Cells meeting our immunophenotypic criteria of endothelial cells are large platelets
Abstract
Background
Circulating endothelial cells (CEC) are shed from damaged vasculature, making them a rational choice to serve as surrogate marker for vascular damage. Currently, various techniques and CEC definitions are in use, and their standardization and validation is needed. A flow cytometric single platform assay defining CEC as forward light scatter (FSC)low-to-intermedate, sideward light scatter (SSC)low, CD45−, CD31++ and CD146+ is a promising approach to enumerate CEC because of its simplicity (Mancuso et al., Blood 2001;97:3658–3661). Here, we set out to confirm the endothelial nature of these cells.
Methods
We isolated cells with a FSClow-to-intermediate, SSClow, CD31++, CD45dim immunophenotype (termed “cells meeting our immunophenotypic criteria for endothelial cells” [CMOIC]) from healthy donors to study the expression of endothelium-associated markers using several techniques. Special attention was paid to reagents identifying the endothelial cell-specific marker CD146. We compared antigen expression patterns of CMOIC with those of the HUVEC endothelial cell line and lymphocytes. Electron microscopy was used to detect the presence of endothelial cell-specific Weibel–Palade bodies in the sorted cells.
Results
CD146 expression was negative on CMOIC for all tested CD146 mAbs, but positive on HUVEC cells and a minor subset of T lymphocytes. Using flow cytometry, we found no expression of any endothelium-associated marker except for CD31 and CD34. HUVEC cells were positive for all endothelial markers except for CD34. Evaluation of CMOIC morphology showed a homogenous population of cells with a highly irregular nucleus-like structure and positive endothelial immunohistochemistry. CMOIC contained neither nuclei nor DNA. Electron microscopy revealed the absence of a nucleus, the absence of endothelial specific Weibel–Palade bodies, and revealed CMOIC to be large platelets.
Conclusion
The vast majority of cells with the immunophenotype FSClow-to-intermediate, SSClow, CD45−, CD31++ do not express CD146 and are large platelets rather than endothelial cells. © 2007 Clinical Cytometry Society.
Circulating endothelial cells (CEC) are mature endothelial cells shed from injured vasculature (1, 2). The concentrations of CEC are reportedly increased in disease conditions associated with vascular damage such as sickle cell anaemia (3), pulmonary hypertension (4), rickettsial infection (5), myocardial infarction (6), ANCA-associated vasculitis (7), and several types of cancer (8). Furthermore, it appeared that the concentrations of CEC frequently correlated with disease severity. Enumeration of CEC may be especially relevant for clinical oncology. Since several new antitumor drugs target tumor angiogenesis or tumor vasculature, CEC counts are a potential surrogate marker for assessing drug efficiency and therefore hold promise to avoid over- or undertreatment of individual patients. In addition, reductions in CEC concentrations may reflect tumor growth and thereby serve as a parameter to reveal disease progression at an early stage (9).
Various enumeration methods for CEC have been reviewed by Blann et al. (1) and Khan et al. (10). Approaches based on cell enrichment (i.e. immunomagnetic sorting, density gradient isolation, and affinity separation), as well as approaches based on flow cytometry only, are used. Currently, there is no consensus about the immunophenotype of CEC and numerous combinations of cell surface and intracellular markers are in use. Commonly used markers include CD31, CD34, CD105, CD144, CD146, CD202b, and CD309, with CD146 being the most widely used as endothelial cell marker. As a consequence of these differences in terms of techniques and definitions, the reported concentrations of CEC in healthy donors and cancer patients vary widely, i.e., ranging between 0.7 and 7,900 cells per mL of peripheral blood (PB) (10). Therefore, and in view of the increasingly recognized importance of CEC, standardization and validation of methods to enumerate CEC are needed.
