Use of TransFix™ cerebrospinal fluid storage tubes prevents cellular loss and enhances flow cytometric detection of malignant hematological cells after 18 hours of storage
Abstract
Flow cytometry is a sensitive method for detection of leptomeningeal localizations of hematological malignancies (LHM) in cerebrospinal fluid (CSF). Rapid processing of CSF is needed, as leukocyte numbers appear to decline quickly after lumbar puncture. The cell-stabilizing agent TransFix™ may enhance the detection of LHM in CSF by preventing cellular loss. To study the effects of TransFix on leukocyte numbers and the detection of LHM, we prospectively collected 99 CSF samples from patients with suspected or proven LHM in tubes with (i) TransFix; (ii) serum-containing medium; and (iii) no cell-stabilizing agents (native CSF). Presence of LHM and absolute leukocyte numbers were determined by flow cytometry after 30 minutes and 18 hours of storage. Leukocyte numbers in TransFix-stabilized CSF were higher than in the corresponding native samples at both time points (1.4× and 2.3× respectively, P < 0.0001 on each occasion). After 18 hours of storage, TransFix enhanced the detection of LHM in CSF. In all discordant paired observations (13/99, P = 0.005), the level of suspicion (classified as positive, suspicious, or negative) in CSF with TransFix was higher than in native CSF. We conclude that the use of TransFix-containing CSF storage tubes prevents cellular loss and enhances flow cytometric detection of LHM after 18 hours of storage. © 2013 International Clinical Cytometry Society.
The use of flow cytometric analysis of cerebrospinal fluid (CSF) in addition to morphologic CSF examination greatly increases the detection rate of leptomeningeal localizations of hematological malignancies (LHM), as compared to the use of morphology alone (1-9). Therefore, flow cytometric CSF analysis is recommended in all patients with suspected primary central nervous system lymphomas (PCNSL), patients with other hematological malignancies complicated by neurological signs and symptoms suggestive of LHM, and patients with aggressive non-Hodgkin lymphomas (NHL) who by themselves have an increased risk of developing LHM (10, 11).
As cell numbers in CSF are low (normally <5 cells/μL) and appear to decrease rapidly ex vivo (12, 13), CSF samples for flow cytometric analysis should be processed immediately after withdrawal (14, 15). Thus, to obtain optimal results, immediate availability of this technique is needed, as any delay in sample transportation or processing will lead to decreased CSF cell numbers and will likely reduce the sensitivity of analysis.
To address this problem, various cell stabilizing methods have been used to reduce cellular loss in CSF samples prior to flow cytometric analysis, as recently reviewed (14). However, only two of these methods have been used in large clinical studies aimed at the detection of LHM: (i) CSF withdrawal in tubes with 2 mL serum-containing medium (1) and (ii) CSF withdrawal in tubes with 0.2 mL of the commercially available fixative agent TransFix™ (4).
Addition of serum-containing medium to CSF prevents cellular loss up to at least 5 hours of storage (12). However, CSF storage tubes with serum-containing medium are not commercially available and have a limited shelf life of around 3 months. The use of TransFix CSF storage tubes may be an attractive alternative for cell stabilization in CSF because of their commercial availability and longer shelf life, that is, 1 year.
TransFix is a cellular stabilization reagent that contains a buffer, an aliphatic aldehyde, and heavy metal salts (16). It was originally designed to stabilize whole blood samples for flow cytometric counting of lymphocytes and their subsets such as CD4+ and CD8+ T cells and was first used by the United Kingdom National External Quality Assessment Service for leukocyte immunophenotyping (17). Later, TransFix became commercially available and was shown to stabilize whole blood samples for up to 10 days (18). TransFix in combination with ethylenediaminetetraacetic acid (EDTA) has also been used to stabilize CSF samples to enable overnight shipping to a central flow cytometry facility (4).
Although the above studies suggest that cell stabilization with serum-containing medium or TransFix may enhance the flow cytometric detection of LHM in CSF (1, 4), no studies have been published that directly compare CSF stabilization with TransFix with the use of serum-containing medium or native CSF. Therefore, the clinical significance of the use of TransFix or serum-containing medium for flow cytometric CSF analysis is unknown.
In this study, we prospectively investigated the effects of TransFix on the detection of LHM in CSF and CSF cell numbers by flow cytometry. CSF samples were processed immediately (i.e., 30 minutes after withdrawal) and after overnight storage (i.e., after 18 hours). Results were compared with those of simultaneously collected CSF samples that were stabilized with serum-containing medium and simultaneously collected CSF samples without cell stabilizing agents (native CSF).
