Efficacy and safety of liraglutide added to insulin therapy in elderly patients with type 2 diabetes
Summary
Introduction
The combination of GLP-1 receptor agonists and insulin is effective in type 2 diabetes (T2D) treatment. However, its longitudinal efficacy and safety in elderly patients have not been established. We evaluated whether liraglutide (Lira) added to insulin therapy safely improved glycaemic control in T2D patients aged >65 years.
Methods
Twenty T2D patients receiving insulin were recruited, and Lira was added to their treatment regimen. Before and 6 months after Lira was added, we assessed the metabolic parameters and continuous glucose monitoring (CGM) data.
Results
Six months after Lira was added, the levels of HbA1c and glycated albumin and body weight were significantly improved, despite the daily doses and number of insulin injections per day being reduced. CGM analysis revealed that the SD and AUC of glucose >180 mg/dL were significantly decreased; the proportion of hypoglycaemic events was not increased.
Conclusion
Lira administration safely improved glycaemic control and reduced body weight. Lira added to insulin therapy may improve the quality of life in elderly T2D patients undergoing insulin therapy, especially those requiring social support.
1 INTRODUCTION
With the ageing of society, the proportion of individuals with diabetes is on the rise.1-4 Glycaemic goals for diabetes patients must be applied after adjustment for individual comorbidities and patient factors. It is necessary to consider other underlying comorbidities and physical activity when setting a glycaemic target and determining appropriate treatment, particularly for older adults.5 Various comorbidities such as renal dysfunction, neuropathy, vision and hearing impairments, and cognitive dysfunction affect the choice of therapeutic approach for diabetes. While insulin treatment is promising in reducing blood glucose levels, it may induce weight gain and hypoglycaemic events that impair the quality of life.6 The combination of GLP-1 receptor agonists (GLP-1RA) and insulin is known to be effective in type 2 diabetes (T2D) treatment, improving glycated haemoglobin (HbA1c) levels, decreasing the relative risk of hypoglycaemia and preventing weight gain.7 Liraglutide (Lira), a once-daily GLP-1RA, in combination with intensive insulin therapy, improves weight control and glycaemic variability.8 Since older adults who develop diabetes may have coexisting chronic illnesses, cognitive dysfunction and functional disabilities, they often need the assistance of caregivers for the injection of insulin, especially in cases where multiple daily injections are required.9 However, the longitudinal efficacy and safety of Lira for elderly T2D patients have not been established. Here, we evaluated whether Lira added to insulin therapy safely improved glycaemic control and whether it enabled the simplification of treatment for T2D patients older than 65 years.
2 MATERIALS AND METHODS
2.1 Study design and ethics
The present study had a single-centre, single-arm, open-label design in a clinical setting and was conducted at the Center Hospital, National Center for Global Health and Medicine, between July 2015 and November 2016. The study protocol was approved by the ethical committee of the National Center for Global Health and Medicine (registration no. NCGM-G-001901-00). Written informed consent was obtained from all participants.
2.2 Participants and study assessment
Twenty patients with T2D who were over the age of 65 years and were treated with insulin in our hospital, with or without oral hypoglycaemic agents, were recruited. Before the administration of Lira, we obtained data on metabolic parameters, such as the total daily dose of insulin (TDD), number of insulin injections, HbA1c level, glycated albumin (GA) level, body weight (BW) and body mass index (BMI). In addition, we performed continuous glucose monitoring (CGM) to obtain data related to fluctuations in the glucose levels, such as the average glucose levels, standard deviation (SD), mean amplitude of glycaemic excursions (MAGE), and the percentage of values below and above the target range (70-180 mg/dL) of glucose. Subsequently, Lira was administered to the participants and titrated up to 0.9 mg in combination with an insulin regimen. If the administration of 0.9 mg of Lira could not be achieved due to patients’ physical issues, the maximum tolerable dose for each patient was administered. If necessary, the dosages of insulin and oral hypoglycaemic agents were adjusted, targeted at the levels of fasting blood glucose (100-140 mg/dL). Six months after Lira was added, data on the metabolic parameters and CGM data were obtained again and were compared to those obtained before Lira administration was started.