Given its minimal sample manipulation, the approach developed by Mancuso et al. (8) appears to be very attractive. This method is based on a single platform whole blood assay without prior cell enrichment, in which CEC are defined as events having low to intermediate forward light scatter (FSC), low sideward light scatter (SSC), and being CD31++, CD45−, and CD146+. Mean CD146+ CEC counts in healthy donors reported were 7,900 cells per mL by using this approach (8), which is in sharp contrast to numbers observed using enrichment-based methods (typically <100 CEC per mL) (1, 10). In view of the large discrepancy between results of single platform methodology and cell enrichment-based methods, we set out to confirm the endothelial nature of FSClow-to-intermedate, SSClow, CD31++, and CD45− cells in the first approach, which we refer to as “cells meeting our immunophenotypic criteria of circulating endothelial cells” (CMOIC).
MATERIALS AND METHODS
Subjects and Sample Collection
PB was obtained from 43 healthy donors. Blood was drawn from the antecubital vein into 7 mL tubes containing ethylenediaminetetraacetic acid (EDTA). All subjects were volunteers and had signed informed consent. The study protocol was reviewed and approved by the institutional Medical Ethical Review Board.
Flow Cytometry
Monoclonal antibodies and dyes.
Various conjugated monoclonal antibodies (mAb), viability stains, and nuclear dyes were used to assess the immunological phenotype of CMOIC in greater detail (Table 1). Antibodies were conjugated to fluorescein isothiocyanate (FITC), R-phycoerythrin (PE), peridinin chlorophyll protein (PerCP), or allophycocyanin (APC). All reagents were diluted based on titration, i.e., absence of nonspecific staining on negative populations and optimal discriminatory power between negative and positive populations (Fig. 1). To assess the presence of nuclei and DNA, we used four nuclear dyes, i.e., DRAQ5 (BioStatus, Leicester, UK), 7-aminoactinomycin D (7-AAD; Sigma-Aldrich, St. Louis, MO), propidium iodide (PI; Sigma-Aldrich), and Laser Dye Styryl-751 (LDS 751; Exciton, Dayton, OH). All dyes were used in concentrations as recommended by their manufacturers. In addition, samples stained for 7-AAD and PI were permeabilized using Fix & Perm (An der Grub, Kaumberg, Austria) in order to stain DNA.
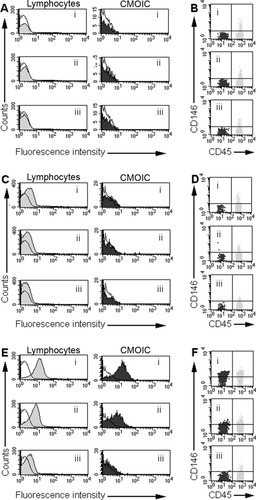
Titration of three CD146 PE mAbs on CMOIC and lymphocytes. A and B: BD Biosciences (clone P1H12); C and D: BioCytex (clone S-Endo-1); E and F: Chemicon (clone P1H12). Blood samples were stained using a stain, lyse, no-wash protocol using 10 μl of antibody per test. The amounts of mAb (μg per test) used were the following: panels Ai–iii, Bi–iii: 0.015–0.008–0.003, panels Ci–iii, Di–iii 0.075–0.038–0.015, panels Ei–iii, Fi–iii: 0.200–0.100–0.025. In each of Panels A, C, and E, open (unstained) histograms show the fluorescence patterns of unstained cells. The filled histograms show CD146 fluorescence patterns on lymphocytes (gray) and CMOIC (black). The BD Biosciences P1H12 clone and BioCytex S-Endo 1 clone do not stain CMOIC and lymphocytes in any dilution (Panels Ai–iii, Ci,iii). Plotting both CMOIC and lymphocytes using CD45 vs. CD146 bivariate histograms reveals the presence of a minor CD146+ lymphocyte subset as expected (11), whilst CMOIC are CD146 negative with these mAb (Panels Bi–iii, Di–iii). In contrast, the Chemicon P1H12 reacts positively with CMOIC at 0.2 μg/test, but this is due to high background fluorescence, as the total lymphocyte population shows a similar staining pattern (Panels Ei–iii). Dim lymphocyte background fluorescence is still visible at a dilution of 1:400 (0.025 μg/test; Panel Eiii); at this concentration, CMOIC are negative.
