A Metadata Checklist and Data Formatting Guidelines to Make eDNA FAIR (Findable, Accessible, Interoperable, and Reusable)
Funding: This work was supported by European Union HORIZON EUROPE Research and innovation programme (101057437); National Oceanic and Atmospheric Administration (NA21OAR4320190) and Commonwealth Scientific and Industrial Research Organisation.
ABSTRACT
The success of environmental DNA (eDNA) approaches for species detection has revolutionized biodiversity monitoring and distribution mapping. Targeted eDNA amplification approaches, such as quantitative PCR, have improved our understanding of species distribution, and metabarcoding-based approaches have enabled biodiversity assessment at unprecedented scales and taxonomic resolution. eDNA datasets, however, are often scattered across repositories with inconsistent formats, varying access restrictions, and inadequate metadata; this limits their interoperation, reuse, and overall impact. Adopting FAIR (Findable, Accessible, Interoperable, and Reusable) data practices with eDNA data can transform the monitoring of biodiversity and individual species and support data-driven biodiversity management across broad scales. FAIR practices remain underdeveloped in the eDNA community, partly due to gaps in adapting existing vocabularies, such as Darwin Core (DwC) and Minimum Information about any (x) Sequence (MIxS), to eDNA-specific needs and workflows. To address these challenges, we propose a comprehensive FAIR eDNA (FAIRe) Metadata Checklist, which integrates existing data standards and introduces new terms tailored to eDNA workflows. Metadata are systematically linked to both raw data (e.g., metabarcoding sequences, Ct/Cq values of targeted qPCR assays) and derived biological observations (e.g., Amplicon Sequence Variant (ASV)/Operational Taxonomic Unit (OTU) tables, species presence/absence). Along with formatting guidelines, tools, templates, and example datasets, we introduce a standardized, ready-to-use approach for FAIR eDNA practices. Through broad collaboration, we seek to integrate these guidelines into established biodiversity and molecular data standards, promote journal data policies, and foster user-driven improvements and uptake of FAIR practices among eDNA data producers. In proposing this standardized approach and developing a long-term plan with key databases and data standard organizations, the goal is to enhance accessibility, maximize reuse, and elevate the scientific impact of these valuable biodiversity data resources.
1 Need for FAIR eDNA Data
The FAIR principles were developed to improve the Findability, Accessibility, Interoperability, and Reusability of scholarly digital research objects such as data and scripts (Wilkinson et al. 2016) (refer to Table 1 for the list of abbreviations, applicable throughout). Alongside open data principles (Mandeville et al. 2021), these principles promote the public good and enhance the transparency and reproducibility of research. Furthermore, the FAIR principles increase the long-term value of data, generate further knowledge at speed, and serve as a cost-saving measure (Beck et al. 2020; Gomes et al. 2022; Hampton et al. 2013; Jenkins et al. 2023; Kim and Stanton 2016; Leigh et al. 2024; Sholler et al. 2019; Soranno and Schimel 2014; Whitlock 2011). Open and FAIR principles have been adopted worldwide by organizations including the Research Data Alliance (RDA, https://www.rd-alliance.org), the Committee on Data of the International Science Council (CODATA, https://codata.org) and the Future of Research Communications and e-Scholarship (FORCE11) (Jacobsen et al. 2020; Martone 2015; Wilkinson et al. 2016). FAIR practices, however, have not yet been fully established in the field of environmental DNA (eDNA), limiting the opportunities to unlock its full potential (Berry et al. 2021; Borisenko et al. 2024; Kimble et al. 2022; Shea et al. 2023).
| Acronyms | Definitions |
|---|---|
| ABS | Access and benefit-sharing |
| ALA | Atlas of Living Australia |
| APIs | Application programming interfaces |
| ASV | Amplicon sequence variant |
| BOLD | Barcode of Life Data System |
| CARE | Collective benefit, Authority to control, Responsibility and Ethics |
| DDBJ | DNA Data Bank of Japan |
| dPCR | Digital Polymerase Chain Reaction |
| DSI | Digital Sequence Information |
| DwC | Darwin Core |
| eDNA | Environmental DNA |
| ENA | European Nucleotide Archive |
| FAIR | Findable, Accessible, Interoperable, Reusable |
| FAIRe | FAIR eDNA (in the context of the current initiative) |
| GBIF | Global Biodiversity Information Facility |
| GGBN | Global Genome Biodiversity Network |
| GSC | Genomic Standards Consortium |
| INSDC | International Nucleotide Sequence Database Collaboration |
| IPLCs | Indigenous Peoples and Local Communities |
| MDT | Metabarcoding Data Toolkit |
| MIDs | Multiplexing identifiers |
| MIEM | Minimum Information for eDNA and eRNA Metabarcoding |
| MIMARKS | Minimum Information about a Marker Sequence |
| MIMS | Minimum Information about Metagenome or Environmental Sequence |
| MIQE | Minimum Information for publication of Quantitative real-time PCR Experiments |
| MIxS | Minimum Information about any (x) Sequence |
| NCBI | National Center for Biotechnology Information |
| OBIS | Ocean Biodiversity Information System |
| OTU | Operational Taxonomic Unit |
| PCR | Polymerase Chain Reaction |
| qPCR | Quantitative Polymerase Chain Reaction |
| SRA | Sequence Read Archive |
| TDWG | Biodiversity Information Standards (formerly Taxonomic Databases Working Group) |
Given increasing pressures on the environment and accelerated species loss (Ceballos et al. 2015; Diaz et al. 2019), FAIR biodiversity data can support robust, real-time biomonitoring, biodiversity inventories, and provide a better foundation for decision making. Reversing the global loss of biodiversity and the associated resources and services requires transformational human actions and robust measurements of the effectiveness of those actions (Berry et al. 2021; Diaz et al. 2019). Yet, environmental measurement and biodiversity assessment at large scale, with high speed and accuracy, is very challenging. Recently, biodiversity science has adopted eDNA to help address these challenges, noting the various advantages of eDNA including non-invasive sampling, broad taxonomic coverage, high sensitivity, accuracy, scalability, and efficiency (Goldberg et al. 2015; Ruppert et al. 2019; Thomsen and Willerslev 2015). eDNA is also effective in situations where conventional methods for biological observation are impractical or impossible, such as in turbid waters, inaccessible subterranean environments, and for detecting elusive or cryptic species (Hashemzadeh Segherloo et al. 2022; Matthias et al. 2021; Saccò et al. 2022; Villacorta-Rath et al. 2022; West et al. 2020; White et al. 2020). As a result of these advantages, it has been widely implemented for biodiversity detection and monitoring, including as part of national and international strategies, for example, National Aquatic Environmental DNA Strategy (Goodwin et al. 2024; Kelly et al. 2024), Fisheries and Oceans Canada (Baillie et al. 2019), DNAqua-net (Blancher et al. 2022), and the Canadian Standards Association (Helbing and Hobbs 2019).
