Elevated unanticipated acoustic startle reactivity in dyslexia
Abstract
People with dyslexia, a neurodevelopmental disorder of reading, are highly attuned to the emotional world. Compared with their typically developing peers, children with dyslexia exhibit greater autonomic nervous system reactivity and facial behaviour to emotion- and empathy-inducing film clips. Affective symptoms, such as anxiety, are also more common in children with dyslexia than in those without. Here, we investigated whether the startle response, an automatic reaction that lies at the interface of emotion and reflex, is elevated in dyslexia. We measured facial behaviour, electrodermal reactivity (a sympathetic nervous system measure) and emotional experience in response to a 100 ms, 105 dB unanticipated acoustic startle task in 30 children with dyslexia and 20 comparison children without dyslexia (aged 7–13) who were matched on age, sex and nonverbal reasoning. Our results indicated that the children with dyslexia had greater total facial behaviour and electrodermal reactivity to the acoustic startle task than the children without dyslexia. Across the sample, greater electrodermal reactivity during the startle predicted greater parent-reported anxiety symptoms. These findings contribute to an emerging picture of heightened emotional reactivity in dyslexia and suggest accentuated sympathetic nervous system reactivity may contribute to the elevated anxiety that is often seen in this population.
1 INTRODUCTION
Dyslexia is a neurodevelopmental disorder that is diagnosed when people exhibit specific and enduring challenges with reading alongside adequate education, motivation and intelligence (Lyon et al., 2003). Reading difficulties in dyslexia are associated with poor phonological awareness (Stanovich, 1988; Swan & Goswami, 1997), slow automatized naming (Wolff et al., 1990) and limited verbal working memory (Berninger et al., 2006). People with dyslexia also differ from those without dyslexia in domains beyond language such as visual perception (Stuart et al., 2006; Talcott et al., 2002), auditory perception (Hämäläinen et al., 2013; Noordenbos & Serniclaes, 2015) and decision-making (Manning et al., 2022; Pereira et al., 2022). Affective symptoms, such as anxiety, are also common in dyslexia (Carroll et al., 2005; Haft et al., 2019; Rodriguez & Routh, 1989), with up to 70% of children with dyslexia reporting elevated anxiety symptoms and 20% meeting diagnostic criteria for an anxiety disorder (Nelson & Harwood, 2011; Wilson et al., 2009). In many children with dyslexia, the co-occurrence of affective symptoms and reading difficulties is associated with deleterious outcomes including lower well-being, self-esteem and academic achievement (Carroll & Iles, 2006; Hossain et al., 2021).
The reasons why affective symptoms so often arise in dyslexia are not well understood, but growing evidence suggests emotion systems are involved (Davis et al., 2018; Sturm et al., 2021). Emotions are brief states accompanied by changes in autonomic nervous system activity, facial behaviour and experience (Cowen et al., 2019; Levenson, 2003; Pasquini et al., 2022; Saper, 2002). Although usually adaptive—shaping our thoughts, feelings and actions—emotions can be problematic when intense or sustained and lead to affective symptoms (Kring & Sloan, 2009). Given that emotions are difficult to evaluate, they are often assessed with questionnaires that rely on verbal descriptions or endorsements of behaviours and internal states. Laboratory-based assessments that measure facial behaviour and autonomic nervous system activity offer an objective, nonverbal window into emotion system functioning, but such studies in dyslexia are limited. In our prior research, we found children with dyslexia had greater facial behaviour and autonomic reactivity than their well-reading counterparts in response to emotion (Sturm et al., 2021) and empathy (Palser et al., 2021) inducing film clips. Not only did the children with dyslexia display higher total facial behaviour than their typically developing peers to emotion-inducing film clips, but they also exhibited greater increases in electrodermal activity (a measure of sympathetic nervous system reactivity) and respiration rate (Sturm et al., 2021). These findings had real-world behavioural correlates: in the children with dyslexia, those with higher total facial behaviour in the laboratory had better parent-reported social skills yet greater symptoms of anxiety and depression in their everyday lives. Taken together, these findings suggested that elevated emotional reactivity in dyslexia may promote interpersonal strengths but also increase vulnerability to affective symptoms.