| Marker | Description | Clone | Manufacturer |
|---|---|---|---|
| CD31 | PECAM-1 | WM59 | BD Biosciences |
| CD34 | Hematopoietic stem cell marker | 8G12 | BD Biosciences |
| CD45 | Pan-leukocyte marker | 2D1 | BD Biosciences |
| CD45 | Pan-leukocyte marker | J33 | Immunotech |
| CD105 | TGF-β1 receptor | 1G2 | Immunotech |
| CD106 | VCAM-1 | 51-10C9 | BD Biosciences |
| CD133 | Progenitor cell marker | AC133/1 | Miltenyi Biotec |
| CD146 | MelCAM | P1H12 | BD Biosciences |
| P1H12 | Chemicon | ||
| S-Endo 1 | BioCytex | ||
| CD202b | Angiopoietin receptor | 83751 | R&D Systems |
| CD309 | VEGFR-2 | 89106 | R&D Systems |
| 7-AAD | DNA specific viability stain | – | Sigma-Aldrich |
| DRAQ5 | DNA specific dye | – | BioStatus |
| LDS 751 | Nuclear and mitochondrial dye | – | Exciton |
| PI | DNA and double-strand RNA binding dye | – | Sigma-Aldrich |
- Abbreviations: PECAM-1, platelet/endothelial cell adhesion molecule; TGF-β1, transforming growth factor β1; VCAM-1, vascular cellular adhesion molecule 1; MelCAM, melanoma-associated cellular adhesion molecule; VEGFR-2, vascular endothelial growth factor receptor 2. Locations of manufacturers: BD Biosciences, San Jose, CA; Immunotech, Miami, FL; Miltenyi Biotec, Bergisch Gladbach, Germany; Chemicon, Temecula, CA; BioCytex, Marseille, France; R&D Systems, Minneapolis, MN; Sigma-Aldrich, St. Louis, MO; BioStatus, Leicester, UK; Exciton, Dayton, OH.
Data acquisition and analysis.
Acquisition was done using a FACSCalibur flow cytometer equipped with a second red-diode laser (BD Biosciences, San Jose, CA). Flow cytometer setup and calibration was performed as described previously (12). Data analysis was performed using CellQuest Pro v5.2 (BD Biosciences). At least 100,000 leukocytes per sample were acquired, and analysis was considered informative if >100 events had been collected that met the CMOIC criteria, i.e., FSClow-to-intermedate, SSClow, CD31++, CD45− (see Results).
HUVEC
The HUVEC-C CRL-1730 cell line was purchased from ATTC (Manassas, VA) and was cultured and harvested as recommended by the supplier. The viability of HUVEC cells was established by staining with trypan blue before each use.
Cell Sorting
For cell sorting, blood samples were prepared using a stain-lyse-wash procedure in order to increase the nucleated cell concentration in the sample. Staining and lysing of whole blood was performed as for absolute cell count enumeration. After lysis, the cell suspension was centrifuged for 5 min at 500g at room temperature (RT). The supernatant was removed from the sample tube. The cell pellet was resuspended in 2 ml of phosphate-buffered saline (PBS) and centrifuged for 5 min at 500g at RT. Finally, the supernatant was removed and the pellet was resuspended in 250 μl PBS. For EM analysis, pre-enrichment of mononuclear cells was done by means of density gradient centrifugation using Lymphoprep (Axis Shield PoC, Norway, density: 1.077).
Individual cellular subsets were sorted using a FACSAria Cell Sorting System (BD Biosciences) equipped with FACSDiva v4.1.2 software (BD Biosciences) and a 70 μm nozzle; sheet fluid pressure was 70 psi (pounds per square inch). For studies based on morphology, immunohistochemistry (IHC), and fluorescence in situ hybridization (FISH), we sorted 4 spots of 200 CMOIC and 4 spots of 200 lymphocytes on standard glass slides. For QRT-PCR and EM, the required amount of cells was sorted in 15 ml tubes containing 750 μl of fetal calf serum.
Morphology
May–Grünewald–Giemsa (MGG) staining was done using a Autostainer XL (Leica Microsystems GmbH, Wetzlar, DE). Images were acquired on a DM2500 microscope equipped with a DC500 digital camera (both from Leica). The acquisition software (Leica IM1000 version 4.0) set focus, brightness, and contrast automatically.
