamination risks during fieldwork (Bruce et al. 2021), the addition of preservation buffers could increase the risk of contamination by introducing an additional handling step. In this study, contamination only occurred during two events—each utilizing Sylphium filters—in the control sample, which was filtered without additional equipment cleaning. Following field sampling, it yielded a marginally positive amplification for cetacean eDNA (Ct 39.34–41.88). These findings emphasize the necessity for careful consideration of DNA preservation, transport procedures, and potential contamination risks to ensure the integrity of eDNA samples that are collected by non-experts.
Numerous studies have drawn correlations between biomass and target eDNA concentration in environmental samples (Takahara et al. 2012; Doi et al. 2017; Muri et al. 2020; Shi et al. 2023). Water samples were collected from close proximity to a variety of species ranging from highly social dolphins, humpback whales, and matriarchal sperm whale groups in the Azores to solitary male sperm whales and fin whales in the Ligurian Sea. Samples collected near whale species (fin, humpback, and sperm) generally yielded lower concentrations of cetacean eDNA (as denoted by the mean Ct values of those samples) than samples collected near any dolphin species (bottlenose, pilot whale, Risso's, striped), which yielded higher amounts of target DNA per sample. While whales have significantly more biomass than dolphins, dolphin samples likely contain eDNA from more than one individual due to their highly social behavior in which they travel at close proximity to one another (Shane et al. 1986; Wells 2003). Dolphins also tend to move faster and have higher metabolic rates than whales (Williams et al. 1993; Williams and Davis 2024), which can result in a higher release of their eDNA into the environment (Thalinger, Rieder, et al. 2021). Therefore, social behavior and activity levels, rather than biomass alone, may drive the concentration of cetacean-specific eDNA detected in marine environments.
Prior research confirms that eDNA rapidly degrades in seawater, making positive detections reliable indications of the recent presence of target species (Port et al. 2016; Collins et al. 2018; Murakami et al. 2019). Understanding the persistence of the eDNA signal after a whale's presence in the sampling area is crucial for the optimization of sampling strategies aimed at detecting cetacean eDNA. Our results suggest that sampling from the flukeprint or surface waters in which the whale breached immediately after the animal's presence yielded the highest concentrations of target eDNA (Figure 5). Interestingly, the chance to obtain a positive detection did not change significantly between immediate filtration and a 5 or 10 min delay in our dataset. This seems to indicate a patchy small-scale distribution of cetacean eDNA and a higher chance of filtering intact cells or cell conglomerates and thus higher levels of target DNA with immediate sampling (Mauvisseau et al. 2021; Kirtane et al. 2023). Sampling as soon as possible from the whale's flukeprint was previously validated in other studies, including the recent Robinson et al. 2025, who collected eDNA from flukeprints of Pacific cetaceans at different time intervals, reinforcing the need for swift collection to maximize detection success, while carefully following the rules of non-disruptive whale watching from onboard a vessel (Gjerdalen and Williams 2000; Székely et al. 2021; Robinson et al. 2023, 2024). Prolonged detectability of cetaceans and prey species via eDNA has only been confirmed in the presence of whale feces at the water sampling location (Suarez-Bregua et al. 2022; Jackson 2022). Though there was no significant difference between the detection rates of sperm whales in the Azores when comparing samples collected from the whale's flukeprint and those from the breaching site, at this point, this pattern cannot be confirmed for all cetacean species and environmental conditions. In general, the flukeprint can be deemed the most optimal sampling location of cetacean eDNA, as it is not species-dependent (i.e., all cetaceans may leave a flukeprint) and is relatively easy to identify on the water's surface. Breaching behaviors, however, are rarer to witness as they are energetically taxing and not a necessary behavior (e.g., hunting; Whitehead 1985; Segre et al. 2020).
The protocol developed in this study has the potential for large-scale implementation in the future. The materials used, such as enclosed filters and easy preservation methods, are designed to be as safe and user-friendly as possible for non-experts to handle, which is crucial for ensuring high data quality from citizen science initiatives (Biggs et al. 2015; Couton et al. 2023). In addition to eDNA sampling, citizen scientists could also be engaged by measuring other parameters, such as dissolved oxygen, conductivity, salinity, dissolved nutrients, and water temperature, using handheld multiparameter instruments at the same locations where water samples are collected (Bastos Gomes et al. 2017; Downie et al. 2024). These efforts (the measurement of eDNA and water quality data) can be combined with long-term photo-identification programs that are already in place on whale-watching vessels, creating a powerful source for cetacean monitoring. For such efforts, the involvement of trained staff will likely be required initially; albeit, with proper training, customers could eventually collect samples independently, minimizing the need for constant supervision.