There are two primary approaches to detect organisms of interest in eDNA studies. The first approach, targeted assays (e.g., quantitative polymerase chain reaction (qPCR), digital PCR (dPCR)), uses taxon-specific primers and probes to detect the presence of DNA from specific taxa. This method typically targets a single taxon (e.g., species or genus) (but also refer to Tsuji et al. (2018) and Wilcox et al. (2020) for multiplex and high-throughput PCR) from which eDNA concentration may be inferred as a proxy for abundance or biomass of the focal taxon (Rourke et al. 2022). The second approach, metabarcoding assays, detects entire taxon assemblages from a sample by amplifying and sequencing genetic marker regions (so-called barcode regions) that are conserved across different taxa (Coissac et al. 2012; Hebert et al. 2003). This approach assigns large numbers of DNA sequences to diverse taxa, offering a broad overview of biodiversity within the sample. Both targeted and metabarcoding assay approaches can produce tremendous volumes of complex data. The exact methodologies employed within each approach can vary significantly (Coissac et al. 2012; Takahashi et al. 2023).
Metabarcode sequences (amplicon sequence variants (ASVs) or operational taxonomic units (OTUs)) are usually taxonomically annotated by comparing them to DNA reference databases, such as those available in the Barcode of Life Data System (BOLD) (Ratnasingham and Hebert 2007), the International Nucleotide Sequence Database Collaboration (INSDC) (Arita et al. 2021), and the Genome Taxonomy Database (GTDB) (Parks et al. 2022). As reference databases are continuously improved, for example through genetic sequencing of vouchered specimens (Delrieu-Trottin et al. 2019; Stoeckle et al. 2020; Weigand et al. 2019), eDNA sequences may receive more precise taxonomic assignment upon reanalysis. The ability to improve in accuracy and precision with time is a feature of eDNA data that is rare among biodiversity data types. This potential is, however, dependent on machine-readable and high-quality metadata, underscoring the importance of compliance with the FAIR principles. Adhering to these will also expand the lifespan and scale of eDNA data, thereby maximizing its power for biodiversity monitoring and providing additional value to existing biodiversity data (Berry et al. 2021; Thompson and Thielen 2023). For instance, FAIR eDNA data can be reused to increase the temporal and spatial scale of species and community observations (Crandall et al. 2023), fill important spatial and taxonomic gaps in our knowledge of global biodiversity, facilitate early invasive species detection (e.g., Helbing and Hobbs 2019), and enhance endangered species monitoring. Furthermore, currently unassigned sequences can contribute meaningful ecological insights, such as the taxon-independent community index (Wilkinson et al. 2024) or in habitat classification and predictive modeling (e.g., Frøslev et al. 2023). Altogether, implementing FAIR principles for eDNA data can maximize its applications and conservation impact.
2 Current State of eDNA Data Practices and Challenges to Achieving FAIR Standards
2.1 Data Sharing Policies
Data sharing is widely regarded as vital for ensuring reproducibility and has been adopted by the majority of ecological researchers (Herold 2015). The current standard practice for eDNA datasets is that upon publication of an associated manuscript, they are stored in digital repositories where they can be accessed by other researchers (Abarenkov et al. 2023; Leigh et al. 2024; Shea et al. 2023), but there is little oversight into which data are archived and how accessible they are. The establishment of public data archiving policies and requirements by scientific journals has been a key contributor to advancing data sharing practices (Sholler et al. 2019; Thrall et al. 2023; Whitlock 2011; Whitlock et al. 2010). Open-access journals such as GigaScience and GigaByte further support these efforts by providing dedicated data repositories (GigaDB) to ensure that publications are accompanied by supporting data that uphold principles of transparency, reproducibility, and FAIR standards (Armit et al. 2022). Additionally, funding agencies have also effectively implemented data sharing policies at national and international levels in various countries (“Australian Research Council,” 2021; “Open Research Europe | Open Access Publishing Platform,” 2025; “Proposal & Award Policies & Procedures Guide (PAPPG) (NSF 24-1) | NSF – National Science Foundation,” 2024; Hutchison et al. 2024).
These proactive approaches have led to a broader adoption of open data practices and a substantial increase in the proportion of published articles sharing their data. For instance, when journals mandate data archiving, there is a nearly 1000-fold increase in the ability to find data online compared to data in journals without a policy (Vines et al. 2013). Despite this progress, challenges to full FAIR principles compliance remain as the process is not yet fully standardized or unambiguously defined. For example, many datasets fall short due to inconsistent formatting and sparse metadata, limiting their interoperability and reusability (Hassenrück et al. 2021; Roche et al. 2015; Tedersoo et al. 2021). This may be due to a lack of awareness and familiarity around data standardization, existing guidelines, and the benefits of FAIR data sharing. However, existing FAIR guidelines for standardized formatting and sharing can be dissonant. Moreover, current metadata checklists (i.e., lists of data terms required and recommended for describing methods and datasets) implemented in standards are not always suitable for documenting the entire eDNA workflow. Nevertheless, each dataset published without meeting the FAIR principles can present a lost opportunity to preserve rich biodiversity data for reuse in research and decision making.
2.2 Data Standards
The establishment and adoption of standardized data formats, vocabularies, and comprehensive metadata checklists provide a robust foundation for ensuring that archived data are machine readable, interoperable, and suitable for reuse (Vines et al. 2013). Darwin Core (DwC, dwc.tdwg.org), developed by Biodiversity Information Standards (TDWG, tdwg.org) and Minimum Information about any (x) Sequences (MIxS, genomicsstandardsconsortium.github.io/mixs), developed by the Genomic Standards Consortium (GSC, gensc.org), are data standards to facilitate FAIR practices for biodiversity and DNA sequence data (Wieczorek et al. 2012; Yilmaz et al. 2011). Widely adopted by major data infrastructures like the INSDC (www.insdc.org) (Arita et al. 2021) and the Global Biodiversity Information Facility (GBIF, www.gbif.org), these standards can be powerful enablers of FAIR data practices. Furthermore, interoperability between DwC and MIxS has been established by a mapping of MIxS terms to a DwC extension (Meyer et al. 2023). The DNA-derived data extension to DwC was developed to accommodate biodiversity data in DwC-based biodiversity platforms like GBIF. The extension is currently maintained by GBIF (http://rs.gbif.org/terms/1.0/DNADerivedData) and consists of terms from MIxS, the Global Genome Biodiversity Network (GGBN) standard (https://www.ggbn.org/ggbn_portal), the Minimum Information for publication of Quantitative real-time PCR Experiments (MIQE) Guidelines (Bustin et al. 2009), and a few custom terms (e.g., atomised versions of the primer terms on MIxS) (Abarenkov et al. 2023).