Immunohistochemistry
Two-step horseradish peroxidase (HRP)-based IHC was performed using the ChemMate Envision Detection Kit (Dako, Glostrup, DK). Sorted populations were fixed in buffered formalin and stained using mouse-anti-human antibodies against CD31, von Willebrand Factor (both from Dako), and Fli-1 (Santa Cruz Biotechnologies, Santa Cruz, CA). Images were acquired on an Axioplan 2 Imaging microscope (Carl Zeiss, Göttingen, Germany).
Fluorescence In Situ Hybridization
Freshly prepared cytospin preparations were used made from 200 sorted CMOIC or 200 lymphocytes. Pretreatment of slides and hybridization of 0.5–1 μl of probes for centromere 7 and 8 (CEP 7 and CEP 8, Vysis, Abbott Laboratories, Des Plaines, IL) was performed as described elsewhere (13). Slides were counterstained with DAPI and embedded in Vectashield/DABCO (Vector Laboratories, Burlingame, VT). For each sample as much as interphase cells as possible were scored. Images were captured with an epifluorescence microscope (Axioplan 2, Carl Zeiss) using MacProbe version 4.3 software (Applied Imaging, Newcastle upon Tyne, UK).
Quantitative Reverse Transcriptase Polymerase Chain Reaction
QRT-PCR for the endothelial cell-specific gene vascular endothelial cadherin (VE-Cadherin, CD144) was performed on sorted CMOIC, HUVEC, and lymphocytes using the protocol and primers described by Rabascio et al. (14). RNA was isolated using the ChargeSwitch Total RNA Cell Kit (Invitrogen, Paisley, UK). Complementary DNA synthesis was done using Reverse Transcriptase Superscript III (Invitrogen). Standard curves were obtained using plasmid kindly donated by Dr. Rabascio (Milan, Italy). RT-PCR was performed on a Taqman RT-PCR instrument with the probe 6-FAM-TGT-GAG-AAC-GCT-GTC-CAT-GGC-CAG-TAMRA (Applied Biosciences, Foster City, CA). For quantification, the 18S ribosomal RNA component was used by using the Taqman Ribosomal RNA Control Reagent (Applied Biosciences) as endogenous control. HUVEC cells were spiked in 5,000 lymphocytes counted by cell sorting. Fifty, 500, and 5,000 HUVEC cells per sample was done to define assay sensitivity. Specificity of the assay was confirmed by comparing a positive control (5,000 HUVEC) with a negative control (5,000 lymphocytes per sample). For detection of VE-Cadherin in CMOIC, 5,000 sorted cells per test sample were used.
Electron Microscopy
Cells were fixed in electron microscopy (EM) fixative 4cF-1G (4% of commercial formalin, 1% of glutaraldehyde) for 24 h at RT. Secondary fixation was done by osmium tetroxide 1% for 24 h. The sample was dehydrated by centrifugation in 50, 70, 90, 95, and 100% aqueous acetone and in equimolar parts of 100% acetone/epoxy resin consecutively (each step: 5 min at 1000g at RT). The dehydrated pellet was embedded in pure epoxy resin (Embed 812, EMS, Hatfield, PA). Polymerization took place at 60°C for 18 h, followed by appropriate sectioning of the embedded cell pellet. Electron micrographs were acquired using a Morgagni 268(D) transmission electron microscope (FEI Electron Optics, NL) at an excitation voltage of 80 kV.
RESULTS
Flow Cytometric Enumeration of CMOIC
Our protocol is based on that of Mancuso et al. (8) with two modifications. Briefly, 100 μL of PB is incubated with 10 μL each of CD31 FITC mAb, CD45 PE, and 10 μL of 7-AAD for 15 min in darkness at RT. After 15 min of lysis in NH4Cl (0.154 mM in purified water), 100 μL of counting beads (Flow-Count fluorospheres, Beckman-Coulter, Miami, FL) were added for calculating absolute cell numbers. The first protocol modification was implementation of back-gating on viable lymphocytes, similar to that used in the ISHAGE guidelines for flow cytometrical CD34+ enumeration of hematopoietic stem cells (15). This approach allows a more reproducible positioning of the lower FSC margin of the analysis gate. The second modification was the exclusion of CD146 from the staining panel as we found it to be negative on CMOIC (see later).