2.3 Statistical analysis
A sample size was calculated based on the TDD and the number of insulin injections. It was found that a sample size of 16 patients would be sufficient to detect a reduction in TDD to 50% of the dosage before adding Lira, assuming a standard deviation of 8, α = 0.05 and power of 85%. Additionally, we determined that the enrolment of six patients would be sufficient to detect a reduction in the number of insulin injections from 4 to 2 times per day, assuming a standard deviation of 1.2, α = 0.05 and power of 85%. Therefore, we aimed to include 20 patients in this study based on those two criteria. Data are presented as median values and interquartile ranges (IQRs). A Wilcoxon test was used to test the differences between the two indicated groups. The numbers of insulin injections were compared using Fisher's exact test. For both analyses, P < 0.05 was considered statistically significant. All statistical analyses were conducted using STATA software (StataCorp LLC, College Station, TX).
3 RESULTS
3.1 Baseline characteristics
A total of 20 patients with T2D participated in this study. The participant characteristics are presented in Table 1. At the baseline, the average age of the patients was 76 (70-77) [median (IQR)] years, diabetes duration was 14 (9.8-25.0) years, BW was 60.0 (55.4-72.8) kg, BMI was 25.2 (20.8-26.8) kg/m2, HbA1c level was 8.4 (7.5-9.2) %, and fasting serum C-peptide level was 2.2 (1.4-2.4) ng/mL, indicating that they tended to be overweight, and their endogenous insulin secretion was relatively preserved. Pancreatic enzyme levels were not evaluated in this study, and haptic enzyme levels were within normal ranges except for one patient with mild liver cirrhosis caused by hepatitis B virus (data not shown). Three patients had a medical history of cardiovascular diseases, such as an old myocardial infarction, atrial fibrillation and peripheral artery disease. Eleven of the 20 patients had been injecting insulin four times per day at the baseline, and the TDD was 17 (11.8-23.3) units/d. The average tolerable dose of the participants was 0.87 mg/d of Lira.
| Age (y) | 76 (70-77) |
| Sex (male/female) | 13/7 |
| Duration of diabetes (y) | 14 (9.8-25.0) |
| Body weight (kg) | 60 (55.4-72.8) |
| Body mass index (kg/m2) | 25.2 (20.8-26.8) |
| Fasting plasma glucose (mg/dL) | 133 (107-158) |
| HbA1c (%) | 8.4 (7.5-9.2) |
| Glycated albumin (%) | 23.4 (20.5-27.8) |
| Fasting plasma C-peptide (ng/mL) | 2.2 (1.4-2.4) |
| C-peptide index | 1.4 (0.95-1.92) |
| History of cardiovascular diseases (yes/no) | 3a/17 |
| Number of insulin injections per day (4/3/2/1) | 11/1/4/4 |
| Total daily dose of insulin (units/d) | 17 (11.8-23.3) |
- Data are expressed as median (interquartile range).
- Each patient had old myocardial infarction, atrial fibrillation and peripheral artery disease, respectively.