Despite dynamic community-driven development (Abarenkov et al. 2023; Bustin et al. 2009; Meyer et al. 2023; Wieczorek et al. 2012; Yilmaz et al. 2011), these existing data standards lack unambiguous and comprehensive terms, vocabularies, and guidance to fully capture some of the distinctive features of eDNA data and workflows. For instance, qPCR-based eDNA workflows, first introduced around 2010 (e.g., Ficetola et al. 2008; Thomsen et al. 2012), have been increasingly used for targeted assay detection (Takahashi et al. 2023). The most comprehensive qPCR checklist (i.e., the MIQE Guidelines) was, however, developed prior to the prevalence of eDNA studies and may need to be revised and updated to align with FAIR principles for qPCR-based eDNA reporting. Furthermore, procedures for monitoring contamination and excluding nontarget taxa, and the parameters used for quality filtering and species detection vary greatly between studies, depending on the project scope and the associated financial and ecological costs of false positives and false negatives (Ficetola et al. 2015; Takahashi et al. 2023). Making detailed workflow descriptions FAIR can enhance future studies' ability to reuse data and ensure high confidence levels in species detection and taxonomic assignment. Increasing efforts have been made to establish minimum reporting requirements to validate eDNA study methods and data (Abbott et al. 2021; Borisenko et al. 2024; Gagné et al. 2021; Kimble et al. 2022; Klymus et al. 2020; Thalinger et al. 2021). As a next step, these requirements could be translated into data standards and formats to enhance human and machine readability, interoperability, and reusability.
Like other machine-derived data, eDNA datasets are comprised of a variety of data components representing degrees of processing and refinement. Therefore, defining the essential data components to be shared is crucial to ensure that shared data are standardized, complete, and reusable. Components of eDNA datasets can be broadly grouped into three categories: (1) raw data, including amplification data (i.e., Ct/Cq values from targeted assay detection studies) and raw DNA sequences (i.e., FASTQ files from metabarcoding studies), (2) interpreted data, including estimated DNA copy numbers from standard curves and detections (1/0) of targeted taxa, raw and curated ASV/OTU tables, and taxonomic assignment of each ASV/OTU, and (3) metadata describing data content, provenance, methods, and associated environmental data for each project and sample. Each data type serves a different purpose and possesses distinct qualities. For example, raw data are essential for documentation and reproducibility, as well as for enabling future re-analysis with different or updated bioinformatic pipelines. In contrast, interpreted data and metadata provide immediate value for broader biodiversity research and data-driven decision-making. Sharing all these data components in standardized formats is crucial to making data FAIR.
2.3 Cyberinfrastructure
There are a range of digital repositories available for depositing eDNA datasets, eliminating the need for a new or single repository. Instead, fostering collaborations among databases could ensure that shared eDNA datasets remain interoperable and reusable. These repositories can broadly be categorized into sequence databases and biodiversity databases. The INSDC sequence databases, which are a collaboration between the US National Center for Biotechnology Information (NCBI, Sayers et al. 2024), the European Nucleotide Archive (ENA, www.ebi.ac.uk/ena) (Yuan et al. 2024), and the DNA Data Bank of Japan (DDBJ, www.ddbj.nig.ac.jp) (Kodama et al. 2024), aim to compile and disseminate DNA sequence data and associated metadata generated worldwide (Arita et al. 2021). Data archived in NCBI, ENA, and DDBJ are routinely exchanged and synchronized among these databases. Biodiversity databases such as GBIF, on the other hand, serve as an open-access online platform for global biodiversity data, with a primary focus on species occurrences. The types of data available in GBIF include digitized specimens from natural history collections, observations from citizen science platforms (e.g., eBird (ebird.org), iNaturalist (inaturalist.org)), and DNA-associated records (e.g., barcoded specimens from the BOLD database (Ratnasingham and Hebert 2007) and records from INSDC (Arita et al. 2021)), as well as metabarcoding and metagenomic datasets from individual studies (e.g., Shea and Boehm 2024) and initiatives such as MGnify (Richardson et al. 2023) (refer to Abarenkov et al. 2023). Other biodiversity databases based on the Darwin Core standard for biodiversity data, such as the Ocean Biodiversity Information System (OBIS, obis.org) and the Atlas of Living Australia (ALA, www.ala.org.au) focus on distinct ecosystems or regions and routinely exchange datasets. Given that eDNA data encompass both biodiversity information (e.g., inferred species occurrences) and molecular sequence data (e.g., raw reads), an optimal data standard would allow for describing eDNA datasets intended to be shared through both biodiversity and nucleotide sequence databases.
Publishing eDNA data in established databases can be a simple and powerful step toward achieving FAIR eDNA, as these platforms provide persistent sample and sequence identifiers across datasets, and offer robust, indexed search functions based on user-defined parameters, such as geographical regions, ecosystem types, taxa and gene regions, and making data highly findable and accessible. Moreover, eDNA data shared in biodiversity databases like GBIF and OBIS contribute to the global biodiversity records by adding DNA-derived species occurrences alongside data from other sources. These databases do not accept all three primary eDNA data components (metadata, raw data and interpreted data) as a whole, and therefore, require linkage if data are to be accessible for re-use. For example, GBIF focuses on inferred biodiversity data, such as detected species or taxa with spatiotemporal metadata, and representative curated DNA sequences. It, however, does not host raw DNA sequencing files (i.e., FASTQ files), but may link to the corresponding INSDC records. Conversely, INSDC databases host raw sequence data, but neither the inferred biodiversity data in an interoperable format nor eDNA-derived species occurrence data without DNA sequences, such as qPCR-based detections. Collaboration efforts between GBIF and INSDC (through ENA) are in progress to ensure cross-linking of related data resources and increase interoperability between the two data infrastructures (“Cross-infrastructure collaboration with ENA improves processing, quality of DNA-derived occurrences” 2021).
In contrast, eDNA practitioners can deposit their eDNA data as a whole in open data repositories, such as Dryad (https://datadryad.org), Zenodo (https://zenodo.org), or FigShare (https://figshare.com), which accept unrestricted data types. Data in these repositories, however, are not fully FAIR, as they are not standardized by default and are not indexed to a degree where they are functionally findable and interoperable. Hence, data reusers are required to manually access and reprocess the data before it can be re-analyzed and/or combined with other datasets. As a consequence, eDNA data published in these repositories are at risk of getting lost and becoming “dark data” (Heidorn 2008).
More recently, various infrastructures have been developed specifically to present eDNA data from particular regions, institutions, or taxa, for example, All Nippon eDNA Monitoring Network (ANEMONE, db.anemone.bio), Wilderlab (www.wilderlab.co.nz/explore), eDNAtlas (www.fs.usda.gov/research/rmrs/projects/ednatlas, (Young et al. 2018)), eDNA Explorer (www.ednaexplorer.org), the Australian Microbiome (www.australianmicrobiome.com), Swedish ASV portal (https://asv-portal.biodiversitydata.se), ASV Board (https://asv.bolgermany.de/metabarcoding), Nonindigenous Aquatic Species (NAS) database (https://nas.er.usgs.gov/eDNA), and the global microbiome database under the National Microbiome Database Collaborative (NMDC, data.microbiomedata.org). These databases utilize custom data structures to serve their particular focus and functionalities, sometimes in combination with terms from more widely established data standards like MIxS and DwC. Publishing data from such databases to global databases (e.g., INSDC and GBIF) requires mapping custom data terms to an open data standard, which can be a lossy process but serves the purposes of discoverability and inclusion in the default global data pools.
