Flow cytometric differential of leukocyte populations in normal bone marrow: Influence of peripheral blood contamination1†
How to cite this article: Brooimans RA, Kraan J, van Putten W, Cornelissen JJ, Löwenberg B, Gratama JW. Flow cytometric differential of leukocyte populations in normal bone marrow: Influence of peripheral blood contamination. Cytometry Part B 2009; 76B: 18–26.
Abstract
Background:
Availability of immunophenotypic reference values for the various leukocyte populations distributed in bone marrow may be helpful to recognize abnormal bone marrow development and, therefore, useful as first screening of individuals with suspected hematological malignancies or other hematopoietic disorders.
Methods:
A single tube four-color staining panel (CD66abce/CD14/CD45/CD34) together with a predefined gating strategy was utilized to immunologically differentiate the distribution of the major leukocyte populations in bone marrow aspirates of healthy donors. The sample-blood erythrocyte ratio was applied to assess the amount of blood contamination of marrow and account for this in the marrow value estimates.
Results:
The frequency of the major leukocyte populations in bone marrow of 134 normal donors were for granulocytes: mean, 69.4%; SD, 10.3%; monocytes: mean, 4.7%; SD, 2.3%; lymphocytes: mean, 18.3%; SD, 8.7%. The frequency of the immature cell population that included precursor cells of each of the cell lineages among other cell types were mean 5.0%; SD 2.2%. The mean percentage of CD34 positive cells was 1.5%; SD 0.7%. Our results showed further that the frequency of cell populations, of which the presence is restricted to the bone marrow (e.g., CD34+ progenitor cells), is influenced by the degree of peripheral blood admixture. Between the total immature cells and purity of the bone marrow, there was a significant positive correlation demonstrated, whereas a negative correlation was found between the percentages of both lymphocytes as monocytes and the purity of the bone marrow.
Conclusions:
With a single tube-staining panel, we obtained reference values for flow cytometric assessment of all relevant leukocyte populations present in bone marrow that can be used as a frame of reference for better recognition of individuals with abnormal hematopoiesis. In addition, we have demonstrated the influence of the degree of peripheral blood admixture in the bone marrow aspirates on those reference values. © 2008 Clinical Cytometry Society
In daily practice, bone marrow aspiration is used for the diagnosis of hematological malignancies. Flow cytometric analysis of bone marrow aspirates allows identification, quantification, and characterization of the various leukocyte subpopulations in suspected abnormal hematopoiesis (1-4).
Bone marrow is a complex tissue composed of multiple hematopoietic lineages of cells at various maturational stages of development per lineage. In healthy subjects, the relative distribution of those lymphocytic, monocytic, granulocytic, and erythroid lineages in the bone marrow shows individual variations within a certain range (5, 6). Knowledge of the normal range in which the various leukocyte subpopulations are distributed in bone marrow facilitates recognition of individuals with abnormal hematopoiesis(2, 7, 8). There are several causes of abnormal distribution of hematopoietic elements in the bone marrow compartment, e.g., (1) differentiation arrest; (2) bone marrow regeneration or increased turnover after immunosuppressive therapy or peripheral depletion or as a response to infection; (3) dysplasia in one or more myeloid lineages; and (4) expansion of neoplastic hematopoietic cells. Most of these abnormalities will present as major shifts in bone marrow composition or as specific in the relative distribution of subsets in one or more lineages (7, 9). In quantitative measurements of subset distributions, peripheral blood contamination of the aspirates (as estimated by accounting for erythrocyte sample-blood ratio's) can influence the outcome of these analyses to a great extent (10-12). Bone marrow biopsies can circumvent the problem, but they are less easy to apply for flow cytometric analysis. As there is still no universal method that is recommended for the evaluation of peripheral blood contamination of bone marrow samples (13, 14), a method described by Holdrinet et al. was used in this study from which peripheral blood contamination in human bone marrow can be calculated from the ratios between the erythrocyte and nucleated cell counts in both the bone marrow aspirate and a simultaneously obtained peripheral blood sample (15).