MATERIALS AND METHODS
Sample Collection and Storage
From January 2011 onward, 99 diagnostic and follow-up CSF samples were obtained at the Erasmus University Medical Center/Daniel den Hoed Cancer Center from 43 patients who (i) underwent lumbar puncture for flow cytometric CSF analysis to test for LHM and (ii) provided written informed consent. This study was performed according to the Declaration of Helsinki, and its procedures were approved by the local ethics committee. In addition to the CSF drawn for conventional tests and procedures, a total of approximately 3 mL extra CSF was directly collected in three polypropylene tubes: ∼1 mL in a tube with 0.2 mL TransFix/EDTA (Caltag Medsystems, Towcester, UK), ∼1 mL in a tube with 2 mL serum-containing medium (Roswell Park Memorial Institute [RPMI]-1640 with 35 mM HEPES, 1 mM l-glutamine, 2% penicillin/streptomycin, 5% heat-inactivated fetal bovine serum, and 2,500 IU heparin), and ∼1 mL in a tube without cell-stabilizing agent (native CSF). CSF was drawn in these three tubes in alternating order to avoid a possible influence of the order in which the tubes were filled. Each of the three tubes was equally split into an additional two sterile polypropylene tubes: one of these tubes was processed 30 minutes after withdrawal; the other was stored at 4°C overnight (18 hours). Before and after splitting of the samples, the weight of the tubes was recorded to verify CSF volumes.
Flow Cytometry
Detailed procedures for CSF processing (15), determination of absolute cell numbers (12, 19), and to test for LHM (1, 14) have been published elsewhere. Briefly, CSF cells were concentrated by centrifugation (8 minutes, 450g) and resuspended in 100 µL phosphate buffered saline (PBS). In cases where surface immunoglobulins (sIg) had to be detected, cells were washed with PBS. Six-color antibody panels were chosen based on clinical information, and—if available—results of previous histological or flow cytometric analyses. All panels included anti-CD45 peridinin chlorophyll protein (PerCP; clone 2D1, Dako, Glostrup, Denmark) to distinguish leukocytes from debris and other cells. In addition, we used anti-CD4 fluorescein (FITC; clones SK3+SK4), anti-CD8 FITC (clone SK1), anti-CD5 R-phycoerythrin (PE; clone L17F12), anti-CD13 PE (clone L138), anti-CD34 PE (clone 8G12), anti-CD10 PE (clone HI10a), anti-CD19 PE (clone 4G7), anti-CD4 PE-Cy5 (clone SK3), anti-CD19 allophycocyanin (APC; clone SJ25C1), anti-CD14 APC-H7 (clone MΦP9), anti-CD19 APC-Cy7 (clone SJ5C1), and anti-CD20 APC-Cy7 (clone L27; all from BD Biosciences); anti-sIg κ PE (clone R0436; Dako), anti-sIgκ FITC (clone HP6156; Lucracon, Stuttgart, Germany); anti-sIgλ FITC (clones HP6062/6054; both from Invitrogen, Carlsbad, CA); anti-sIgλ PE (cat. 2072-09) and anti-sIgM PE (cat. 2020-09; both from SouthernBiotech, Birmingham, AL); anti-CD5 PE-Cy7 (clone BL1a) and anti-CD10 PE-Cy7 (clone ALB1; both from Beckman Coulter, Fullerton, CA); and anti-CD7 APC (clone 124-1D1) and anti-CD3 APC-eFluor780 (clone UCHT1; both from eBioscience, San Diego, CA). Fluorochrome-conjugated antibodies were added to 100 µL of the cell suspension, incubated for 15 minutes in the dark, washed, and resuspended in 100 µL PBS. To determine absolute cell numbers, 100 µL of Cyto-Cal Count Control counting beads (Duke Scientific Corporation, Palo Alto, CA) was added. Listmode data were acquired on a FACSCanto flow cytometer (BD Biosciences).