While the aforementioned databases and infrastructures share the goal to promote open and data-driven science, they also cater to specific focuses and requirements. Consequently, a spectrum of repositories exists, ranging from those accepting any research data in unrestricted formats (e.g., Dryad, Zenodo, FigShare) to those exclusively processing eDNA-derived data from a single source using standardized protocols (e.g., ANEMONE and Wilderlab). Because of the numerous repositories, there is likely not a need for a new or single repository. Instead, fostering collaborations among eDNA communities, databases, data standard organizations, and journal editors and publishers could ensure that shared eDNA datasets are comprehensive, interoperable, and reusable. This involves integrating eDNA data into existing databases and standards and mapping it across past, current, and future standards. This approach retains flexibility and interoperability with existing repositories while supporting the formatting of shared datasets into common standards. This, in turn, facilitates data mobilization, collation, and reuse.
3 Project Scope
Fine-tuning, optimizing, and extending existing data and metadata standards specifically for eDNA-based studies (both targeted and metabarcoding detection approaches) is an important first step toward achieving FAIR eDNA.
- Formation of an international, multidisciplinary working group comprising eDNA practitioners, journal editors (e.g., the Environmental DNA journal), and experts in biodiversity and ‘omics data science’.
- Identification of data components and formats suited for FAIR eDNA practice for both targeted and metabarcoding detection approaches.
- Establishment of a comprehensive, FAIR eDNA (FAIRe) metadata checklist, with corresponding guidelines by adapting domain-specific usage of existing terms and adding new terms to existing standards (i.e., DwC, MIxS, DNA-derived extension).
- Development of a customisable metadata template generator and an error-checking validation tool.
Together, these efforts aim to guide data providers and facilitate the unambiguous description of eDNA datasets that are aligned with FAIR data practices (Figure 1). Our goal is to provide a FAIRe metadata checklist and guidelines as resources for existing data standards, infrastructures, and databases, helping them to address gaps to better meet their own objectives. The guidelines will also benefit individual eDNA science practitioners and labs by improving data management standardization, making their data more FAIR not only for reusers but also for internal organization.

Finally, we discuss additional steps to further advance FAIR practices within the eDNA community and maximize the utility of eDNA data. The proposed FAIRe guidelines will evolve based on user feedback and the emergence of new methods and technologies, with the most up-to-date version available at https://fair-edna.github.io/index.html.
The proposed FAIRe guidelines currently focus on the two major eDNA detection approaches: targeted (e.g., qPCR) and metabarcoding assay detections. While we acknowledge the rapid development of diverse techniques, such as multiplex and high-throughput PCR and DNA metagenomics, these two approaches encompass the major eDNA workflows (Takahashi et al. 2023).
We also recognize that there are critical aspects of FAIR eDNA data that are not covered here. For example, the importance of FAIR code for reproducibility in bioinformatics and analysis workflows, as well as for enhancing the FAIRness of data supporting any science, is not covered here, but it has been addressed in recent studies by Ivimey-Cook et al. (2023), Thrall et al. (2023), Thalinger et al. (2021), Cadwallader et al. (2022) and Jenkins et al. (2023). This paper does not take a position on access and benefit-sharing (ABS) issues related to Digital Sequence Information (DSI) (https://www.cbd.int/dsi-gr). While ABS concerns often focus on the use of publicly available DSI, considerations around how DSI is shared also play a role in compliance with international, regional, and national regulations. Any data management plan can carefully account for these rules to ensure proper adherence.
4 FAIR eDNA (FAIRe) Practice Guidelines
- Darwin Core Quick Reference Guide. https://dwc.tdwg.org/terms/.
- Minimum Information about any (x) Sequence (MIxS) standard. https://genomicsstandardsconsortium.github.io/mixs/.
- Darwin Core extension of DNA derived data. https://rs.gbif.org/extension/gbif/1.0/dna_derived_data_2024-07-11.xml.
- Abarenkov et al. (2023) Publishing DNA-derived data through biodiversity data platforms, v1.3. Copenhagen: GBIF Secretariat. https://doi.org/10.35035/doc-vf1a-nr22.
- The OBIS manual. https://manual.obis.org/GBIF Metabarcoding Data Toolkit. https://mdt.gbif.org/.

4.1 Data Formats and Components
-
Project metadata (projectMetadata)
- ○
Contents: Information that applies to an entire project and dataset. This includes a project name, reference associated with the datasets from which the datasets were derived (e.g., bibliographic references to studies, DOI of published, and associated data), and metadata describing workflows including PCR, sequencing, and bioinformatic steps.
- ○
Purpose: To describe project information and methodologies that apply to all samples, thereby avoiding the need to propagate identical entries to all samples in sample metadata. Information, such as bioinformatic quality filtering parameters and thresholds, enables data reusers to assess whether the data quality meets the specific requirements for certain types of reuse. Standardization of such information is particularly important as minimum requirements for amplification analysis, bioinformatic workflows, and quality assurance levels vary between studies and applications, including those reusing shared data.
- ○
Note: Multiple primer sets are often applied within one project to target more than one taxon. In this case, practitioners would follow the format in Figure 5 to record assay-specific workflows and outputs.
- ○
-
Sample metadata (sampleMetadata)
- ○
Contents: Information for each sample (where this cannot be stated at the project level in project metadata), including sampling date, location, methodologies (e.g., sample collection, preservation, storage, filtration, and DNA extraction), and environmental variables (i.e., temperature, water depth).
- ○
Purpose: To record sample-specific information through the workflows from sampling to bioinformatics.
- ○
Note: Including control samples in sample metadata can enhance data transparency and FAIRness. The metadata checklist includes terms such as samp_category, neg_cont_type, pos_cont_type, and rel_cont_id to describe control types and their links to eDNA samples.
- ○
-
Experiment/run metadata (experimentRunMetadata)
- ○
Contents: Sample-specific information for PCR, library preparation (experiment) and sequencing (run) workflows.
- ○
Purpose: To record workflows, from PCR onward, that are specific to each sample and assay.
- ○
Note: Each entry (row) has a unique library ID (lib_id) and multiplexing identifiers (MIDs) (mid_forward, mid_reverse).
- ○
-
PCR standard data (stdData)
- ○
Contents: Detailed information of PCR standards, including starting input quantity, Ct/Cq values, and standard curve parameters (e.g., efficiency, R2).
- ○
Purpose: To improve reproducibility and allow data reusers to regenerate the estimated copy numbers using their own approach. The entries in assay_name and pcr_plate_id in the PCR standard data and amplification data allow linking of the standards and eDNA samples.
- ○
-
eLow Quant data (eLowQuantData)
- ○
Contents: Outputs from the eLow Quant binomial approach for PCR standards if applicable (refer to Lesperance et al. 2021).
- ○
Purpose: To improve reproducibility and allow data reusers to regenerate the estimated copy numbers using their own approach.
- ○
-
Targeted assay amplification data (ampData)
- ○
Contents: Raw amplification data of each PCR technical replicate performed on eDNA samples, as well as estimated copy numbers based on standard curves (if applicable) and detection status of targeted taxon in each biological replicate (field sample).