Film clips are a powerful means of eliciting emotions (Levenson et al., 2008), but even simple film stimuli require viewers to process dynamic streams of verbal and nonverbal information. Given that children with dyslexia can have difficulties in language as well as other areas of cognitive functioning, it is possible that the elevated behavioural and autonomic reactions that we found in our prior studies were due to differences in how the children with dyslexia interpreted or understood the film clips. To rule out this alternative explanation, we conducted the present study to examine whether children with dyslexia also have heightened reactivity to simpler affective stimuli. The unanticipated acoustic startle—a loud aversive noise that occurs without warning—is an effective paradigm for assessing a form of emotional reactivity that requires less higher-order cognitive and verbal processing. There are two primary approaches in the startle literature. Unanticipated acoustic startle, which presents a single trial to provide a robust measure of baseline startle reactivity (Goodkind et al., 2010; Hagemann et al., 2006; Roberts et al., 2004; Soto et al., 2005), and startle modulation, which examines whether affective information modifies startle responses to repeated acoustic stimuli across multiple trials (e.g., Bradley et al., 2018; Filion et al., 1998; Lang et al., 1990). Here, in the first investigation of startle reactivity in dyslexia, we used an unanticipated acoustic startle task, providing a measure of emotional reactivity free from anticipation. As reactions to the unanticipated acoustic startle stimulus are less influenced by task demands, this paradigm is ideal for investigating whether behavioural and autonomic reactions to simple affective stimuli are elevated in dyslexia.
The startle response lies at the interface of reflex and emotion (Ekman et al., 1985). Although some people startle more easily than others (Brown et al., 1991), the startle response is a highly evolutionarily conserved defensive reaction that is present across species (Davis, 1984; Hoy et al., 1989; Koch, 1999; Liu & Hale, 2014) and in humans of all ages including infants (Balaban, 1995), children (Grillon et al., 1997), adults (Grillon et al., 1994) and elders (Sturm et al., 2006). Mediated by brainstem circuits that do not require input from cortical systems (Davis et al., 1982), the startle response is accompanied by a rapid sequence of involuntary motor and autonomic nervous system changes that are highly consistent across people, contexts and cultures (Sokolov, 1963; Soto et al., 2005). The initial reaction begins within 500 ms of the stimulus and includes behaviours such as lip stretching, eye closure, neck flexion, forward movement, shoulder raising and head jerking, actions that may help to protect from potential threat (Ekman et al., 1985). In the seconds that follow the initial reaction, a secondary self-conscious reaction may arise as a person self-reflects and imagines others evaluating their initial response (Ekman et al., 1985; Roberts et al., 2004; Sturm et al., 2006). When people are startled, increases in sympathetic nervous system activity (Sturm et al., 2006) mobilize internal resources and promote ‘fight or flight’ behaviours that are important for survival (Gray, 1998, 2003). While robust sympathetic reactivity to a potential threat can prepare an individual to respond to danger, dysregulated sympathetic reactivity that does not quickly return to baseline can be maladaptive and underlie affective symptoms such as anxiety (Grillon et al., 1994; Grillon & Baas, 2003; Kaviani et al., 2004; Rosen & Schulkin, 1998). Whether heightened sympathetic nervous system reactivity contributes to anxiety in dyslexia is unknown.
In the present study, we investigated whether startle reactivity was elevated in children with dyslexia. We measured facial behaviour, electrodermal activity and emotional experience in children with and without dyslexia in response to a 105 dB acoustic stimulus that was presented without warning. To examine whether children with higher sympathetic reactivity during startle had higher anxiety symptoms, we related the children's electrodermal reactivity during the startle task to parent-reported measures of anxiety.
2 METHODS
2.1 Participants
Fifty participants, 30 children with dyslexia and 20 typically developing children without dyslexia, were included in the present study. All participants were fluent English speakers between the ages of 7 and 13 years of age. The sample size for the current study was based on our previous work in children with dyslexia (Sturm et al., 2021), which found medium to large effect sizes (Cohen's D = 0.60–0.80; Cohen, 2016) for elevated emotional reactivity in similarly structured analyses (i.e., same number of covariates and interaction terms). The current study protocol was approved by the University of California, San Francisco (UCSF) Human Research Protection Program. Participants provided verbal assent, and their guardians provided written informed consent.
Participants were recruited through the UCSF Dyslexia Center, and those with a history of learning differences underwent a comprehensive multidisciplinary evaluation including a clinical interview and neurological examination as well as academic, neuropsychological and language testing. For inclusion in the dyslexia cohort, children were required to have a prior diagnosis of dyslexia and a confirmed diagnosis of dyslexia at the time of the study. Many of the children with dyslexia (63%) attended specialist schools for children with learning differences. The typically developing children, who did not have diagnosed or suspected learning differences, were recruited through local schools. They completed an abbreviated evaluation that included abridged academic, neuropsychological and language testing. All participants completed Matrix Reasoning, a test of nonverbal reasoning from the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), and performed above the 9th percentile (i.e., above the impaired range). Children were excluded if they had a history of acquired brain injury, known genetic condition that impacts cognition and development, psychiatric disorder or neurodevelopmental condition other than dyslexia.