We compared our protocol with that of Mancuso et al. (8) by testing 43 samples side-by-side. There was a strong correlation between the counts of CEC (8) and CMOIC (Pearson's correlation of r = 0.93). Specifically, the median CEC count was 31,000/mL (range, 12,000–64,000) and the median CMOIC count was 33,000/mL (range, 9,500–65,000). The two-tailed t-test for paired samples revealed no significant difference between both methods (P = 0.21).
CMOIC Do Not Express CD146
We studied the expression of CD146 by CMOIC (i.e., FSClow-to-intermediate, SSClow, CD31++, CD34+, CD45−, 7-AAD− events) using 3 PE-conjugated mAb. Expression of CD146 is known to be limited to endothelial cells (1, 16, 17) and a subset of activated T-cells (11, 18). Two mAb derived from the P1H12 clone (one from BD Biosciences and one from Chemicon [Temecula, CA]), and a third mAb derived from the S-Endo 1 clone (BioCytex [Marseille, France]) were titrated on HUVEC, CMOIC, and lymphocytes (CD45bright, SSClow).
No expression of CD146 was detected within CMOIC and the majority of lymphocytes using the BD Biosciences and BioCytex mAb (Fig. 1, panels A and C). The small CD146+ subset of lymphocytes consisted of activated CD3+ T cells (Ref.22 and data not shown). As expected, all CD146 mAb showed bright and homogenous staining on HUVEC (Fig. 2, left panels). In contrast to the other two CD146 mAb, the Chemicon reagent stained both CMOIC and lymphocytes when applied in the same concentration as originally described by Mancuso et al. (8), i.e., 0.2 μg per test (Fig. 1, panels Ei). A negative signal with this mAb on the majority of lymphocytes was only obtained at a much lower concentration, i.e., 0.025 μg/test (Fig. 1, panel Eiii). At that concentration, no reactivity was observed against CMOIC (Fig. 1, panel Eiii), whilst HUVEC (Fig. 2) and a small subset of lymphocytes (Fig. 1, panel Fiii) remained positive. Based on visual comparison of the BD Biosciences and Chemicon CD146 PE conjugates, we suggest that the reactivity of CMOIC with the latter conjugate is due to large amounts of free fluorochrome in this reagent (Fig. 3).
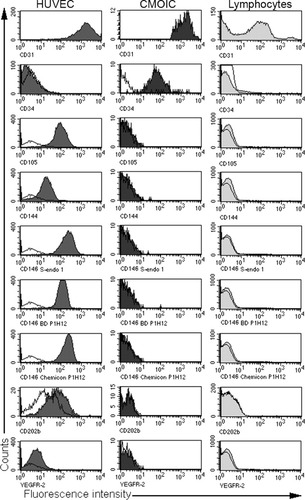
Immunological characterization of HUVEC, CMOIC, and lymphocytes. The open histograms show the fluorescence patterns of unstained cells. The filled histograms show the fluorescence patterns after staining with the mAb as indicated at the bottom of each panel. HUVEC show homogenous and positive staining patterns for all endothelium-associated markers except for CD34. Lymphocytes show heterogeneous staining for CD31, but are negative for the other markers. CMOIC are only positive for CD31 and CD34, but are negative for all other endothelial specific markers.

Vials containing various dilutions of PE-conjugated CD146 mAb (Chemicon, clone P1H12). On the left, CD146 PE (clone P1H12, BD Biosciences) is shown as comparison. The BD Biosciences reagent is colorless, whilst the Chemicon reagent is pink in all dilutions up to 1:400 (0.025 μg/test).
Cell Morphology
Evaluation of cell morphology of ∼150 sorted CMOIC using light microscopy and May–Grünewald–Giemsa staining revealed a homogenous cell population. These cells were typically around 5 μm in diameter, and contained an irregularly shaped nucleus-like structure and variable amounts of cytoplasm (Fig. 4).