Data Analysis
An example of our gating strategy to detect LHM and determine absolute numbers of leukocytes and their subsets is shown in Figure 1. To detect LHM, listmode data were analyzed with FACSDiva software (BD Biosciences). Clusters of >25 events fulfilling criteria for malignant populations were classified as positive, clusters of 10–25 events as suspicious, and below 10 events as negative, as described before (1, 15). Absolute cell numbers and fluorescence intensities were determined with FCS express software (De Novo Software, Los Angeles, CA). Relative cell numbers were calculated by dividing the absolute cell number of a particular subset, time point, and storage condition by the absolute cell number of the same subset in CSF with serum-containing medium at 30 minutes. Discordances in the detection of LHM in CSF with TransFix versus the simultaneously collected control CSF samples were compared with the McNemar test using SPSS version 17.0.2 (IBM, Chicago, IL). Cell numbers as well as fluorescence intensities of TransFix-treated CSF cells versus CSF cells from the paired control samples were compared with the Wilcoxon signed rank test using Prism version 5 (GraphPad, San Diego, CA). The effects of TransFix or serum-containing medium on the detection of LHM, cell numbers, and fluorescence intensities in follow-up samples were assumed to be independent of those in the initially obtained samples of the same patients. Results of continuous variables were expressed as median and range, unless otherwise specified. Two-sided P values <0.05 were considered significant.
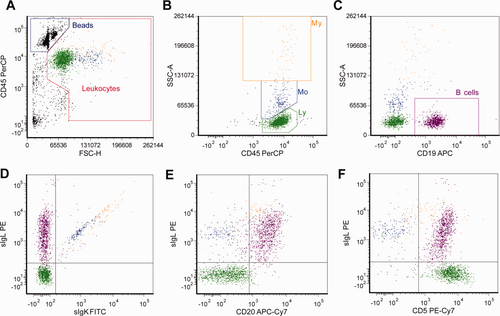
Gating strategy to detect B lymphocyte clonality and enumerate leukocyte subsets in CSF by six-color flow cytometry. Leukocytes were distinguished from nonleukocyte events and debris by gating on forward scatter (FSC) and CD45; microspheres were used for absolute counting and defined as FSClow, FLhigh (panel A). Panels B–F show further analysis of the events selected in the leukocyte gate in panel A. Leukocyte subsets were defined with high CD45 expression and side scatter (SSC) (panel B) and show three subsets: lymphocytes (Ly; CD45+, SSClow; green dots), monocytes (Mo; CD45+, SSCintermediate; cyan dots), and granulocytes (My [myeloid cells]; CD45+, SSChigh; orange dots). B cells were gated using the lineage-specific marker CD19 and side scatter (SSC) (purple dots, panel C). The B cells in this analysis were monoclonal sIgL+ (panel D) and expressed CD20 (panel E) and CD5 (panel F). Data are shown of an 18-hour-old CSF sample in TransFix™ from a patient with a B cell non-Hodgkin lymphoma (small lymphocytic lymphoma).
RESULTS
Samples and Patients
A total of 99 CSF samples were obtained, 43 diagnostic samples and 56 follow-up samples from 43 patients (24 male patients, 19 female patients), all tested for the occurrence of LHM. The median age of these patients was 60 years (range 22–77 years). Of the 43 patients, 26 patients had a systemic localization of a hematological malignancy while 17 patients had a PCNSL. In most cases (30/43, 70%), a B cell NHL was present, usually a diffuse large B cell lymphoma (23/30, 77%). Other cases included patients with acute myeloid leukemia (n = 5), T lymphoblastic leukemia/lymphoma (n = 3), T cell NHL (n = 2), plasma cell myeloma (n = 1), and B lymphoblastic leukemia (n = 2). The median volumes of CSF drawn were: 1.14 mL (0.48–2.45) CSF in TransFix/EDTA, 1.28 mL (0.47–2.66) CSF in serum-containing medium, and 1.25 mL (0.45–2.33) of native CSF. The median leukocyte count of the CSF samples was 5 cells/μL (range <1–552 cells/μL); the median protein content was 0.38 g/L (range 0.15–2.42 g/L).