In this study, we have defined a single tube four colors staining panel in combination with a standardized gating strategy as a reliable and simple method to immunologically differentiate the major leukocyte populations present in bone marrow aspirates. This method was than utilized to obtain normal reference values for flow cytometric assessment of the relative size of precursor cells, lymphocytes, monocytes, and granulocytes present in bone marrow aspirates of normal healthy individuals. Availability of those values are discussed with a view of how they can be used as a frame of reference for better recognition of individuals with abnormal hematopoiesis and are therefore useful as a first screen to initiate further flow cytometric analyses. Moreover, we evaluated the influence of the degree of peripheral blood admixture in the bone marrow aspirates on those reference values.
MATERIALS AND METHODS
Bone Marrow Aspirates
Bone marrow samples obtained from healthy allogeneic stem cell transplantation donors were collected in K2EDTA anticoagulant tubes (Becton Dickinson, Oakville, ON). Informed consent was given at the time of donor screening and before collection of marrow aspirates and blood. All donors were included as healthy based on medical history, physical examination, cytomorphologic white blood cell differential and serology. The number of males and females within the total donor group was equally distributed and the age distribution between sexes almost similar (median age of total group was 48 years; range 15–79). Each aspirate was obtained by a single pull from a single puncture. A total number of 134 aspirates were obtained with a mean volume of 3.25 ml (range 0.5–9 ml).
Determination of Peripheral Blood Admixture in the Bone Marrow Aspirates
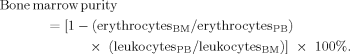
Preparation of Bone Marrow Cells
Erythrocytes present in the bone marrow suspension were bulk lysed by ammonium chloride lysing solution for 15 min. in the dark at RT to prevent selective loss of cells and to conserve light scatter characteristics (16). Afterward, cells were washed twice with PBS (pH 7.2) to remove cell debris of lysed erythrocytes. Pelleted cells were resuspended in PBS, 1% bovine serum albumin, 0.268 mM EDTA, and pH 7.2 at a final concentration of 20 × 106 leukocytes/ml. The median recovery after lysis was 65% (range: 28–99). One hundred microliters of cell suspension per staining was used for subsequent immunophenotyping.
Immunostaining Panels
Mixture of four appropriately titered monoclonal antibodies was used to stain the cells, consisting of CD66abce-FITC (Dako Cytomation, Glostrup, Denmark: Kat4c), CD14-PE (BD Biosciences, San Jose, CA: mϕp9), CD45-PerCP (BD Biosciences: 2D1), and CD34-APC (BD Biosciences: 8G12). Monoclonal antibody Kat4c recognizes three myeloid-associated molecules (CD66a, b, and c) that are expressed by myeloid cells of differing stages of maturity from promyelocytes to granulocytes but is mainly expressed by end-stage granulocytes (17, 18). The four-color panel was used to determine the immunological differentiation of the various leukocyte populations present in the bone marrow of normal healthy donors.
Multiparameter Flow Cytometry
Four-color cytometry was performed using a FACS-calibur flowcytometer (Becton Dickinson Biosciences, San Jose, CA). Instrument setup, calibration, and interpretation were performed as previously described (19). Data were collected by adjusting the FSC threshold on live cells to exclude platelets and debris. List mode data of 5000 lymphocytes (CD45+, SSC low) were collected from each staining with the immunological differentiation panel. Analyses of leukocyte populations were done according to predefined gate settings (Table 1 and Fig. 1). Data analyses were done with Cell Quest software (Becton Dickinson Biosciences, San Jose, CA).