By testing various protocols for cetacean eDNA sampling onboard whale-watching vessels, this study establishes a strong foundation for large-scale, non-invasive research that can be applied to a variety of conservation goals and research questions, including abundance monitoring, trophic interactions, and potentially even population genetics. The ability to detect species via eDNA provides a powerful tool for monitoring elusive or endangered cetacean species without the need for physical interaction (e.g., collecting a biopsy), making it an ideal approach for whale-watching vessels. This study has refined several critical factors of the sampling process, including sampling timing, water volume, and filter type, offering valuable insights into best practices for fieldwork. However, additional parameters still need exploration, such as incorporating biological replicates to account for natural variability in surface water eDNA or investigating how sampling at different depths might influence cetacean eDNA detection and persistence in the water column (Drummond et al. 2021; Jeunen et al. 2024; Rozanski et al. 2024). Despite the need for exploring these additional factors, the results of this study present a gateway for expanding the use of eDNA sampling toward citizen science opportunities. Through the integration of eDNA sampling into whale-watching operations, more comprehensive and sustainable monitoring programs can be implemented in the future to support conservation efforts on a large, international scale.
Author Contributions
(i) Contributions to the conception or design of the study: B.T., M.T., M.H.R., A.A., E.V. (ii) Acquisition, analysis, or interpretation of the data: L.K.R., B.G.O., E.B., J.R., T.S.P., A.C.-C., C.L., M.J., E.V., A.A., M.H.R., B.T. (iii) Writing of the manuscript: L.K.R., B.G.O., E.B., C.L., M.J., E.V., A.A., M.H.R., M.T., B.T.
Acknowledgments
This research was funded by Biodiversa+, the European Biodiversity Partnership under the 2021-2022 BiodivProtect joint call for research proposals, co-funded by the European Commission (GA No. 101052342) and with the funding organizations: Fundo Regional para a Ciência e Tecnologia (FRCT), Governo Regional dos Açores; M2.2/eWHALE/001/2023; Ministry of Universities and Research (MUR), from the FIRST and IGRUE special account current account relating to the European partnership Biodiversa+ Call 2021–eWHALE project, Italy; Rannís (Rannsóknamiðstöð Íslands), Iceland; and additionally, this research was funded in part by the Austrian Science Fund (FWF) [doi:10.55776/I6389]. For open access purposes, the author has applied a CC BY public copyright license to any author accepted manuscript version arising from this submission. We would like to express our gratitude to the people employed by the research institutes and tour providers involved in the eDNA sampling campaign and the scope of the present research, namely Sabina Airoldi, Valentina De Santis, and all the Tethys Research Institute’s team; Dania Tesei, Michael Costa, Rita Norberto, Marlene Schwandt, Rita Leitão, Katharina Leeb, Anthony Le Floch, Francisco Nunes, Renato Cardoso, Faustine Darius, Monique Schouten, Laura Biet, Johanna Franke, Silva Ruppert, Romane Renou, and Małgorzata Aleksandra Szałęga. We would also like to thank Daniela Sint and Sandra Schallhart for their generous support in the laboratory. Open access funding provided by Universitat Innsbruck/KEMÖ.
Ethics Statement
This study was conducted in strict accordance with ethical guidelines and best practices for environmental DNA (eDNA) research. All fieldwork and sampling procedures were carried out in compliance with local and international regulations, including whale-watching guidelines designed to minimize disturbance to animals. Samples were collected non-invasively, with no direct interaction with the target species. All data reported in this manuscript are original, and no material has been plagiarized or previously published elsewhere. The authors confirm that all analyses were performed with scientific rigor, and the results presented are an accurate reflection of the findings without any fabrication, falsification, or manipulation. Any limitations or uncertainties associated with the data have been transparently disclosed. Furthermore, the use of appropriate controls and replication was ensured throughout the study to maintain data integrity and reproducibility. All contributors have been duly acknowledged, and any potential conflicts of interest have been disclosed. This manuscript adheres to the ethical guidelines outlined by the Committee on Publication Ethics (COPE) and the journal's policies.
Conflicts of Interest
E.V. is co-founder and manager of Cetacean Watching Lda. (CW Azores), a for-profit company dedicated to whale watching. C.L. serves on the Board of Directors, and M.J. is a member of the College of Arbiters of the Tethys Research Institute, a non-profit organization supporting marine conservation through science and public awareness mainly in the Mediterranean Sea. B.G.O. is the founder and project manager of Ocean Missions, and T.S.P. and A.C.-C. are affiliated with the organization, a non-governmental entity focused on conservation, education, and research in the Arctic region. M.T. is the co-founder of Sinsoma GmbH, a for profit company dedicated to DNA analyses of environmental samples. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
Data Availability Statement
All data regarding samples, their Qubit total DNA measurements, and their quantitative PCR measurements are available on Figshare: https://doi.org/10.6084/m9.figshare.27205371.v1.