The reported annual household incomes ranged from $60,000 to >$500,000, with a median income bracket of $250,000–299,999 (n = 12 declined to state their income), suggesting a relatively high socioeconomic status across the sample. Medication usage in the children was low. Two participants were taking allergy medication (one with dyslexia, one without), and one participant with dyslexia was taking stimulants at the time of the study. No participants reported taking anxiolytics, antidepressant medications or beta-blockers. See Table 1 for demographic and cognitive information.
| Measure | Dyslexia (n = 30) | Without dyslexia (n = 20) |
|---|---|---|
| Sex (male:female) | 12:18 | 10:10 |
| Race | 73% white, 3% multiracial, 23% NA | 75% white, 15% Asian or Pacific Islander, 10% multiracial |
| M (SD) | ||
| Agea | 10.24 (1.73) | 10.30 (1.74) |
| Nonverbal reasoningb | 70.32 (22.54) | 69.47 (22.23) |
| Letter-word identificationc | 31.22 (24.53) | - |
| Word attackc | 34.32 (26.31) | - |
| Sight word efficiencyd | 31.97 (30.82) | - |
| Phonemic decoding efficiencyd | 27.47 (26.54) | - |
| Reading ratee | 28.11 (24.40) | - |
| Reading accuracye | 15.18 (15.63) | - |
| Reading fluencye | 19.59 (17.62) | - |
| Reading comprehensione | 30.12 (20.53) | - |
- Note: Ratio is provided for sex and mean (M) and standard deviation (SD) are provided for all other variables.
- a Age reflects chronological age reported in years.
- b Nonverbal reasoning reflects the percentile score on Matrix Reasoning subtest of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999).
- c Letter-word identification and word attack reflect percentile scores on the Woodcock-Johnson IV (Schrank et al., 2014) subscales.
- d Sight word efficiency and phonemic decoding efficiency reflect percentile scores on the Test of One-Word Reading Efficiency—second edition (TOWRE-2; Torgesen et al., 2012) subscales.
- e Reading rate, accuracy, fluency and comprehension scores reflect percentile scores on the Grey Oral Reading Ability—fifth edition (GORT-5; Wiederholt & Bryant, 2012).
2.2 Academic assessment
Single-word reading was assessed with untimed Letter-Word Identification and Word Attack measures from the Woodcock-Johnson IV (Schrank et al., 2014) and the Test of One-Word Reading Efficiency—Second Edition, a timed measure (TOWRE-2; Torgesen et al., 2012). Paragraph reading was measured using the Grey Oral Reading Ability—Fifth Edition (GORT-5; Wiederholt & Bryant, 2012). Testing confirmed that all children with dyslexia had at least one low reading score (≤25th percentile). We used a relatively liberal cut-off because most of the children had received extensive remediation. Nevertheless, most of the children with dyslexia (87%) fell below the 10th percentile on at least one reading measure. Four participants did not complete all academic testing due to time constraints, but all had at least one very low reading score (<10th percentile) on the measures that were completed and so were retained in the sample.
2.3 Anxiety symptoms
Parents completed the Behaviour Assessment System for Children—Second Edition (BASC-2) child and adolescent parent rating scale forms (Reynolds & Kamphaus, 2004), based on the age of their child. This scale is a standardized, well-validated, multidimensional rating system that assesses a broad range of skills and personality traits in addition to adaptive and problem behaviours. The child form (ages 6–11) consists of 160 items, and the adolescent form (ages 12–21) consists of 150 items. The BASC-2 scoring algorithm standardizes participants' scores within their age group, allowing comparison of scores across a range of ages and both child and adolescents versions. Parents rated each item according to the frequency of the behaviour on a four-point scale, ranging from N (never), through S (sometimes), O (often), to A (almost always). Item raw scores were summed to obtain subscale scores for 14 behavioural domains. We focused on the ‘Anxiety’ subscale, which includes items such as: ‘Worries about things that cannot be changed’, ‘Is nervous’, ‘Appears tense’ and ‘Has panic attacks’. Item raw scores were summed, and subscale scores were converted into standardized T scores (M = 50; SD = 10). High scores represent more problematic behaviours; T scores between 60 and 69 are considered at-risk; and scores ≥70 are considered clinically significant. Scores were available for a total of 29 participants (22 with dyslexia, 7 without dyslexia); parents of the remaining participants declined to complete this measure.