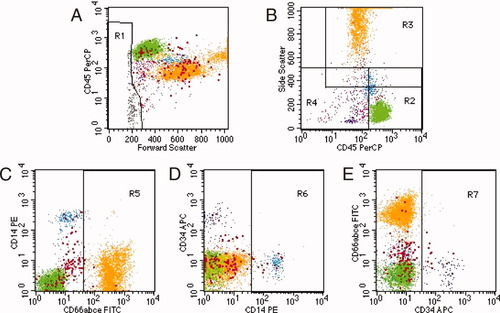
Representative dot plots of a normal bone marrow aspirate sample in which the major leukocyte subpopulations are flow cytometric analyzed after staining with CD66abce-FITC, CD14-PE, CD45-PerCP, and CD34-APC. (A) In the first step, cell debris and platelets are excluded from the analysis; region R1. (B) In the second step, regions were set according to the right angle light scatter (SSC) and CD45 phenotype characteristics of the leukocyte populations: R2: lymphocytes/monocytes, CD45+, SSCuntil mature myeloid; R3: mature myeloid, CD45(+)/+, SSChalfway monocytes until upper channels; R4: immature/monocytes, CD45−/until R2, SSCuntil mature myeloid. (C–E) Regions R5, R6, and R7 are set on the negative populations. Determination of the frequency of the various leukocyte populations is done according to the definitions described in Table 1.
| Populationa | Parameter | ||||||
|---|---|---|---|---|---|---|---|
| FSC | SSC | CD45 | CD66 | CD14 | CD34 | ||
| Lymphocytes (green) | Definition: | int./hgh | low | pos | neg | neg | neg |
| Gate: | not R1 | R2 | not R5 | not R6 | not R7 | ||
| Monocytes (blue) | Definition: | int./hgh | low/int. | dim/pos | – | pos | neg |
| Gate: | not R1 | R2 or R4 | – | R6 | not R7 | ||
| Mature myeloid (orange) | Definition: | int./hgh | int./high | dim | pos | – | neg |
| Gate: | not R1 | R3 | R5 | – | not R7 | ||
| Immature CD34− (pink) | Definition: | int./hgh | low/int. | neg/dim | neg | neg | neg |
| Gate: | not R1 | R4 | not R5 | not R6 | not R7 | ||
| Immature CD34+ (purple) | Definition: | int./hgh | low/int. | neg/dim | neg | neg | pos |
| Gate: | not R1 | R4 | not R5 | not R6 | R7 | ||
- a Colors correspond to the dot plots in Figure 1. The depicted red-colored dots in all dot plots are remaining cells that were excluded from the definitions of the analysis.
Statistical Analysis
Data were entered in a database and analyzed by STATA statistical software Version 9.2 StataCorp, College Station, TX). The frequency of a given population was calculated as the percentage of total gated leukocytes. The influence of the covariates age, sex, and purity of bone marrow on the frequency of the various leukocyte populations was assessed by Kruskal-Wallis analysis. Peripheral blood admixture related differences were determined by Spearman Rank analysis. Differences were considered significant if P ≤ 0.05.
RESULTS
Immunological Differential of Leukocyte Populations in Normal Bone Marrow by Flow Cytometry
In this study, we immunostained leukocytes with the pan-leukocyte marker CD45, lineage-specific markers CD14 (monocytic) and CD66abce (granulocytic), and the nonlineage restricted immature cell marker CD34 to determine a so-called immunological differentiation of the various leukocyte populations present in bone marrow aspirates, obtained from normal healthy donors. An example of such a multiparametric flow cytometry analysis is shown in Figure 1. First, a gating region R1 (FSC low and FSClow/CD45low) was set on plot A on all events with a lower forward scatter than lymphocytes and low to negative CD45 staining to exclude any dead cells, erythroblasts, and cell debris remaining after the erythrocyte lysis step (Fig. 1A). Subsequent analysis was done on all depicted “not R1′” events, i.e., leukocytes. In the second step, gating regions R2–R4 were set in plot B, according to the right angle light scatter (SSC) and CD45 phenotype characteristics of the leukocyte populations(20, 21). R2 was established to include lymphocytes and monocytes with bright CD45 staining and low to intermediate side scatter (CD45+, SSClow-int) R3 was established to include mature myeloid cells with high side scatter (CD45(+)/+, SSCint-high). Finally, R4 was set to include immature cells and monocytes with negative to dim CD45 staining and low to intermediate side scatter (CD45neg/dim, SSClow-int). These gating region settings