Qualitative Detection of Hematological Malignancies
Thirty minutes from CSF withdrawal, we compared the flow-cytometric detection of LHM in TransFix-treated CSF samples with CSF samples with serum-containing medium (Table 1) and native CSF samples (Table 2). Compared to CSF with serum-containing medium and native CSF, CSF with TransFix showed concordant results in 86/99 (87%) and 83/99 (84%) of cases, respectively. Discordant results included paired observations in which the level of suspicion was higher (i.e., positive vs. suspicious, positive vs. negative, and suspicious vs. negative) in CSF with TransFix as well as paired observations in which the level of suspicion was higher in CSF with serum-containing medium or native CSF. Hence, 30 minutes after withdrawal, use of TransFix had neither a significant beneficial nor significant unfavorable effect on the detection of LHM, as compared to serum-containing medium or native CSF. The flow-cytometric detection of LHM after 18 hours in CSF samples with TransFix versus CSF samples with serum-containing medium and native CSF samples is shown in Tables 3 and 4. Compared to CSF with serum-containing medium and native CSF, CSF with TransFix showed concordant results in 92/99 (93%) and 86/99 (87%) of cases, respectively. In all discordant pairs of observations, the level of suspicion was higher in CSF with TransFix than in CSF with serum-containing medium (7/99 [7%], P = 0.07) or native CSF (13/99 [13%], P = 0.005). To exclude that the asymmetrical distribution of the discordances seen between TransFix-treated CSF and native CSF was due to a higher volume of TransFix-stabilized CSF than that of native CSF, we analyzed the CSF volumes of the discordant pairs of samples separately and found no significant difference (0.56 mL [0.31–0.81] vs. 0.56 mL [0.23–0.92], respectively, P = 0.80). All samples that scored positive in CSF with TransFix were derived from patients who were also diagnosed with LHM by our conventional diagnostic procedure involving flow cytometric analysis of >2 mL CSF with serum-containing medium (1).
| Medium | ||||
|---|---|---|---|---|
| t = 30 minutes | Positive | Suspicious | Negative | Total |
| TransFix™ | ||||
| Positive | 25 | 3 | 0 | 28 |
| Suspicious | 3 | 1 | 2 | 6 |
| Negative | 1 | 4 | 60 | 65 |
| Total | 29 | 8 | 62 | 99 |
- P = 0.64 (McNemar test).
| Native | ||||
|---|---|---|---|---|
| t = 30 minutes | Positive | Suspicious | Negative | Total |
| TransFix™ | ||||
| Positive | 21 | 3 | 4 | 28 |
| Suspicious | 2 | 0 | 4 | 6 |
| Negative | 0 | 3 | 62 | 65 |
| Total | 23 | 6 | 70 | 99 |
- P = 0.23 (McNemar test).
| Medium | ||||
|---|---|---|---|---|
| t = 18 hours | Positive | Suspicious | Negative | Total |
| TransFix™ | ||||
| Positive | 28 | 3 | 3 | 34 |
| Suspicious | 0 | 1 | 1 | 2 |
| Negative | 0 | 0 | 63 | 63 |
| Total | 28 | 4 | 67 | 99 |
- P = 0.07 (McNemar test).
| Native | ||||
|---|---|---|---|---|
| t = 18 hours | Positive | Suspicious | Negative | Total |
| TransFix™ | ||||
| Positive | 23 | 6 | 5 | 34 |
| Suspicious | 0 | 0 | 2 | 2 |
| Negative | 0 | 0 | 63 | 63 |
| Total | 23 | 6 | 70 | 99 |
- P = 0.005 (McNemar test).
Cell Numbers
Thirty minutes after withdrawal in CSF with medium, lymphocytes showed the highest absolute cell numbers (0.50 cells/µL [0.01–110.3]), while numbers of monocytes and granulocytes were generally very low (0.27 cells/µL [0.01–9.65] and 0.27 cells/µL [0.04–8.95], respectively). Figure 2 shows the cell numbers of leukocytes, lymphocytes, monocytes, and granulocytes after 30 minutes and 18 hours of storage, relative to their cell numbers in CSF with serum-containing medium after 30 minutes. Thirty minutes from withdrawal, the median number of leukocytes in CSF with TransFix was similar to those in CSF with serum-containing medium and 1.4 times higher than in native CSF. After 18 hours of storage, the median leukocyte number in CSF with TransFix was 1.8 times higher than in CSF with serum-containing medium and 2.3 times higher than in native CSF (Fig. 2A). These higher total leukocyte numbers in CSF with TransFix as compared to medium (after 18 hours) and native CSF (both time points) were mainly due to higher lymphocyte numbers (Fig. 2B). Monocyte numbers in CSF with TransFix were similar to those in serum-containing medium and higher than those in native CSF after 18 hours of storage (Fig. 2C), while no significant differences in granulocyte numbers were found between CSF with TransFix, CSF with serum-containing medium, and native CSF (Fig. 2D).