- ○
Purpose: The Ct/Cq values of each technical replicate, combined with the standard information above, enable the re-conversion of the raw amplification data to concentration or detection status in future studies. Detect/non-detect records of a target taxon at a biological replicate level allow prompt reuses of taxon occurrence records in future studies, combined with sample metadata. They also standardize eDNA data in the context of biodiversity data produced using other methods and consequently make the collective data and underlying research discoverable and reusable for wider audiences.
- ○
Note: “NA” (not zero) should be used under the term quantificationCycle (i.e., Ct/Cq values) to indicate no amplification occurred, because zero could be interpreted as an extremely high DNA concentration, which may or may not be target DNA that would be unquantifiable due to being outside the standard curve range.
- ○
-
Raw DNA sequences
- ○
Contents: DNA sequences in FASTQ format, demultiplexed for each sample. The sequences of primers, adapters, and MIDs must be removed.
- ○
Purpose: To allow data reusers to repeat quality filtering, denoising, and taxonomic assignment using their own algorithms and thresholds to fit their purposes. To enable re-analyses using different or improved pipelines/algorithms. To enhance the reproducibility of studies.
- ○
-
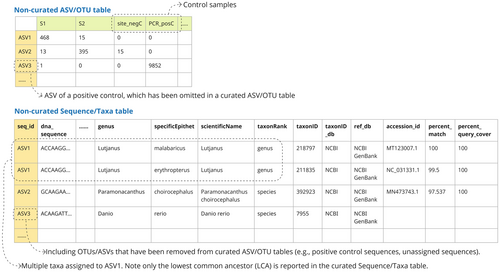
Non-curated ASV/OTU table (otuRaw) (Optional) (Figure 4)
- ○
Contents: The initial ASV/OTU table produced in a bioinformatic pipeline, containing absolute sequence read counts of all ASV/OTUs (including contaminant/nontargeted taxa and unassigned ASV/OTUs) across all samples (including controls). Each ASV/OTU will be provided with a unique seq_id at this stage.
- ○
Purpose: To communicate objective information of read counts of all OTUs/ASVs in all samples, and allow data reusers to modify or re-run the data curation steps if required. The inclusion of control samples, suspected contaminants, and nontargeted taxa allows the investigation of common contamination sequences, taxa, and sources across multiple studies, and aids in potentially identifying how to minimize them.
- ○
Note: The column names in ASV/OTU tables should be either samp_name or lib_id, making sure they maintain clear links between the experiment/run metadata and the ASV/OTU table.
- ○
Note: Although it is called a “raw” ASV/OTU table, some level of compression, quality filtering, and curation may already have been applied, such as filtering based on minimum length, read counts, error rates, and OTU clustering. The extent of data curation performed on the non-curated ASV/OTU table can vary between studies depending on the bioinformatics pipeline used, and this should be described under the term otu_raw_description in the project metadata.
- ○
-
Curated ASV/OTU table (otuFinal)
- ○
Contents: An ASV/OTU table resulting from various data curation processes, including denoising (i.e., custom or LULU curation), removing control samples, and eliminating suspected contamination and nontarget taxa. This table should ideally be identical to, or correspond closely to, the one used for the final (e.g., ecological) analyses published in associated scientific papers.
- ○
Purpose: To facilitate reproducibility of original (ecological) analyses and general reuse of a curated/cleaned version of the data. To improve interoperability with other types of biodiversity data, and thereby facilitate incorporation in general biodiversity databases, and make the data and underlying research discoverable and reusable for wider audiences.
- ○
Note: The column names in ASV/OTU tables should be either samp_name or lib_id, making sure they maintain clear links between the experiment/run metadata and the ASV/OTU table.
- ○
Note: In some studies, researchers may choose to collapse multiple ASVs/OTUs assigned to the same taxon, summing their read counts. We, however, strongly encourage data providers to submit ASV/OTU tables in their original, non-collapsed form. Preserving each ASV/OTU with its individual read counts and sequence ensures better interoperability and prevents the loss of potentially valuable ASVs/OTUs due to uncertain or erroneous taxonomic annotations resulting from incomplete or imperfect reference databases at the time of analysis.
- ○
Note: Descriptions on how data were curated to produce the curated ASV/OTU table should be recorded under the term otu_final_description in the project metadata.
- ○
-
Non-curated Sequence/Taxa table (taxaRaw) (Optional) (Figure 4)
- ○
Contents: In addition to the contents in the curated Sequence–Taxa table (below), this table includes ASVs/OTUs that were excluded from the curated ASV/OTU table (e.g., nontarget taxa, contaminants, positive control sequences). It may also contain multiple rows for a single ASV/OTU assigned to multiple taxa, with information for each assigned taxon.
- ○
Purpose: To enhance data transparency by providing access to ASVs, OTUs, and taxa excluded from the curated ASV/OTU table. To allow data reusers the opportunity to reassess and rerun specific data curation steps.
- ○
Note: When assigning a lowest common ancestor (LCA) to a single ASV/OTU, multiple rows should be generated for the ASV/OTU to capture information about each of the assigned taxa. Users may apply a similarity threshold (e.g., 97%) and include all reference sequences within this range, or a representative subsample of them, in the output.
- ○
Refer to the example in Figure 4 (ASV1 in the non-curated Sequence/Taxa table).
- ○
-
Curated Sequence/Taxa table (taxaFinal)
- ○
Contents: DNA sequence of each seq_id listed in the curated ASV/OTU table. If a taxon was assigned, also include assigned taxonomy and assigned quality assurance parameters (e.g., % identity, % query coverage). If multiple taxa are assigned to a single ASV/OTU, the LCA should be used as the assigned taxon.
- ○
Purpose: To allow data reusers to assess the specificity and accuracy of the inferred taxonomy, and to perform taxonomic re-annotation using different or updated sequence reference databases.
- ○


4.2 Consistent Identifiers and File Naming
To ensure machine readability and long-lasting reference to a digital resource, consistent, persistent sample and sequence identifiers (samp_name, lib_id and seq_run_id) across datasets (Damerow et al. 2021; McMurry et al. 2017) must be used. Similarly, each file must have a clear, unambiguous name, consisting of project_id, assay_name, and seq_run_id (refer to Table 2 and example datasets) to maintain clear links between files. For example, a curated ASV/OTU table should be named as otuFinal_<project_id>_<assay_name>_<seq_run_id> (e.g., otuFinal_gbr2022_MiFish_lib20230922.csv). If multiple data components are stored within a single spreadsheet workbook, the file name should be the project_id (e.g., gbr2022.xlsx). In this case, each worksheet name should follow the format in Table 2 without the project_id as it already appears in the main file name (e.g., otuFinal_MiFish_lib20230922).