ensured analysis of all acquired events (Fig. 1B). Subsequently, in plot C and D, regions R5 and R6 were set on the negative populations to include the mature granulocytes and monocytes. Finally, in plot E, region R7 was established by which all CD34 positive cells could be identified. Determination of the frequency of the various leukocyte populations was done according to the definitions described in Table 1. Four subpopulations consisting of lymphocytes, monocytes, granulocytes, and precursor cells of the various lineages were recognized in bone marrow according to their right angle light scatter (SSC) and CD45 phenotype characteristics in combination with positive and/or negative surface expression of CD66abce, CD14, and CD34. The lineage-specific markers CD66abce and CD14 enabled us to better distinguish the immature myeloid population (i.e.,CD66abce neg. and CD14 neg.) from the mature granulocyte (i.e., CD66abce pos.) population (Fig. 1C) and mature monocytes (i.e., CD14 pos.) (Fig. 1D) than CD45 staining in combination with side scatter (18). The CD34 positive precursor cells within the leukocyte population are depicted in Figure 1E. Finally, within the gated leukocyte population, there remained a small population of cells that did not fulfill the definitions of the analysis, e.g., plasma cells, megakaryocytes, and nonhematopoietic cells. Those remaining cells were named undefined cells and are depicted in all dot plots as red-colored dots.
Characterization of interassay variability using the combination of the four-color staining panel together with the above-described defined gating strategy showed that each time a reproducible frequency of the various leukocyte populations within a normal bone marrow sample was obtained (data not shown).
Reference Ranges of Leukocyte Subpopulations in Normal Human Bone Marrow Aspirates
With the combination of the immunological differentiation single tube 4-color staining panel, a total number of 134 normal bone marrow aspirates were evaluated for the relative distribution of the described major subpopulations. Kruskal-Wallis statistical analysis revealed that the covariates age and sex were of no significant influence on the relative distribution of all the various leukocyte populations in normal bone marrow (data not shown). However, in the analysis, the influence of age was limited to only an adult population of donors as normal pediatric marrows were not available for this study. In the aspirates, the median percentages of the mature lymphocyte, monocyte, and granulocyte subpopulations were 15.9%, 4.1%, and 71.2%, respectively. The median frequency of the total immature cell population as defined by CD45 dim and SSC low/intermediate in the bone marrow was 4.7%, and the median percentage of CD34 positive cells was 1.4%. Interquartile ranges (p25–p75) of the analyzed leukocyte populations are depicted in Figure 2. Analysis of each acquired bone marrow sample revealed a small heterogeneous population of cells that did not fulfill the definitions of the analysis. The median percentage of those remaining undefined cells was 2.4%.
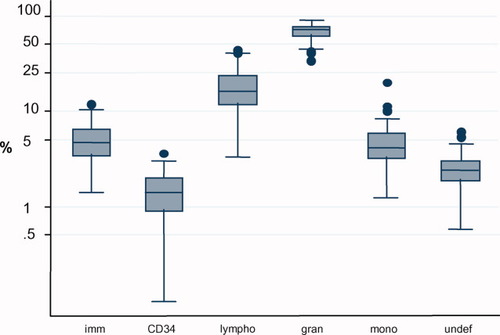
Distribution of the major leukocyte subpopulations present in bone marrow aspirates obtained from healthy individuals. The line in the middle of each box represents the median of the data. The boxes extend from the 25th to the 75th centiles (i.e., the interquartile range). The lines emerging from the boxes (i.e., whiskers) extend to the upper and lower adjacent values, which are defined as 1 [1/2] × the interquartile range rolled back to where there are data. Observed points more extreme than the adjacent values (i.e., outside values) are plotted individually. Imm: total immature cell population; CD34: CD34+ precursor cells; lymph: lymphocytes; gran: granulocytes; mono: monocytes; undef: remaining undefined cells that were excluded from the analysis.