3.2 Changes in the metabolic parameters after the addition of Lira to insulin therapy
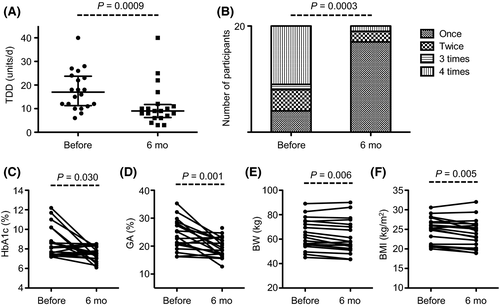
Six months after Lira was added, the TDD significantly decreased from 17.0 (11.8-23.3) [median (IQR)] to 9.0 (6.8-11.3) units/d (P = 0.0009) (Figure 1A). The number of insulin injections per day also significantly decreased (P = 0.0003). While 11 out of 20 patients received insulin injections four times per day at the baseline, 17 received them only once a day 6 months after Lira was added (Figure 1B). Despite the reduction in the TDD and number of insulin injections, the levels of HbA1c [8.4 (7.5-9.2) to 7.5 (7.0-8.2) %, P = 0.030] and GA [23.4 (20.5-27.8) to 19.1 (17.1-22.3) %, P = 0.001] were significantly improved. Furthermore, the BW [60.0 (55.4-72.8) to 56.7 (52.2-70.5) kg, P = 0.006] and BMI [25.2 (20.8-26.8) to 23.5 (20.2-26.1) kg/m2, P = 0.005] were significantly decreased 6 months after Lira was added (Figure 1C-F). Notably, all four patients who were administered sulfonylurea could discontinue the agent in the process of increasing the doses of Lira. These data suggest that Lira improves glycaemic control despite the reduction in the number of insulin injections, the dosage of insulin and the amount of oral hypoglycaemic agents.
3.3 Favourable effects of Lira on glucose fluctuation
Continuous glucose monitoring was performed to evaluate the degree of fluctuations in the glucose levels. In the CGM analysis, the SD of the glucose levels significantly improved from 35.1 (29.7-42.8) to 29.1 (20.7-40.1) [median (IQR)] (P = 0.018) and the average glucose levels tended to decrease [163 (137-179) to 146 (127-154) mg/dL, P = 0.076] 6 months after the addition of Lira. Moreover, the percentage of glucose levels above 180 mg/dL was significantly decreased [5.6 (2.55-17.4) to 1.65 (0.0-7.85), P = 0.003] (Table 2). These data indicate that Lira improves not only HbA1c levels, but also the degree of blood glucose fluctuations, even after a decrease in the dosage of insulin injections and cessation of sulfonylurea in elderly T2D patients.
| Before the addition of Lira | 6 mo after the addition of Lira | P value | |
|---|---|---|---|
| Average glucose (mg/dL) | 163 (137-179) | 146 (127-154) | 0.076 |
| SD (mg/dL) | 35.1 (29.7-42.8) | 29.1 (20.7-40.1) | 0.018 |
| MAGE (mg/dL) | 73.8 (56.3-91.4) | 49.7 (41.2-87.2) | 0.332 |
| % < 70 (%) | 0 | 0 | NS |
| % > 180 (%) | 5.6 (2.55-17.4) | 1.65 (0-7.85) | 0.003 |
- CGM, continuous glucose monitoring; SD, standard deviation; MAGE, mean amplitude of glycaemic excursions; NS, not significant; % < 70, the percentage of glucose levels below 70 mg/dL; % > 180, the percentage of glucose levels above 180 mg/dL.
- Data are expressed as median (interquartile range).
3.4 Safety of the combination therapy using Lira and insulin
After the addition of Lira, the dosages of oral hypoglycaemic agents and insulin were appropriately decreased, targeted at the levels of fasting or pre-meal blood glucose (100-140 mg/dL). Consequently, there were no hypoglycaemic events, hypoglycaemic symptoms and/or self-monitoring blood glucose levels <70 mg/dL not only before but also during the administration of Lira. Furthermore, in the results of the CGM analysis, glucose levels below 70 mg/dL were not detected at all during the administration of Lira (Table 2). In terms of the adverse events related to gastrointestinal disorders, although 2 out of 20 patients experienced nausea and diarrhoea, they could continue receiving Lira by reducing the dose to 0.6 mg/d. No patients complained of constipation. No other adverse events, such as liver dysfunction, kidney dysfunction and cardiovascular complications, were reported.