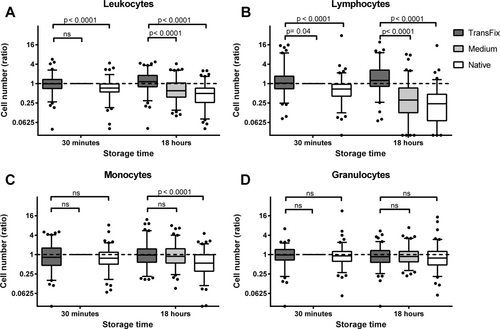
Relative numbers of leukocytes (A) and their subsets (B–D) after 30 minutes and 18 hours of storage in CSF with TransFix™, CSF with serum-containing medium, and native CSF. Relative numbers were calculated by dividing the absolute numbers (in cells/µL) by those in CSF with serum-containing medium at 30 minutes. A reference line is drawn at a relative cell number of 1, to indicate the cell number in serum-containing medium at 30 minutes. Boxes represent medians and quartiles, whiskers 5th and 95th percentiles. P values were calculated with the Wilcoxon signed rank test. ns, not significant.
Surface Antigen Labeling and Light Scatter
Figure 3 shows the measured fluorescence intensities after staining with fluorochrome-conjugated antibodies against the leukocyte marker CD45, B cell markers (CD19 and CD20), and surface immunoglobulins (sIgκ and sIgM). Thirty minutes after withdrawal, a small but significant decrease in fluorescence intensity is seen for CD45-PerCP in CSF treated with TransFix (panel A). At both time points (30 minutes and 18 hours), the fluorescence intensities of B cells labeled with anti-CD19 APC and anti-CD20 APC-Cy7 were significantly lower in TransFix-treated CSF samples than in CSF samples with serum-containing medium (panels B and C). Similarly, lower fluorescence intensities were seen in CSF with TransFix after staining for surface immunoglobulin kappa (panel D). We did not observe any significant increase in background fluorescence in CSF with TransFix or serum-containing medium (data not shown). Figure 4 shows the light scatter properties of diffuse large B cell lymphoma cells and other lymphocytes in CSF with TransFix (panels A and D), CSF with serum-containing medium (panels B and E), and native CSF (panels C and F) in a representative sample. Although forward and sideward scatter signals of the depicted cells tended to be lower after stabilization with TransFix, diffuse large B cell lymphoma cells could still be discriminated from other lymphocytes based on their higher forward light scatter signal.
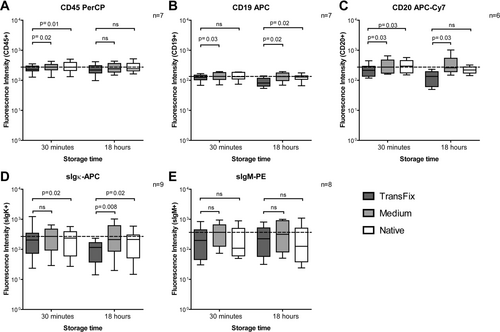
Fluorescence intensities after staining for the leukocyte marker CD45 (A), the B cell markers CD19 and CD20 (B, C), and the surface immunoglobulins kappa and M (D, E) in CSF with TransFix™, CSF with serum-containing medium, and native CSF after 30 minutes and 18 hours of storage. A reference line is drawn to indicate the median fluorescence intensity in serum-containing medium at 30 minutes. Boxes represent medians and quartiles, whiskers 5th and 95th percentiles. P values were calculated with the Wilcoxon signed rank test. ns, not significant.
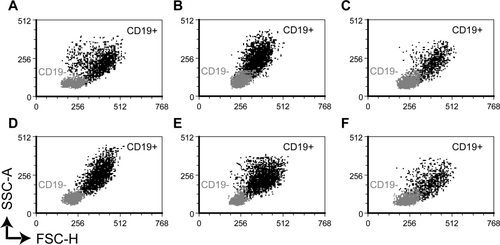
Light scatter properties of CD19+ diffuse large B cell lymphoma cells (black dots) and CD19− nonmalignant lymphocytes (grey dots) derived from CSF with TransFix™ (A, D), CSF with serum-containing medium (B, E), and native CSF (C, F) after 30 minutes (A–C) and 18 hours (D–F) of storage. All CD19+ cells (black dots) also expressed CD10 and were monoclonal sIgK+, consistent with the diagnosis diffuse large B cell lymphoma. In all six conditions, diffuse large B cell lymphoma cells could be discriminated from other lymphocytes based on their higher forward light scatter. SSC-A, sideward scatter (area); FSC-H, forward scatter (height).
DISCUSSION
In this study, we compared the detection of LHM by flow cytometry using TransFix-stabilized CSF with the detection of LHM using CSF stabilized with serum-containing medium and native CSF. We found that, after 18 hours of storage, use of TransFix significantly enhanced the detection of LHM as compared to native CSF and CSF with serum-containing medium, while 30 minutes after withdrawal, detection rates under the three conditions were similar.