Relation Between the Relative Distributions of the Leukocyte Subpopulations in Bone Marrow Aspirates and the Amount of Peripheral Blood Contamination
The peripheral blood contamination in the marrow samples was calculated according to the formula described in the Materials and Methods section. The mean bone marrow purity as determined by the Holdrinet method was 77% (range 23–97%). First, we assessed whether or not there was a correlation between the aspirated volume and the amount of peripheral blood contamination. The results in Figure 3 significantly show that the larger the aspirated volume obtained the higher the peripheral blood contamination. Subsequently, the relative percentages of the major subpopulations in each aspirate were plotted against the purity of the bone marrow (Fig. 4). As shown in Figure 4A, the percentage CD45-gated immature cells was positively correlated with the purity of the bone marrow. A similar correlation was observed between CD34+ progenitor cells and peripheral blood contamination (Fig. 4B). In contrast, there was a significant (P < 0.01) negative correlation between the percentages of both lymphocytes as monocytes and the purity of the bone marrow aspirates (Figs. 4C and 4D). Thus, in many cases, the majority of monocytes and lymphocytes may derive from peripheral blood contamination. Moreover, we found a positive correlation between the percentage of granulocytes and purity of the bone marrow aspirate (Fig. 4E).
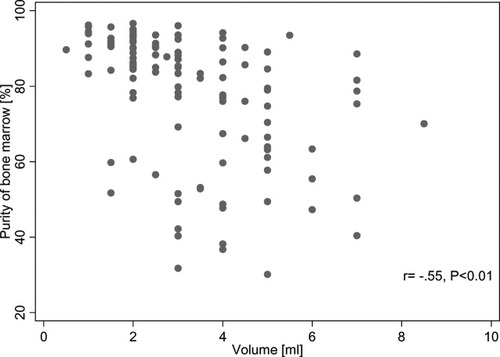
Relation between the bone marrow aspirated volume and the purity of the bone marrow.
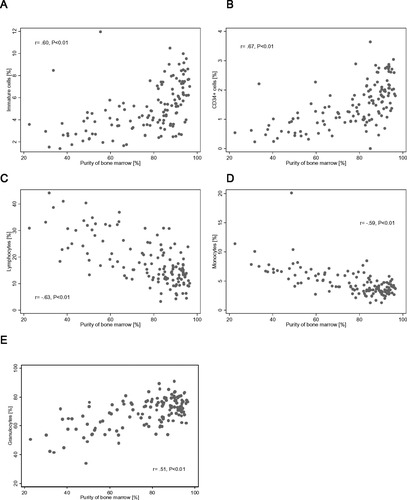
Scatter diagrams of the major leukocyte subpopulations present in 134 bone marrow aspirates obtained from healthy individuals. Relation between the purity of the bone marrow and immature cells (A), CD34+ cells (B), lymphocytes (C), monocytes (D), and granulocytes (E).
Finally, the data generated in this study were further analyzed, to provide normal ranges for leukocyte subsets in the least contaminated specimens. Reference ranges are presented in Table 2 for 78 normal bone marrow specimens with a purity of 80% and higher.
| Cell type | na | Min. | P5 | Median | P95 | Max. | Mean | SD |
|---|---|---|---|---|---|---|---|---|
| CD34+ cells | 78 | 0.1 | 0.7 | 1.8 | 2.8 | 3.6 | 1.8 | 0.7 |
| Immature cells | 78 | 2.4 | 2.5 | 5.9 | 9.2 | 10.5 | 5.9 | 2.0 |
| Lymphocytes | 78 | 3.3 | 5.7 | 13.4 | 25.0 | 30.9 | 14.2 | 5.8 |
| Granulocytes | 78 | 53.9 | 59.5 | 73.7 | 85.5 | 91.0 | 73.4 | 7.6 |
| Monocytes | 78 | 1.3 | 2.0 | 3.5 | 5.8 | 8.5 | 3.7 | 1.2 |
| Remaining cells | 78 | 0.9 | 1.4 | 2.5 | 4.5 | 18.8 | 2.8 | 2.0 |
- a Selected specimens with >80% purity of the bone marrow as determined by the Holdrinet method.