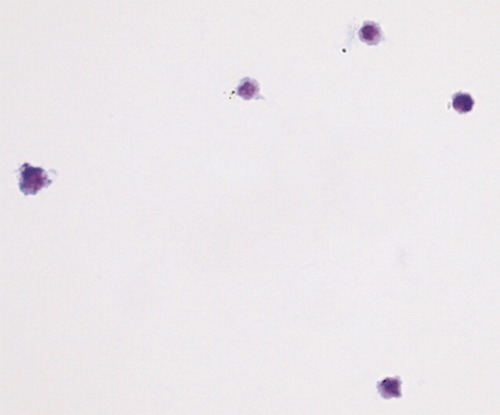
May–Grünewald–Giemsa staining of sorted CMOIC. This representative sample shows cells that contain an irregularly shaped structure and variable amounts of cytoplasm. Cell size ranges between 4 and 6 μM.
Immunological Phenotyping and Comparison to HUVEC
Because of the absence of CD146 on CMOIC, we tested various mAb commonly used in CEC enumeration: CD31, CD34 (class III), CD105, CD144, CD146, CD202b, and CD309 (VEGFR-2) (8, 10, 19, 20) on PB from six healthy donors for their expression on CMOIC. These expression patterns were compared to those of HUVEC, a cultured cell line used as model for endothelial cells, and to those of lymphocytes, which were used as a negative control (Fig. 2). All these mAb except CD34 were reactive with HUVEC. Lymphocytes showed a very heterogeneous expression pattern of CD31, and were negative for all other endothelial markers. CMOIC were only dimly positive for CD34 (Fig. 2). The expression of several other lymphocytic, myeloid, and platelet markers, i.e., CD3, CD4, CD8, CD14, CD19, CD33, CD41, CD56, CD57, and CD61, was compared between CMOIC and leukocyte subsets. The expected staining patterns were observed for each leukocyte subset, whilst all markers except for the platelet markers CD41 and CD61 were negative on CMOIC (data not shown).
In addition to immunophenotyping by flow cytometry, we also addressed the expression of endothelial specific antigens by means of IHC. We sorted CMOIC based on our definition of FSClow-to-intermediate, SSClow, CD31++, and CD45dim. Lymphocytes were sorted as a reference. Unstained cells were used as negative control. CMOIC showed an intense staining for CD31, vWF, and Fli-1, which is consistent with the phenotype of endothelial cells and platelets (21). Expectedly, lymphocytes did not stain for CD31 and vWF, and only dimly for Fli-1 (Fig. 5).
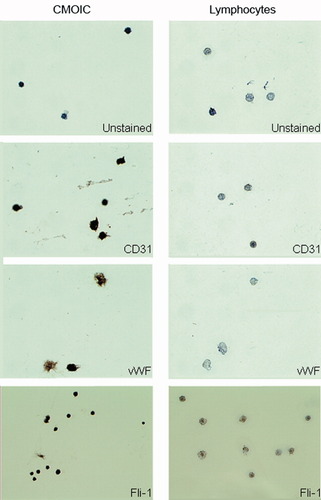
Analysis by IHC. Expression of the endothelium-associated antigens vWF, CD31, and Fli-1 of sorted cells (left panels) were compared to lymphocytes (right panels). Unstained samples were used to assess positivity. Magnification, ×400. CMOIC show an intense but highly irregular staining for all tested markers. Lymphocytes do not stain for any of these markers.
QRT-PCR for VE-Cadherin
Because of the apparent lack of endothelial marker expression by CMOIC, we studied the presence of VE-Cadherin mRNA in these cells. We obtained a positive signal for VE-Cadherin mRNA from a minimum of 50 HUVEC spiked in 5,000 leukocytes. However, we did not detect a positive signal for VE-Cadherin mRNA when 5,000 CMOIC or 5,000 lymphocytes were tested (data not shown).