2.4 Laboratory-based assessment
2.4.1 Procedure
Procedures were conducted in a well-lit testing room. Participants were familiarized with the testing environment and seated in a comfortable chair. Stimuli were presented on a 21.5 inch computer monitor placed 4.25 feet in front of them. All audio-visual instructions were presented using E-Prime (version 3.0, Psychology Software Tools, Pittsburgh, PA). During the assessment, the experimenter left the testing room, observing the participant from a nearby control room with a semi-concealed camera and communicating via an intercom system. Participants were informed they would be video-recorded prior to the start of the testing session. They completed a battery of tasks in the same order, which assessed resting baseline, startle reactivity and emotional reactivity, among other domains of emotion. Only the startle task was analysed in the present study. Following completion of the laboratory assessment, participants were debriefed by the experimenter during which they were given the opportunity to ask any questions.
2.4.2 Unanticipated acoustic startle task
Participants were instructed to relax and watch the screen but were not told what to anticipate during the task. An ‘X’ appeared on the screen when the pre-trial baseline began and remained for 60 s. After 60 s, the participants heard a 100 ms, 105 dB burst of white noise that was presented without warning through speakers located directly behind them. The volume of the stimulus was verified using a RadioShack Digital Sound Level Meter (RadioShack, San Salvador, El Salvador) in the testing room. Following the acoustic startle stimulus, the ‘X’ remained on the screen for a post-startle baseline that lasted 60 s.
2.4.3 Measures
Facial behaviour
Videotaped recordings of the unanticipated startle task were coded with the Dynamic Affective Behavioural coding system (DAB) using Noldus Observer version 14.0 software (Noldus Technologies, Leesburg, VA). We (SRH, CRV and VES) developed the DAB coding system to build on the Emotional Expressive Behaviour coding system (Gross, 1996), which captures a broad range of facial behaviours and bodily movements but lacks anatomically specific criteria for facial behaviours. The DAB coding system includes an expanded set of emotion categories and their corresponding facial movements, or action units, as defined by the Facial Action Coding System (FACS; Ekman et al., 2002).
The DAB coding system quantifies behaviours that correspond to 16 emotion categories for which there is empirical evidence for emblematic facial behaviours (e.g. Campos et al., 2013; Cordaro et al., 2018; Keltner et al., 2019; Matsumoto et al., 2008): interest, concentration, boredom, anger, sadness, disgust, fear, contempt, happiness/amusement, surprise, awe, pain, embarrassment, shame, sympathy and pride (see Supplementary Table 1 for details). All behaviours were rated on a three-point intensity scale: 1 (slight but noticeable), 2 (moderate) or 3 (strong). When no behaviour was present, the face was coded as ‘neutral’, and a 0 was recorded. Codes were mutually exclusive, and thus, blends of emotion were not permitted. Coders provided continuous ratings of the 30-second trial beginning with the startle stimulus, and the data were later exported on a second-by-second basis. One participant was missing facial behaviour data due to failure of the video recording equipment. Ninety four percent of the videos were rated by multiple coders; interrater reliability was excellent (Cohen's kappa = 0.85; Cohen, 1960; Fleiss, 1981).
To test our hypothesis that children with dyslexia would have greater emotional reactivity, we computed a total facial behaviour score by summing the total intensity scores of each code, as in previous studies (e.g., Sturm et al., 2021), across 17 s that began with and followed the startle stimulus. This time window, which was used in our previous work examining behavioural and physiological reactivity during the unanticipated startle task (Sturm et al., 2006), was sufficient to capture both the initial and secondary self-conscious startle responses. Higher scores indicated greater total facial behaviour. In follow-up analyses, we compared the groups on the total scores for each emotion category. Outliers were defined as participants with a total facial behaviour scores that fell outside ± three standard deviations from the group mean; this resulted in the removal of one participant with dyslexia from the facial behaviour analyses.
Electrodermal activity
Electrodermal activity was measured throughout the unanticipated startle task using Biopac Systems Inc. (biopac.com; California, USA) MP150 bioamplifiers and a computer equipped with data acquisition software. A Biopac GSR100c amplifier was used to pass a small voltage between two Ag/ACl Silver 8 mm EL258s shielded electrodes (using an electrolyte of sodium chloride) attached to the palmar surface of the middle phalanges of the ring and index fingers of the non-dominant hand. Room temperature in Fahrenheit was also recorded by the Biopac amplifier.