| Data type | Name format | Example |
|---|---|---|
| Project metadata | projectMetadata_<project_id> | projectMetadata_gbr2022.csv |
| Sample metadata | sampleMetadata_<project_id> | sampleMetadata_gbr2022.csv |
| PCR standard data | stdData_<project_id> | stdData_gbr2022.csv |
| eLow Quant data | eLowQuantData_<project_id> | eLowQuantData_gbr2022.csv |
| Amplification data | ampData_<project_id>_<assay_name> | ampData_gbr2022_eSERUS.csv |
| Experiment/run metadata | experimentRunMetadata_<project_id> | experimentRunMetadata_gbr2022.csv |
| Non-curated ASV/OTU table | otuRaw_<project_id>_<assay_name>_<seq_run_id> | otuRaw_gbr2022_MiFish_run20230922.csv |
| Curated ASV/OTU table | otuFinal_<project_id>_<assay_name>_<seq_run_id> | otuFinal_gbr2022_MiFish_run20230922.csv |
| Non-curated Sequence/Taxa table | taxaRaw_<project_id>_<assay_name>_<seq_run_id> | taxaRaw_gbr2022_MiFish_run20230922.csv |
| Curated Sequence/Taxa table | taxaFinal_<project_id>_<assay_name>_<seq_run_id> | taxaFinal_gbr2022_MiFish_run20230922.csv |
4.3 Metadata Checklist
We developed a FAIR eDNA (FAIRe) metadata checklist, providing a comprehensive vocabulary for describing different data components and methodologies (Appendix S1). Thorough documentation promotes improved transparency and reproducibility of studies, as well as enabling the evaluation of data suitability for specific reuse cases. The FAIRe checklist consists of 337 data terms (38 mandatory, 51 highly recommended, 128 recommended and 120 optional terms), organized into workflow sections (e.g., sample collection, PCR, bioinformatics). Most of the mandatory, highly recommended, and recommended information is generated naturally during the course of any project. As a result, data meeting the minimum reporting requirements can be readily made available and submitted to public repositories.
4.3.1 Vocabulary Basis
The FAIRe metadata checklist consists of terms sourced from existing data standards such as MIxS, DwC, and the DNA-derived data extension to DwC. MIxS offers several checklists and extensions relevant for eDNA data, including Minimum Information about a Marker Sequence (MIMARKS), Minimum Information about Metagenome or Environmental Sequence (MIMS), and extensions for various environments (e.g., water, sediment, soil, air, host associated, and symbiont associated). These standards were reviewed and incorporated into the FAIRe metadata checklist where applicable to ensure comprehensive coverage of eDNA data needs. We have also incorporated terms from MIQE guidelines (Bustin et al. 2009), the single species eDNA assay development and validation checklist (Thalinger et al. 2021) and Minimum Information for eDNA and eRNA Metabarcoding (MIEM) guidelines (Klymus et al. 2024). The source and Uniform Resource Identifier (URI) for each term from existing standards are documented in the FAIRe metadata checklist under the columns “source” and “URI”. Where necessary, modifications have been made to term names, descriptions, and examples for easier interpretation by the eDNA community. A total of 158 new terms were proposed to accommodate some diverse attributes of eDNA procedures and datasets, and to improve the possibility of evaluating fitness for reuse. Among these are terms related to targeted assay detection workflows (e.g., lod_method, std_seq), bioinformatic tools, filtering parameters and cutoffs (e.g., demux_tool, demux_max_mismatch), taxonomic assignment metrics (e.g., percent_match), and taxon screening methods (i.e., screen_contam_method, screen_nontarget_method). The FAIRe checklist also includes a range of environmental variables relevant to eDNA samples, sourced from the MIxS sample extensions including Water, Soil, Sediment, Air, HostAssociated, MicrobialMatBiofilm, and SymbiontAssociated. These variables are typically recorded during sampling events, and sharing them could enable more advanced modeling approaches among other applications.
If suitable term names are not available in the FAIRe checklist, users should search for them in existing standards, such as MIxS (https://genomicsstandardsconsortium.github.io/mixs) and DwC (https://dwc.tdwg.org/terms), and use these standardized terms where possible. If relevant terms cannot be found in these resources, users may add new terms using clear, concise, and descriptive names within related tables.
4.3.2 Specified Use of Terms and Controlled Vocabularies
Several approaches were implemented to increase standardization of terms. Firstly, for some free-text terms from existing standards, we propose using controlled vocabularies. For example, the value for the target_gene term should be selected from a list of 28 gene regions used for barcoding. This standardization prevents variations of COI such as CO1, COXI, COX1, Cytochrome oxidase I gene, Cytochrome c oxidase I, and spelling mistakes/variants of these from being entered. If a suitable value cannot be found among the specified values of the vocabulary, it is possible to enter “other:” followed by a free-text description. Secondly, units of numeric variables are strictly specified based on the International System of Units wherever possible, and only numeric entries without units are allowed. When units are necessary for unambiguous communication, separate terms for measurement value and measurement unit are provided, with the unit restricted to a controlled vocabulary (e.g., samp_size for the numeric value and samp_size_unit with options of mL, L, mg, g, kg, cm2, m2, cm3, m3, and other). Thirdly, formats for some data terms are restricted, generally following other standards (i.e., DwC, MIxS) and data infrastructures (i.e., GBIF, OBIS, INSDC). For example, decimalLatitude and decimalLongitude are required terms and must be in decimal degrees using WGS84 datum. If records in an original datasheet follow other formats (e.g., degree minute second, UTM, datums other than WGS84), they should be stored under the terms verbatimLatitude and verbatimLongitude while decimalLatitude and decimalLongitude are also required to be entered. Similarly, sampling date and time are summarized under the term eventDate.
Which utilizes the ISO 8601 format (https://www.iso.org/iso-8601-date-and-time-format.html), followed by the difference from UTC time (e.g., “2008-01-23T19:23-06:00” in the time zone 6 h earlier than UTC). Original records of date and time before conversion into the ISO 8601 format should be stored under the terms verbatimDate and verbatimTime.
4.3.3 Missing Values
- Not applicable: control sample
- Not applicable: sample group
- Not applicable (other reasons not applicable)
- Missing: not collected: synthetic construct
- Missing: not collected: lab stock
- Missing: not collected: third party data
- Missing: not collected (other reasons not collected)
- Missing: not provided: data agreement established pre-2023
- Missing: not provided (other reasons not provided)
- Missing: restricted access: endangered species
- Missing: restricted access: human-identifiable
- Missing: restricted access (other reasons restricted access)
4.4 Data Submission/Publication
Publishing eDNA datasets to databases such as GBIF, OBIS, and INSDC is an essential step in ensuring FAIR data practices. These platforms ensure data findability and accessibility for scientific research and decision making that rely on open data, either through application programming interfaces (APIs) or sophisticated web-browser interfaces. They offer data validation procedures during submission and provide additional standardization to ensure interoperability. Persistent sample and sequence identifiers are provided upon the submission of data to these databases, which are essential for machine-readability and long-lasting reference to the data. For example, in GBIF, dataset authors and publishers receive credit through unique dataset Digital Object Identifiers (DOIs) that enable citation tracking and automatic usage reporting.
- Submit raw DNA sequence data and metadata to nucleotide databases such as INSDC (ENA, NCBI, or DDBJ). For instance, NCBI allows submission of project metadata via BioProject, sample metadata via BioSample, and raw sequences via the Sequence Read Archive (SRA) (Barrett et al. 2012). This links metadata with sequence data, enabling users to retrieve complete datasets by querying BioProject or BioSample records.