DISCUSSION
During the past decades, flow cytometric immunophenotyping has been playing an important role in establishing the diagnosis of hematological malignancies (22). Availability of reference values for the various leukocyte populations distributed in the bone marrow of healthy individuals can be helpful to identify normal and abnormal variations in hematopoiesis and are therefore useful as first screening of individuals with suspected malignancies or other hematological disorders (1, 2, 8). Especially, reference values are helpful as a first screen to initiate further immunophenotypic analyses when, for instance, an increased number of immature cells, CD34 positiveprecursor cells, or other leukocyte populations are observed in bone marrow of a patient that is suspected for a hematological malignancy. Analysis of bone marrow aspirates have been reported with several automated hematology analyzers (23, 24), but sufficient accuracy has not been obtained due to various technical problems. Recent studies have demonstrated that white blood cell differential by flow cytometry is at least as reliable as by standard cytology (25, 26). In our study, we determined reference values by flow cytometric analysis of more than hundred bone marrow aspirates obtained from normal healthy donors. Those values were obtained by the use of a single tube four colors staining panel in combination with a standardized gating strategy. This method allowed us in a reliable and simple way to immunologically differentiate the major mature leukocyte populations (granulocytes, monocytes, and lymphocytes) and to determine the relative distribution of the immature compartment present in normal bone marrow (Figs. 1 and 2). Other mature nucleated leukocytes such as plasma cells, basophils, and megakaryocytes were not evaluated, as we focused on the major leukocyte populations and immature population of precursor cells in bone marrow of which changes in the relative distribution could indicate an underlying hematological malignancy.
The median frequency of the total compartment of immature cells was found to be 4.7% of total leukocytes. Within the CD45dim SSClow/intermediate gate settings not only precursor cells of the various lineages are acquired but also plasma cells and basophils; however, the frequency of these are very low. A part of the hematopoietic progenitor cells express CD34 and are restricted to the immature region in CD45 gating. The median frequency of these CD34 positive cells in bone marrow was 1.5% of total leukocytes, including mainly myeloid and B cell precursors (2, 27). Analysis of each acquired bone marrow sample revealed also a small number of cells that did not fulfill the definitions of the analysis. These remaining undefined cells are on one hand the result of aggregates of cells or cells that have bound aspecific the fluorchrome-conjugated antibodies and on the other hand leukocytes that could not be identified by the used gating strategy. Thus, the immunological differentiation can be very helpful in investigations of pathological bone marrow samples but in fact also in other samples, (e.g., blood, aspirates, or lymph node biopsies) because it enables us to define all the acquired events of a stained patient specimen.
Methods have been described to evaluate the amount of peripheral blood admixture in bone marrow aspirates (10, 28). However, often the identification and quantitative evaluation of different bone marrow-restricted compartments of nucleated cells (e.g., plasma cells, erythroblasts, and CD34 precursor cells) in the sample is used for a rough semiquantitative evaluation of the representativeness of the sample. For instance, when the lymphocyte + monocyte counts were higher than 30%, this level was used to exclude bone marrow aspirates with an unacceptable degree of peripheral blood admixture (29). Because of the simplicity and reproducibility of the assay, we utilized the method described by Holdrinet et al. (15). This method is based on the fact that the number of erythrocytes derived from the bone marrow compartment is relatively small to the total number of erythrocytes measured in an aspirate. Hence, one may calculate the peripheral leukocyte cell fraction from the ratios between the erythrocytes and leukocyte count in both the bone marrow aspirate and a simultaneously obtained sample of peripheral blood.