Assessment of DNA and RNA Content of CMOIC
The lack of expression of endothelial specific surface antigens, the positive expression of thrombocyte markers CD41 and CD61, the immunohistochemical positivity of CD31, vWF, and Fli-1 and the absence of endothelial-specific VE-Cadherin mRNA prompted us to address whether or not CMOIC contained DNA or RNA. The DNA specific dyes 7-AAD and DRAQ5 stained brightly positive on all leukocytes but not on CMOIC (Fig. 6, panels A and B). Both PI, which stains double-stranded DNA and RNA, and LDS 751, which stains RNA in nuclei and mitochondria, showed bright reactivity with leukocytes, but relatively dim reactivity with CMOIC (Fig. 6, panels C and D). We then performed FISH using probes against the centromeres of chromosomes 7 and 8 in combination with counterstaining by DAPI. This approach revealed no signal in CMOIC but showed two copies of each centromere and a positive DAPI staining in lymphocytes (data not shown). These combined results indicate that CMOIC do not contain DNA.
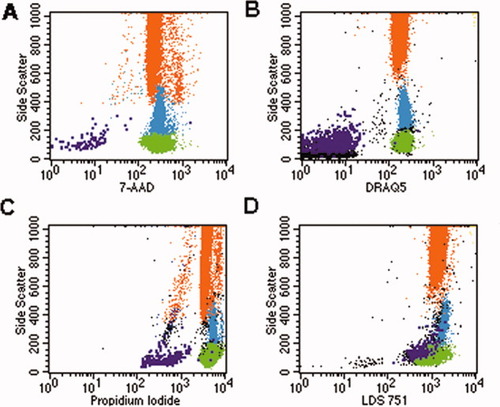
Assessment of presence of DNA, RNA, and mitochondria in CMOIC and leukocyte subsets. One hundred microliter of blood were stained with the stain, lyse, no-wash protocol using 10 μl of CD31 FITC, 10 μl of CD45 PE, and 10 μl of the nuclear dyes in appropriate concentrations. Granulocytes are shown in orange, monocytes in blue, lymphocytes in green, CMOIC in purple, and cellular debris in black. 7-AAD and DRAQ5 are positive on all leukocytes, demonstrating the presence of DNA in these cells. The negative staining of CMOIC implicates that these cells do not contain DNA (Panels A and B). PI and LDS751 are brightly positive on leucocytes. A less intense staining is observed on CMOIC, which is the result of staining double strand RNA in the case of PI (panel C) and the staining of mitochondria in the case of LDS751 (Panel D).
Electron Microscopic Analysis
Weibel–Palade bodies are rod-shaped organelles that are specific for vascular endothelium in vivo (22) and in vitro (23). Presence of these organelles would provide definite proof of the endothelial origin of our sorted CMOIC. For this purpose, 600,000 CMOIC were isolated by flow sorting, and a similar number of lymphocytes were sorted as control. A homogenous population of both CMOIC and lymphocytes was observed (Fig. 7, upper 2 panels). At higher magnification (Fig. 7, lower 2 panels), CMOIC appeared to be 4 μm in size not nucleated and to lack Weibel–Palade bodies. Instead, CMOIC were found to contain mitochondria, an open canalicular system and centrally located electron dense granulae: these morphological characteristics are consistent with large platelets (24-26).
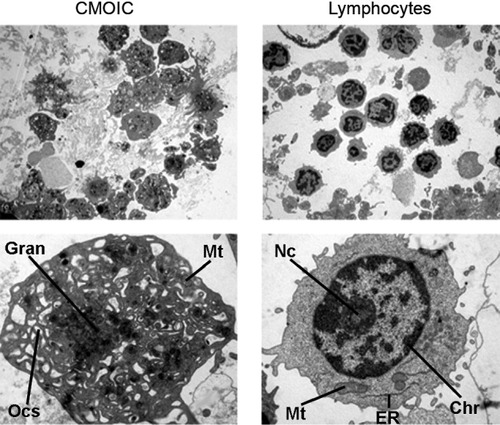
Electron micrographs of CMOIC (left panels) and lymphocytes (right panels). The upper panels show a representative overview of each sample at ×2,200 magnification. The corresponding lower panels show a single CMOIC and a single lymphocyte at ×11,000 magnification. Here, the lymphocyte shows a clearly distinguishable nucleus with chromatin (Chr) and a nucleolus (Nc). In the cytoplasm, mitochondria (Mt) and endoplasmatic reticulum (ER) are present. The CMOIC lacks a nucleus. A wide open canalicular system is present (Ocs), as well as some mitochondria (Mt). In the middle, mainly electron dense granulae (Gran) are present.