4 DISCUSSION
The present study demonstrated that the administration of Lira could safely improve glycaemic control in elderly patients, in spite of the decrease in the insulin dose and number of insulin injections. A previous study showed that GLP-1RA in combination with basal insulin therapy was an effective treatment option with respect to weight loss and fewer hypoglycaemic events.10
Our study focused on T2D patients older than 65 years, for the evaluation of the efficacy and safety of the combination therapy using GLP-1RA and insulin, since hypoglycaemic events related to diabetes treatment are relatively common in older patients.11 In particular, multiple insulin injections and sulfonylurea administration are associated with a risk for hypoglycaemia. Throughout the present study, no hypoglycaemic events were detected even in the data from CGM, suggesting that the addition of the GLP-1RA, Lira, to insulin therapy could safely improve glycaemic control even in elderly patients, if the dosages of insulin and oral hypoglycaemic agents are appropriately adjusted.
As demonstrated in the present study, the decreased number of insulin injections may improve the quality of life in elderly T2D patients. Furthermore, the formulation of a simplified therapeutic option for diabetes may be beneficial in terms of treatment adherence for elderly individuals, particularly those who require social support. In addition, the TDDs were decreased 52.9%, which was a greater reduction than that found in a previous study. 12 GLP-1RAs could reduce fasting blood glucose through the inhibition of glucose-dependent glucagon secretion from pancreatic alpha cells and gluconeogenesis in the liver and reduce postprandial blood glucose by delaying gastric emptying.13 Those effects of GLP-1RAs may be associated with the reduction in TDD. The reduction in the degree of fluctuation in glucose levels, as shown in the CGM analysis results, may also improve the quality of life and treatment adherence among elderly T2D patients and may be helpful for medical staff members who provide support to elderly people with diabetes. In our CGM analyses, the frequencies of hyperglycaemia were significantly decreased. The reduction in both fasting and postprandial blood glucose by Lira might contribute to the reduction in hyperglycaemia.14
The addition of Lira to insulin therapy led to reduced BWs in our study. This reduction may have been achieved because of the effects of Lira itself and reductions in the doses of insulin or sulfonylurea. Insulin therapy induces a certain amount of BW gain in T2D patients,15 whereas GLP-1 RAs might reduce BW by inducing satiety in the hypothalamus.13, 16 In our study, the reduction in TDD may be partially responsible for BW reduction. The effects of Lira on weight loss are also associated with delays in gastric emptying.17 It has been demonstrated that the administration of Lira can reduce the proportion of visceral adipose tissue.18 With respect to weight loss for the elderly, attention should be paid to disuse syndrome which leads to a loss of skeletal muscle volume. Among our study's participants, the daily activity was not impaired, perhaps because they were originally relatively overweight. Moreover, a recent study showed that Lira may be beneficial for cardiovascular and renal function.19, 20
The present study had certain limitations. Firstly, it had a single-arm design, with a relatively low number of participants and short period (6 months) of observation. In addition, although our target glucose level was 100-140 mg/dL before each meal, we did not use any strict algorithm for glucose adjustment. Additionally, the dosage of insulin was adjusted according to the glucose levels during the study period. Therefore, randomized studies with a higher number of participants, longer periods, and strict algorithms for the adjustment of insulin and oral hypoglycaemic agents should be considered in future.
In conclusion, the addition of Lira to insulin therapy improved glycaemic and BW control, without increasing the number of hypoglycaemic events in elderly T2D patients. The reduction in the number of insulin injections may be beneficial for the elderly individuals, especially those who require social support.
ACKNOWLEDGEMENTS
The authors are grateful to Maya Matsushita, Koji Maruyama and Keisuke Ueno for their technical assistance as well as patient volunteers who took part in this study.
CONFLICT OF INTEREST
The authors declare that there are no conflict of interests associated with this manuscript.
AUTHOR CONTRIBUTIONS
M.T. designed the study, researched the data and wrote the manuscript. D.C. contributed to the organization of the study and discussion of the data and reviewed/edited the manuscript. M.N. contributed to discussions and the critical review of the manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
ETHICS STATEMENT
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki Declaration (revised in 2008). The study protocol was approved by the ethical committee of the National Centre for Global Health and Medicine (registration no. NCGM-G-001901-00). Written informed consent was obtained from all participants.
DATA AVAILABILITY
The data sets collected in this study are available from the corresponding author on request.