- Publish the derived biodiversity data—that is, inferred taxon occurrences with sequences and metadata—to biodiversity databases such as GBIF or GBIF and OBIS (if marine data). Both GBIF and OBIS rely on data being formatted according to the DwC standard with the DNA-derived data extension as described in the co-authored guide (Abarenkov et al. 2023). The dataset is converted into so-called Darwin Core Archive (DwC-A) that can be indexed by GBIF and OBIS.
- Archive the remaining data components (i.e., standard data for targeted assay studies and raw ASV/OTU table for metabarcoding studies), formatted according to the FAIRe guidelines, in open data repositories such as Dryad, Zenodo, and Figshare or as Supporting Information in journals. This approach ensures comprehensive storage of raw data that are not addressed in the above steps. However, datasets in these repositories are not indexed to the extent required for effective searchability and interoperability within larger eDNA, biodiversity, or nucleotide data platforms.
- Publishing protocols and sharing bioinformatics and analytical codes (Jenkins et al. 2023; Samuel et al. 2021; Teytelman et al. 2016) through open-source platforms such as protocols.io (https://www.protocols.io), WorkflowHub (https://workflowhub.eu), and Better Biomolecular Ocean Practices (BeBOP) (https://github.com/BeBOP-OBON) can further support FAIR principles.
- The linkage between all the above data, protocols, and codes can be strengthened by assigning DOIs and documenting them in data accessibility statements within journal articles, as well as in the metadata terms such as seq_archive, code_repo, and associated_resource.
The guidelines provided here result in data formatted slightly different from those accepted by nucleotide and biodiversity databases. Hence, minor reformatting is required. One example is the necessary data format when multiple assays were applied in a single project. GBIF recently launched the Metabarcoding Data Toolkit (MDT), a user-friendly web application (https://www.gbif.org/metabarcoding) that reshapes tabular metabarcoding data—similar to the format proposed here—and publishes it to GBIF and OBIS complying with the guidelines in Abarenkov et al. (2023). The GBIF MDT currently requires a separate input dataset for each assay (hence information from project and sample metadata are repeated), whereas the FAIRe checklist allows the metadata from multiple assays to be combined (Figure 5). An R-script (FAIRe2MDT) has been developed (R Core Team 2024) to convert FAIRe data templates for GBIF and OBIS publication via MDT, bridging the differences in data formats between FAIRe and GBIF standards (refer to section ‘Available example datasets, scripts and tools’). Using these tools in combination streamlines the publication of eDNA data to GBIF and OBIS. Overall, there is currently a strong effort by biodiversity data platforms to improve the suitability of depositing eDNA-based data.
4.5 Available Example Datasets, Scripts and Tools
- FAIRe-ator (FAIR eDNA template generator): This R function creates data templates based on user-specified parameters, such as assay type (i.e., targeted or metabarcoding assay), sample type (e.g., water, sediment), and the number of assays applied. Additionally, the function allows users to input project ID and assay name(s), which ensures correct file name formatting and pre-fills the project_id and assay_name terms in the template. While the full template (Appendix S4) includes data components and metadata terms for all assay and sample types, FAIRe-ator produces study-specific templates tailored to individual project needs.
- FAIRe-fier (FAIR eDNA metadata verifier): This planned tool has a working prototype already in place. The plan is to make this metadata verifier publicly accessible through a web interface, allowing users to upload their metadata and validate it without requiring any scripting knowledge. The tool checks various components, including whether mandatory terms are completed or, if not, whether a valid reason has been provided following the missing value guidelines. It also verifies that controlled vocabulary entries and fixed-format terms are correctly formatted. If any issues are found, users receive output with warning and error messages, indicating where corrections are needed. Formatted output will be produced when there is no warning message, allowing users to submit it to the respective publishers.
- FAIRe2MDT: This R script converts FAIR eDNA data templates for GBIF submission via MDT.
5 Planned Next Steps
The current guidelines can substantially empower users to enhance the FAIRness of eDNA data, but other contributions will also make a difference.
5.1 Raising Awareness
Our next step focuses on raising awareness of FAIR principles, existing guidelines, and available tools to promote their adoption with the eDNA communities. To achieve this, the lead author will host workshops with eDNA practitioners over the coming year, offering hands-on opportunities to introduce FAIR data principles and provide practical guidance on implementing standardized formatting protocols. The first workshop took place at the eDNA conference in Wellington, New Zealand, in February 2025. More than a training exercise, these workshops enable establishing direct communications, identifying communities' needs, and addressing challenges to adopting FAIR data practices within their workflows. This step is particularly important as open and FAIR data can only be achieved through eDNA practitioners' collaborative efforts and relies on their commitment to making data accessible for broader use.
Several studies have explored Open Science behaviors and identified the barriers among scientists (e.g., Norris and O'Connor 2019; Tenopir et al. 2015). These barriers include technical and resource limitations, such as lack of standards, tools, time and skills to navigate required data management systems (Tedersoo et al. 2021; Tenopir et al. 2015). Additionally, multiple perceived barriers exist, including unawareness of the value of data for others, fear of scrutiny due to potential mistakes, resistance to openly sharing data given large efforts invested to secure funding in the face of limited resource for scientific research and resistance to changing existing practices (Norris and O'Connor 2019; Tenopir et al. 2015).
To address these challenges, communicating the diverse benefits of data sharing beyond reproducibility and documentation is important. In addition to contributing to the public good, data sharing leads to various benefits to data providers. These include increased visibility and citation of associated work (Colavizza et al. 2020; Piwowar et al. 2007; Wood-Charlson et al. 2022), direct citation metrics of data accessed through databases (e.g., GBIF), and enhanced collaborations and co-authorships, which all lead to improved professional stature (Bethlehem et al. 2022; McKiernan et al. 2016; Piwowar and Vision 2013; Whitlock 2011). Many eDNA practitioners, however, may not yet be fully aware of these benefits. Establishing open and direct communications with them through workshops will help us identify their unique barriers and needs, work collaboratively to overcome them, and foster a culture of FAIR data practices in the community.
5.2 Revising, Updating and Integrating the Guidelines
As eDNA science is relatively new and rapidly evolving, new methods and technologies will emerge. To improve and adjust the FAIRe metadata checklist and formatting guidelines and keep them relevant, regular revisions and updates are essential. Input from data providers and reusers is vital to this process, and we welcome feedback at any time via https://github.com/FAIR-eDNA/FAIR-eDNA.github.io/issues. Updated guidelines will be available at https://fair-edna.github.io.
Leveraging the network of the co-authors (e.g., GSC, TDWG, ENA, GBIF, OBIS, and ALA), we aim to continuously revise and co-develop the FAIRe metadata checklist to integrate with established data standards and databases (Figure 6). Implementation and alignment of FAIRe procedures and formats will be supported through contributions to the existing guidelines for publishing DNA-derived biodiversity data (Abarenkov et al. 2023), and development of scripts, like the FAIRe-ator and FAIRe2GBIF, and tools like the GBIF MDT. Integration of the FAIRe checklist into the GSC suite (i.e., MIxS), that will facilitate its adoption within the INSDC system, will be pursued through collaboration with the GSC MIxS Compliance and Interoperability Working Group (CIG) and with input from TDWG (Figure 6).