The fraction of peripheral nucleated cells in bone marrow aspirates may be considerable in hematological diseases. Therefore, it is important to consider a possible artifact in relation to the relative distribution of the various leukocyte populations. For example, in measuring the number of erythrocytes and the total leukocyte count in patients with hematological malignancies, one has to realize that distribution of erythrocytes and leukocytes between the periphery and the bone marrow compartment can be disturbed and therefore of influence on the calculation of peripheral blood contamination.
To obtain a representative bone marrow sample, it is first of all important to aspirate small volumes. Our results namely showed that the larger the aspirated volume obtained the higher the peripheral blood contamination. Therefore, we recommend from this study to aspirate a maximum volume of 2 ml by a single pull from a single puncture. Moreover, as shown in this report, peripheral blood contamination of the bone marrow aspirate is of influence on the reference values, especially on the quantitative measurements of leukocyte populations that predominantly are distributed in the bone marrow. Therefore, we presented also normal reference values in those bone marrow specimens with a purity of at least 80% (Table 2). CD45-gated immature cells and CD34 positive progenitor cells were positively correlated with the purity of the bone marrow, as those populations are primarily distributed in the bone marrow. In contrast, lymphocytes and monocytes are predominantly distributed in the periphery and therefore showed a negative correlation with the purity of the bone marrow. Furthermore, there was a positive correlation found between the percentage of granulocytes and purity of the bone marrow. These findings can be explained by the use of the granulocytic-specific markers CD66abce, a combination of four CD66 family members of which antigen expression varies according to the degree of granulocyte maturation (17, 18). CD66 isotypes are mainly expressed by end-stage granulocytes, but there is also expression at the (pro)myelocyte and metamyelocyte stage. The frequency of those early myelocyte stages is higher in the bone marrow than in the periphery (30, 31).
Bone marrow is considered as the preferred type of sample to be analyzed in patients suspected of having myelodysplastic syndrome (MDS). Several studies have provided evidence that flow cytometry may add significantly in the refinement of the diagnosis and prognostication of MDS (32-34). Although, MDS patients frequently show a hypercellular marrow with peripheral cytopenias, contamination with peripheral blood is still a limiting factor for accurate characterization of maturation-associated abnormalities of hematopoietic precursors in the bone marrow. Therefore, in cases of flow cytometric analysis of MDS, it is recommended to use a method for the evaluation of peripheral blood contamination of bone marrow samples. Further studies on bone marrow aspirates and simultaneously obtained peripheral blood samples of MDS patients are necessary to assess the value of the Holdrinet method. This method will also be of help to determine whether or not the bone marrow sample is representative in cases when it is necessary to identify and accurately quantify bone marrow-restricted compartments of cells, such as plasma cells, erythroblasts, and CD34+ precursor cells. Moreover, since it is now possible to reliably identify low numbers of malignant cells during and after therapy, i.e., to detect minimal residual disease by multiparameter flow cytometry (35), it is of utmost importance to be sure about the representativeness of the bone marrow sample.
From this study, we conclude that the obtained reference values for flow cytometric assessment of all relevant leukocyte populations in normal bone marrow can be used as a frame of reference for better recognition of individuals with abnormal hematopoiesis. Furthermore, in our daily immunophenotyping practice, we nowadays always include the immunological differentiation panel with each pathological patient specimen that is evaluated for a possible blood cell disorder as it helps us in the decision, for which additional panels are going to be used for further characterization, and we have demonstrated that when it is necessary to identify and accurately quantify bone marrow-restricted compartments of cells, it is helpful to determine whether or not the obtained bone marrow sample is representative or contaminated with peripheral blood.
Finally, from the results of this study, we propose the following recommendations: (1) include an immunological differentiation method with each pathological patient sample, (2) use references values obtained from normal bone marrow for flow cytometric assessment of all relevant leukocyte populations, and (3) determine the blood admixture of the obtained bone marrow sample.
Acknowledgements
We are grateful to A. Bijkerk for help in the preparation of the flow cytometry figures. We also thank A. A. van de Loosdrecht and T. M. Westers for critically reading the manuscript.