Currently, it is recommended to immediately process CSF samples for flow cytometric detection of LHM, preferably within 60 minutes after withdrawal (15, 20). This implies immediate availability of a staffed flow-cytometry facility, which is not within the reach of many institutions. A method that enables storage of CSF for later analysis with diagnostic accuracy similar to immediately processed native CSF is therefore highly desirable. Cell-stabilizing agents such as TransFix and serum-containing medium have been tested by some laboratories (1, 4). In this study, we tested the use of TransFix, serum-containing medium, and native CSF side-by-side, to enable direct comparison of these methods.
Quijano et al. (4) used TransFix to stabilize CSF samples of patients with aggressive B cell NHL for overnight shipment to a central flow cytometry facility and detected LHM in 22% of cases, the same percentage as found in a previous study on fresh native CSF (3). However, they did not directly compare their results with those of immediately processed native CSF, and hence, a possible negative influence of CSF storage in TransFix on the detection of LHM can not be excluded. Our study shows that the diagnostic accuracy of the flow cytometric detection of LHM in 18-hour-old TransFix-stabilized CSF is similar to that of immediately processed CSF.
TransFix may stabilize leukocytes in different ways: a buffer may prevent cell death due to an increase in pH (21), while an aliphatic aldehyde would fixate the cells by crosslinking of amino-acid residues (16). TransFix also contains heavy metal salts that, according to the inventors, further stabilize leukocytes, and reduce excessive autofluorescence caused by aliphatic aldehydes (22). TransFix was originally designed for the stabilization of whole blood samples. In blood, TransFix has shown to reduce cellular loss of lymphocytes up to 10 days of storage (18). In addition, light scatter properties of lymphocytes were well maintained, autofluorescence levels were low, and fixation did not interfere with surface antigen labeling for CD45, CD3 and other markers, enabling reliable enumeration of major lymphocyte subsets (17, 18). However, TransFix was less effective in preventing granulocyte and monocyte loss over time. In these leukocyte subsets, it caused a decrease in forward and sideward scatter signals and negatively affected surface antigen labeling (18). The effects of TransFix on leukocytes in CSF and malignant hematological cells have not been studied in detail so far. Here, we have shown that, similar to blood, TransFix potently reduced cellular loss of lymphocytes. In addition, the effects of TransFix on light scatter signals seemed not to interfere with the identification of diffuse large B cell lymphoma cells based on their higher forward scatter signal than other lymphocytes. Also the slightly negative effect on surface antigen labeling did not interfere with proper identification of cellular subsets or malignant cells.
The acquired CSF volumes are potential limitations of this study. First, the average volume of CSF studied per condition and time point was ∼0.5 mL, as it was considered unethical to collect more than 3 mL of extra CSF in addition to the CSF needed for conventional tests and procedures. Normally, it is recommended to analyze a minimum of 2 mL of CSF, to obtain sufficient sensitivity (15). Theoretically, the discrepancies in test results found in this study might not apply to higher CSF volumes. However, we believe that it is reasonable to assume that CSF-stabilizing reagents that enable more sensitive detection of LHM in 0.5 mL of CSF will also have a beneficial effect on larger CSF volumes.
Second, we chose to directly collect CSF in TransFix CSF storage tubes, as previous data suggested that significant cellular loss may occur directly after withdrawal (12). As a consequence, some variation in the acquired CSF volumes was seen between samples with TransFix and paired samples with serum-containing medium or native CSF. However, we do not believe that the higher detection rates of LHM in CSF with TransFix were caused by a larger test volume, because median CSF volumes in TransFix were not higher than in the paired control samples, and in particular in discordant pairs of CSF samples, CSF volumes did not differ significantly.
To conclude, we showed that TransFix enables flow-cytometric detection in 18-hour-old CSF samples with a similar or higher detection rate than in rapidly processed CSF with serum-containing medium or native CSF. We propose that the use of TransFix may facilitate flow cytometric analysis of CSF samples that were collected outside office hours and enable the use of external flow cytometry facilities in institutions without a flow cytometry facility of their own. In addition, TransFix may save costs, as it allows storage and batch-processing of CSF samples instead of immediate processing that requires continuous availability of a flow cytometry facility.
Acknowledgments
The authors would like to thank the participating patients and their physicians. This research was supported by The Gratama Foundation (Harlingen, The Netherlands).