In the scope of existing agreements between TDWG and GSC to collaborate on the development of specifications for sequence-based biodiversity data, we plan to support the integration of the FAIRe checklist into the DwC DNA Derived Data extension. Continued revision, testing, and alignment with these organizations' objectives will help ensure successful implementation. Important milestones include the publication of updated guidelines for publishing DNA-derived biodiversity data (Abarenkov et al. 2023), the publication of the eDNA MIxS checklist, and the publication of the FAIRe-aligned DwC DNA Derived Data extension. Achieving these milestones will serve as evidence that the FAIRe checklist is adequate and accepted by the eDNA community. Long-term maintenance is planned to be managed by these consortia and organizations, but will depend on sustained engagement and interest from the eDNA science community in the FAIRe checklist.
We have developed the current guidelines with great interest to integrate them into journal publication requirements. We have collaborated with Wiley and Environmental DNA to develop a roadmap for integrating FAIR data principles into their publication procedures. This roadmap involves three key steps: (1) strongly recommending adherence to the FAIRe guidelines upon journal article publication, (2) making compliance mandatory in due course, and (3) extending these requirements to additional journals including Molecular Ecology and Evolution. While most journals mandate data sharing, submitting data in a FAIR manner is not yet a widespread practice (Roche et al. 2015). Implementation of the FAIR data guidelines could be an effective strategy given the large number of eDNA studies published each year with the tremendous volume of genetic and species occurrence data they generate (Takahashi et al. 2023).
5.3 Harmonizing FAIR and CARE
The CARE principles, which stand for Collective benefit, Authority to control, Responsibility, and Ethics, were developed by the Global Indigenous Data Alliance to protect the Indigenous rights and interests in Indigenous data, which include information, data, and traditional knowledge about their resources and environments (Carroll et al. 2021, 2020). The people- and purpose-oriented CARE principles are designed to complement the data-centric FAIR principles by ensuring that Indigenous data remain FAIR while centering Indigenous sovereignty (Mc Cartney et al. 2023). Implementing the CARE principles into FAIR data initiatives can ensure that data use respects Indigenous rights, serves a meaningful purpose, and promotes the wellbeing of Indigenous Peoples and Local Communities (IPLCs) (Carroll et al. 2021; O'Brien et al. 2024).
Mc Cartney et al. (2023) established a roadmap for the lifecycle of eukaryotic biodiversity sequencing data following the CARE principles. It identifies actions required from researchers throughout each study to build sustainable partnerships with IPLCs. In the context of FAIR initiatives, it is important to develop culturally aware linked metadata. For example, generalizing or withholding culturally sensitive data, such as geographical coordinates of culturally important sites, is respected and accepted, which can be mapped under the terms dataGeneralization and informationWithheld in the FAIRe metadata checklist and other standards and databases (Chapman 2020). Information on access, use permission, and the person or organization owning and managing data rights, should be defined at the start of each project, and recorded under the terms accessRights and rightsHolder. Further works are needed in contextual metadata to document Traditional Knowledge Labels and Notices (Liggins et al. 2021; “Local Contexts – Grounding Indigenous Rights” 2023), provenance about place and people (Mc Cartney et al. 2023), and the cultural importance of species (Reyes-García et al. 2023). Meetings, workshops and collaboration across various stakeholders including IPLCs and eDNA communities can elucidate the benefits of eDNA data sharing in various aspects, exchange more information and raise discussions. All these efforts can harmonize FAIR and CARE, establish and retain the link between people and nature, and serve as a vehicle for knowledge transfer, capacity development, two-way science and biodiversity conservation.
6 Conclusion
Our work advances the goal to make eDNA data FAIR by identifying essential data components and formats, developing an eDNA-specific, comprehensive metadata checklist that builds on existing biodiversity and molecular data standards, and providing data formatting guidelines for eDNA communities. We also outline future steps that support effective integration of FAIR practices within these communities. Looking ahead, we are focused on creating tools to make FAIR procedures automated and hassle-free, ensuring that FAIR data practices become the norm among eDNA practitioners. This is an ongoing, community-driven project, continuously refined with input from broad communities to enhance and adapt the proposed guidelines presented here, which is necessary in the rapidly evolving eDNA field.
Author Contributions
Conceptualization: M.T., O.B., M.L., G.J., T.G.F., and J.P. Validation: all authors. Data curation/visualization: B.D., C.V.R., K.S., K.W., L.R.H., L.R.T., M.L.D.L., M.T., N.A.P., N.D., C.C.H., and R.H. Software: M.T., N.D., S.F., and S.Y.Y., with feedback provided by K.S., K.W., L.R.H., L.R.T., M.L.D.L., N.A.P., R.H., S.P.J., T.S.J., and T.G.F. Writing – Original Draft: M.T. Writing – Review and Editing: all authors. Project administration: M.T. Funding acquisition: O.B.
Acknowledgments
We deeply and gratefully acknowledge the invaluable contributions of Prof. Louis Bernatchez, who passed away in 2023. Prof. Bernatchez's enthusiasm for the topic, plus his extensive networks, were pivotal in establishing the FAIRe working group. His commitment to the fields of molecular ecology, conservation, and eDNA, as well as his dedication to integrating FAIRe guidelines into journal data-sharing policies, laid the foundation for this initiative. We extend our thanks to all participants of the eDNA biodiversity data workshop, co-hosted by GBIF and CSIRO in Canberra in October 2023. The workshop facilitated vibrant discussions on the opportunities and challenges in advancing FAIR eDNA practices, fostering collaboration across the global eDNA and biodiversity database communities. Special thanks go to Jodie van de Kamp, Nerida Wilson, Haylea Power, Chloe Anderson, and Alyssa Budd for their technical expertise and valuable advice on eDNA, taxonomy, and database development. We acknowledge Ooid Scientific (https://ooidscientific.com/) for their design of the infographic (Figure 1). This project was funded by the Environomics Future Science Platforms of the Commonwealth Scientific and Industrial Research Organization (CSIRO). Additionally, the contributions of T.G.F. and T.S.J. to this project have received funding from the European Union's Horizon Europe research and innovation programme under grant agreement No 101057437 (BioDT project, https://doi.org/10.3030/101057437). Views and opinions expressed are those of the author(s) only and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them. L.R.T. was supported by award NA21OAR4320190 to the Northern Gulf Institute from NOAA's Office of Oceanic and Atmospheric Research, U.S. Department of Commerce.
Disclosure
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data and resources (including the FAIRe metadata checklist, templates, example datasets, codes) derived from this article are available in the Supporting Information of this article and the GitHub repositories (https://github.com/orgs/FAIR-eDNA/repositories). All the above resources are also accessible via https://fair-edna.github.io/index.html.