DISCUSSION
As the number of new molecules inhibiting angiogenesis increases, a monitoring tool for their effectiveness is needed in order to avoid over- or undertreatment with these compounds (1, 10). For this purpose the enumeration of CEC, which are thought to be shed from mature normal and tumor vasculature, is a promising (3-7, 27) option. The single-platform flow cytometric approach as described by Mancuso et al. (8, 28-30) appeared to be highly attractive for this purpose, as it would obviate the need for prior cell enrichment and, at the same time, generate absolute CEC counts. However, review of the results obtained with various methods of CEC enumeration revealed that the single-platform flow cytometric method yielded 100- to 1,000-fold higher CEC counts than methods using CD146-based enrichment of CEC (3-7, 27). Therefore, we considered it necessary to confirm the endothelial nature of the cells identified by the single-platform flow cytometric approach. To this end, we investigated CMOIC, i.e., FSClow-to-intermediate, SSClow, CD45dim and CD31++.
Here, we showed that CMOIC are actually not CEC but large platelets. First, their reactivity with CD146 mAb was only demonstrated using the Chemicon CD146 PE conjugate and not with two other CD146 PE conjugates (i.e., the P1H12 from BD Biosciences and S-Endo 1 from BioCytex). This result, combined with our observation that the Chemicon CD146 PE conjugate reacted with most lymphocytes in the same concentration as which they stained CMOIC (i.e., 0.2 μg/test), but were negative with CMOIC at a much lower concentration where lymphocytes showed only dim reactivity, led us to conclude that CMOIC do not express CD146. Using immunophenotyping and sorting of CMOIC followed by morphology, IHC, and QRT-PCR, we were not able to detect cells with endothelial characteristics among these cells. The positive staining of CMOIC for vWF and Fli-1 was not conclusive with respect to their endothelial origin, as cells from the megakaryocytic lineage express these antigens as well (21, 31). Furthermore, nuclear staining and FISH failed to detect cells with a nucleus in this population and finally, electron microscopy revealed CMOIC to be large platelets. The absence of CMOIC (and platelets) in patients with severe aplastic anemia and chemoradiotherapy-induced bone marrow aplasia, and their reappearance in the circulation of engrafting recipients of an allogeneic stem cell transplant (data not shown) further suggests that CMOIC are derived from hematopoietic stem cells and not from endothelial cells lining the vasculature.
Our findings provide an explanation for the significantly higher CD146+ CEC counts as reported using the single platform flow cytometric assay (8, 28-30) and those determined by CD146-based enrichment methods (3-7, 27). We suggest that the CD146 positivity of CEC as defined by Mancuso et al. (8)., which largely overlap with CMOIC, is due to nonspecific reactivity of the Chemicon CD146 PE conjugate used in the published concentration (8). These results emphasize the importance of appropriate mAb titration using well-defined positive and negative controls, and the use of internal negative controls (such as lymphocytes) in addition to unstained cells.
Recent approaches to CEC enumeration use immunomagnetic enrichment using paramagnetic particles coated with CD146 mAb in combination with markers to exclude hematopoietic cells (i.e., CD45) (32), or in combination with markers that confirm endothelial origin (Ulex Europaeus Lectin-1 and CD105) (32, 33), as enrichment for CD146 also yields CD146+ T cells. With these approaches, CEC counts in healthy individuals generally do not exceed 20 cells per mL (32, 33).
Acknowledgements
We are grateful to Dr. Reno Debets for his advice, to Mr. Berend Hooibrink, Mr. Renier van der Linden, and Mrs. Danielle Hijdra for cell sorting, to Mrs. Brigitte van Krimpen for performing PCR assays, to Mrs. Greet Bikker for the morphological studies, to Mr. Piet van der Heul for electron microscopy, and to Mrs. Berna van Beverloo for the cytogenetic analyses.