Electrodermal data were processed offline using a custom pipeline scripted in AcqKnowledge software (v5, biopac.com). The measures we focused on were skin conductance level (SCL; a tonic measure of basal sympathetic outflow) and skin conductance response (SCR; a phasic measure of sympathetic fluctuations). For SCR, algorithms identified and marked the signature components of the waveform, and these markers were then visually inspected for errors and noise.
Outliers in the raw physiological data were considered to be ± three standard deviations from the mean level during the trial; these periods were interpolated if their duration was 3 s or less and deleted if their duration was greater than 3 s. For each physiological channel, second-by-second averages were then exported for analysis. Two children without dyslexia were missing SCL data. We computed reactivity scores for each measure by subtracting the maximum level during the 60 s pre-startle baseline from the maximum level in a 17 s window that began with the startle stimulus. Any reactivity scores that fell outside ± three standard deviations from the group mean were also considered outliers and deleted, which resulted in the removal of one child with dyslexia from the SCL analyses.
Emotional experience
After the post-startle baseline period, participants responded to a series of questions. They rated how strongly they experienced various emotions (afraid, amused, anger, awe, disgust, embarrassment, excited, love, proud, sad and surprised) to the startle stimulus on a three-point scale of ‘Not at all’ (0), ‘A little’ (1) or ‘A lot’ (2) (e.g., ‘Did you feel afraid when you heard the sound?’). Participants provided verbal responses to these questions, which the experimenter recorded. We summed each participant's responses to the 11 emotional experience questions to generate a total emotional experience score.
2.5 Data analysis
Analyses were conducted in R Project, version 3.6.0 (R Core Team, 2017). We used independent samples t-tests (or the non-parametric Mann–Whitney equivalent when the data showed a significant deviation from the normal distribution on Kolmogorov–Smirnov tests) to assess group differences in age, nonverbal reasoning and anxiety. We used a Chi-square analysis to assess group differences in sex. We used multiple regression analyses controlling for age, sex and nonverbal reasoning to test for group differences in facial behaviour, electrodermal reactivity (i.e., SCR and SCL) and emotional experience. In the analyses of electrodermal reactivity, we included room temperature, time of day (AM/PM; Venables & Mitchell, 1996) and the interaction between time of day and sex (e.g., Venables & Mitchell, 1996) as additional covariates. Cohen's f2 is provided as an estimate of effect size (Cohen, 1988). We used Spearman correlations to examine associations among the emotion measures and their associations with anxiety. As our analyses of electrodermal activity included additional covariates, we performed leave-one-out cross-validation on models assessing group differences in SCR and SCL to assess the robustness of our results. Root mean squared error (RMSE) and mean absolute error (MAE) scores that are low indicate good model fit and that the models provide a good explanation of the data.
2.6 Transparency and openness
We report how we determined our sample size and all data exclusions, manipulations and measures in the study. This study design and analysis were not pre-registered. All analysis code is available at https://osf.io/xma7h/?view_only=c77a762e789a4556acf62cd09d1da5bc. Data are not publicly available because they contain information that could compromise the privacy of participants but will be shared with others for the purpose of research upon completion of a formal data sharing agreement, as required by our institution.
3 RESULTS
3.1 Demographic and group comparisons
There were no differences between the groups in age, W = 298, p = 0.976, sex, X2(1) = 0.17, p = 0.684, nonverbal reasoning, W = 254.5, p = 0.601, or anxiety, t(11.8) = 0.38, p = 0.707. The anxiety scores in both groups were low on average but ranged from the 2nd to 98th percentile, suggesting our sample captured a wide range of anxiety symptom burden (see Table 2).
| Dyslexia | Without dyslexia | |
|---|---|---|
| M (SD) | M (SD) | |
| Total facial behavioura | 14.15 (6.85) | 10.81 (7.01) |
| Skin conductance response reactivityb | 0.55 (0.42) | 0.33 (0.31) |
| Skin conductance level reactivityb | 1.36 (1.28) | 0.79 (0.44) |
| Total emotional experiencec | 3.20 (1.56) | 3.50 (1.54) |
| Anxietyd | 41.59 (30.03) | 37.71 (25.98) |
- Note: Means (M) and standard deviations (SD) are provided.
- a Total facial behaviour reflects summed behavioural responses across all emotion codes during the first 17 s following the acoustic startle stimulus.
- b Electrodermal activity reflects the group mean of maximal reactivity relative to maximal baseline levels during the first 17 s following the acoustic startle stimulus.
- c Total emotional experience is the summed total experience of all self-reported emotions.
- d Standardized scores of the parent-reported BASC-2 anxiety subscale.
3.2 Facial behaviour
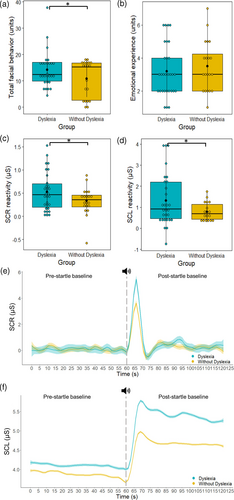
Children with dyslexia had greater total facial behaviour during the unanticipated startle task than children without dyslexia, Β = −4.36, t(44) = −2.22, p = 0.032, f2 = 0.12 (see Figure 1a). There was also a main effect of nonverbal reasoning whereby those with higher nonverbal reasoning had greater total facial behaviour, Β = 0.12, t(44) = 2.67, p = 0.011, f2 = 0.16. There were no main effects of age, Β = 0.38, t(44) = 0.65, p = 0.521, f2 = 0.01, or sex, Β = −1.52, t(44) = −0.75, p = 0.457, f2 = 0.01, on total facial behaviour. Follow-up analyses revealed both groups exhibited moderate to high levels of fear, interest and amusement, but there were no group differences in any specific facial behaviours (see Supplementary Table 2).
3.3 Electrodermal reactivity
Children with dyslexia had greater SCR reactivity during the unanticipated startle task than children without dyslexia, Β = −0.26, t(42) = −2.40, p = 0.021, f2 = 0.13 (see Figure 1c). There were also main effects of sex, Β = 0.53, t(42) = 2.84, p = 0.007, f2 = 0.17, with greater SCR reactivity in females than males; time of day, Β = 0.52, t(42) = 3.07, p = 0.004, f2 = 0.19, with greater reactivity in the afternoon; and an interaction between sex and time of day, Β = −0.53, t(42) = −2.38, p = 0.022, f2 = 0.12, with males experiencing greater variability in SCR reactivity between morning and afternoon than females. There were no main effects of age, Β < 0.01, t(42) = 0.10, p = 0.919, f2 = <0.01, nonverbal reasoning, Β < 0.01, t(42) = 1.16, p = 0.253, f2 = 0.02, or room temperature, Β = −0.14, t(42) = −1.49, p = 0.144, f2 = 0.05, on SCR reactivity. We verified our findings using leave-one-out cross-validation, which indicated good model fit for predicting SCR reactivity, RMSE = 0.38, MAE = 0.30.
Children with dyslexia had greater SCL reactivity during the unanticipated startle task than children without dyslexia, Β = −0.72, t(39) = −2.28, p = 0.029, f2 = 0.12 (see Figure 1d). There was also a main effect of sex, with females showing greater reactivity than males, Β = 1.15, t(39) = 2.08, p = 0.045, f2 = 0.10. There were no main effects of age, Β = 0.12, t(39) = 1.29, p = 0.206, f2 = 0.04, nonverbal reasoning, Β = 0.01, t(39) = 1.56, p = 0.127, f2 = 0.07, time of day, Β = 0.94, t(39) = 1.83, p = 0.075, f2 = 0.09, or room temperature, Β = −0.31, t = −1.11, p = 0.274, f2 = 0.03, on SCL reactivity. There was also no interaction between sex and time of day on SCL reactivity, Β = −1.19, t(39) = −1.79, p = 0.081, f2 = 0.08. Leave-one-out cross-validation indicated good model fit for predicting SCL reactivity, RMSE = 1.08, MAE = 0.86.
3.4 Emotional experience
There was no difference between the groups in total emotional experience during the unanticipated startle task, Β = 0.23, t(45) = 0.51, p = 0.610, f2 = 0.01 (see Figure 1b). There was a main effect of sex, with males reporting greater emotional experience than females, Β = −1.12, t(45) = −2.49, p = 0.017, f2 = 0.13, but no main effects of age, Β = −0.06, t(45) = −0.42, p = 0.680, f2 = <0.01, or nonverbal reasoning, Β < 0.01, t(45) = 0.04, p = 0.967, f2 = 0.03. Follow-up analyses revealed no group differences in the experience of specific emotions, but both groups reported feeling strong feelings of surprise and moderate feelings of fear on average (see Supplementary Table 3).
3.5 Associations among emotional reactivity measures and anxiety
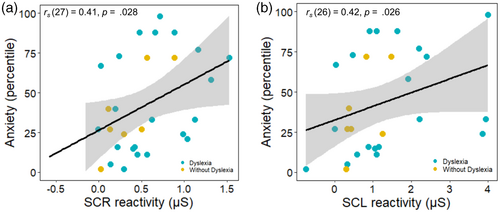
Across the sample, greater SCR reactivity, rs (27) = 0.41, p = 0.028 (see Figure 2a), and SCL reactivity, rs (26) = 0.42, p = 0.026 (see Figure 2b), during the unanticipated startle task were associated with higher anxiety. Higher anxiety was not associated with greater total facial behaviour, rs (26) = −0.10, p = 0.608, or total emotional experience, rs (27) = 0.12, p = 0.545, however.
4 DISCUSSION
In this study, we found children with dyslexia had greater total facial behaviour and electrodermal reactivity to an unanticipated acoustic startle task than children without dyslexia. Follow-up analyses revealed no group differences in any one type of facial behaviour, but both groups displayed moderate to high levels of fear, interest and amusement following the startle stimulus. The children with and without dyslexia reported similar emotional experiences during the startle and endorsed predominant feelings of surprise and fear. Although the children with dyslexia did not have higher parent-reported anxiety on average, higher electrodermal reactivity during the unanticipated startle task was associated with higher anxiety symptoms across the sample.
Our findings advance current knowledge of emotion system functioning in dyslexia. In our previous research, we found children with dyslexia had heightened emotional reactivity while viewing emotion-inducing film clips (Sturm et al., 2021). Although compelling, this finding left open the possibility that extraneous group differences in a cognitive process such as attention, working memory or appraisal during the task contributed to our results. Investigations of the startle response, a defensive reaction that lies at the interface of reflex and emotion (Ekman et al., 1985), provide an opportunity to examine emotional reactivity while invoking less cognitive processing. We found children with dyslexia have heightened behavioural and autonomic reactivity to a simple unanticipated acoustic stimulus, which is consistent with the elevated emotional reactivity that we have found in our previous studies of dyslexia. In many people, a secondary self-conscious response follows the initial startle reaction, which unfolds as they reflect on how they first responded to the noise (Ekman et al., 1985; Sturm et al., 2006). As we did not find group differences in any specific negative, positive or self-conscious facial behaviours, our results do not point to a singular elevation in the initial or secondary startle response in dyslexia. Given the accumulating evidence for accentuated emotional and social sensitivity in dyslexia (Davis et al., 2018; Palser et al., 2021; Sturm et al., 2021), future studies are warranted that more explicitly evaluate self-conscious emotional reactivity (Robins & Schriber, 2009).
During the startle task, electrodermal reactivity, as measured by changes in SCL and SCR, was higher in children with dyslexia than in those without, a result that suggests elevated sympathetic nervous system reactivity in dyslexia. While these findings are consistent with our prior work (Sturm et al., 2021), the present study uncovered a novel association between electrodermal reactivity and anxiety. Children with dyslexia experience anxiety symptoms at greater rates and severity than typically developing children (Carroll et al., 2005; Carroll & Iles, 2006; Haft et al., 2019; Rodriguez & Routh, 1989), but the causal associations between anxiety and reading difficulties are unknown. While some studies suggest anxiety results from academic struggles in dyslexia (Hossain et al., 2021; Riddick et al., 1999), others argue that anxiety interferes with reading and thereby increases risk for dyslexia (Bryan et al., 2004), and still others conclude there is a bidirectional relationship between the two domains (Grills-Taquechel et al., 2012; Van der Ende et al., 2016). The close connection between reading difficulties and affective symptoms is also evident in familial studies (Francis et al., 2019), which reveal shared genetic risk for dyslexia and anxiety (Willcutt, 2014). As early stress exposure may increase risk for dyslexia (Espin et al., 2019; Kershner, 2020; Zakopoulou et al., 2019), future longitudinal studies will be needed to understand how and why dyslexia and anxiety so often co-occur during development.
In children with dyslexia, autonomic nervous system functioning relates to distinct strengths and vulnerabilities. The sympathetic and parasympathetic branches of the autonomic nervous system act in concert to support numerous bodily functions including mobilizing resources for action as well as maintaining homeostasis (Craig, 2005; Levenson, 2003; Porges, 2001; Saper, 2002; Taylor et al., 2015). In a previous study, we found children with dyslexia had higher baseline respiratory sinus arrhythmia, a measure of vagal inhibition on the heart, than those without dyslexia (Palser et al., 2021). Greater resting activity in the parasympathetic nervous system, in general, is associated with a host of socioemotional advantages including greater compassion (Stellar et al., 2015), positive emotions (Isgett et al., 2017; Lischke et al., 2018), cooperation (Beffara et al., 2016) and creativity (Palser et al., under review). Consistent with the role of the parasympathetic nervous system in socioemotional engagement (Porges, 2001), we found children with dyslexia had greater cardiac deceleration during an empathy task, which suggested heightened interest in the emotions of others (Palser et al., 2021). While higher baseline respiratory sinus arrhythmia may promote strengths in empathy, we have also found elevated emotional reactivity (i.e., facial behaviour) relates to greater social skills in dyslexia (Sturm et al., 2021). Heightened responsivity in emotion systems, and in the sympathetic nervous system in particular, is not always advantageous; however, as suggested by the positive association, we detected between electrodermal reactivity and anxiety in the present study. As basal set points and phasic responses in the sympathetic and parasympathetic nervous systems may contribute to real-world behaviour, additional studies are needed to elucidate how autonomic nervous system functioning promotes adaptive social functioning or predisposes children with dyslexia to affective symptoms.
4.1 Limitations
The study has limitations to consider. Much remains unknown about the neural underpinnings of enhanced startle reactivity in dyslexia, and the present study did not include neuroimaging measures. The unanticipated startle response reflects involuntary activity in brainstem pathways, putatively originating in the nucleus reticularis pontis caudalis (Brown et al., 1991; Davis et al., 1982; Wilkins et al., 1986). While a brainstem-mediated reaction to an unanticipated startle stimulus may be modulated by input from other emotion-relevant regions including the orbitofrontal cortex (Roberts et al., 2004) and amygdala (Bechara et al., 1995; LaBar et al., 1995; Tranel & Hyman, 1990), the stereotyped autonomic and motor changes that characterize the startle response do not require higher-order cerebral input (Ashwal et al., 1990; Kurauchi et al., 1995). As systems with predominant representations in the right hemisphere of the brain support electrodermal reactivity (Critchley et al., 2000), future neuroimaging studies will need to tease apart whether right-lateralized systems that influence the brainstem (Davis et al., 2018), or brainstem-mediated systems themselves, underlie the greater unanticipated startle reactivity that we found in dyslexia.
We found evidence that startle reactivity is accentuated in dyslexia, but we cannot rule out the possibility that differences in previous life experiences influenced our results. Prior studies have found heightened startle responses in clinical disorders such as post-traumatic stress and panic disorders, which negative affective states can exacerbate (Grillon et al., 1998; Ray et al., 2009). Although the children with and without dyslexia did not differ in their mean anxiety levels, it is possible that those with dyslexia had more aversive experiences associated with learning differences that made them more sensitive to the startle stimulus than the children without dyslexia. While there is some evidence that dyslexia-associated risk genes alter brainstem responses to auditory stimuli including speech (Neef et al., 2017), it is likely that biological and experiential factors play a role in heightened startle reactivity in dyslexia. Future studies that investigate whether early life stressors in dyslexia influence startle reactivity, anxiety and sympathetic nervous system functioning are needed to elucidate how early experiences interact with biological vulnerability to increase affective symptom risk in dyslexia.
4.2 Conclusions
The current findings contribute to an emerging picture of emotion system differences in dyslexia. Our studies to date have shown that children with dyslexia not only have elevated emotional reactivity to emotion-inducing film clips (Sturm et al., 2021) but also have accentuated behavioural and sympathetic nervous system reactivity to an unanticipated acoustic task. Anxiety symptoms are highly prevalent, and often unremitting, in dyslexia and may reflect involvement of the sympathetic nervous system. Whether alterations in emotions are core diagnostic features of reading difficulties or secondary effects that emerge after aversive life experiences that children with learning differences face is unknown but is worthy of future investigation.
FUNDING INFORMATION
This work was supported by the Charles and Helen Schwab Foundation, including a Schwab Innovation Fund awarded to Eleanor R. Palser; the John Douglas French Alzheimer's Foundation; the National Institute on Aging, with awards to Virginia E. Sturm (R01AG073244, R01AG057204) and Bruce L. Miller (P30 AG062422); the National Institute of Neurological Disorders and Stroke, awarded to Maria Luisa Gorno Tempini (R01NS050915); and the National Institute on Deafness and Other Communication Disorders, awarded to Maria Luisa Gorno Tempini (K24DC015544).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data are not publicly available because they contain information that could compromise the privacy of participants but will be shared with others for the purpose of research upon completion of a formal data sharing agreement, as required by our institution.




