DNA methylation profiles reveals STAB1-mediated endothelial cell and immune cell interactions in Moyamoya disease
Shihao He, Zhenyu Zhou, and Rui Liang are co-first author.
Dear editor
This investigation provides a detailed characterisation of the multifaceted pathological processes driven by DNA methylation in Moyamoya disease (MMD). The Stabilin-1 (STAB1)-mediated interaction between endothelial and immune cells modulates vascular homeostasis, potentially contributing to MMD pathogenesis.
MMD is a chronic cerebrovascular disease which is characterised with distal stenosis of internal carotid artery.1 Yet the marked clinical heterogeneity of Moyamoya disease suggests that genetic variants alone are insufficient to explain its pathogenesis, underscoring the need to interrogate complementary epigenetic mechanisms such as DNA methylation. Sung et al. employed the Illumina 450K array on a small cohort of endothelial cells and validated their findings in a HUVEC tube formation assay, revealing overexpressed Sort1's role in MMD angiogenesis.2 However, their limited sample size and exclusive focus on endothelial cells constrain the generalisability of these results, underscoring the need for broader investigation. Tokairin et al. used the Illumina 850 K to conduct genome-wide DNA methylation in female cohorts. In MMD, hypomethylation was observed in genes regulating natural killer cell signalling, cell growth and migration, DNA methylation and so on.3 The study did not stratify by clinical subtype, lacked male participants, and provided no functional validation. In contrast, the current work employs a balanced, subtype-defined discovery set, couples methylome data with intracranial RNA-seq, and experimentally demonstrates that STAB1 hypomethylation enhances endothelial ECM production and tube formation. These additions not only corroborate Tokairin's immune-inflammatory theme but also position STAB1 as a mechanistic link between aberrant immunity and intimal thickening in Moyamoya disease.
In the discovery cohort, we enrolled 30 participants, comprising 10 with haemorrhagic (HEM) and 10 with ischemic (IS) Moyamoya disease, alongside 10 healthy controls (HC). We mapped the DNA methylation profiles of ischemic and haemorrhagic Moyamoya disease patients via the Illumina 850K chip (Figure S1). The middle cerebral artery (MCA) sequencing data of 11 MMD patients and 9 control patients from GSE157628 were used as the validation cohort. Through weighted gene co-expression network analysis (WGCNA) and differential analysis of the discovery cohort and the validation cohort, the most potential biomarker, STAB1, was obtained (Figures S1, S2 and 1A).
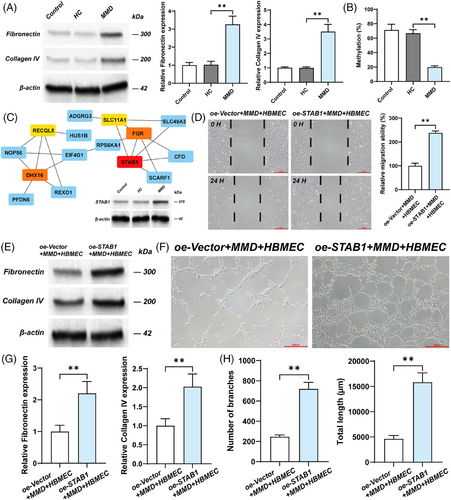
The extracellular matrix (ECM) is a macromolecular reticular structure synthesised and secreted by cells to the extracellular space. Matsuo et al. conducted immunohistochemical analysis on the specimens of the middle cerebral arteries (MCA) from autopsies of MMD patients and found that ECM was significantly accumulated in the thickened intima.4 The ECM components (fibronectin, collagen IV) were also found to be upregulated in ECs cultured with MMD serum and overexpressing STAB1 EC (Figure 1A, E, and G). This suggests that STAB1 may promote the thickening of the vascular intima by enhancing the synthesis of ECM by ECs. Ye et al. explored the effect of RNF213 on human brain microvascular endothelial cells (HBMEC) in Moyamoya disease.5 They found that RNF213 knockdown promoted the proliferation, migration and tube formation of HBMEC. This suggests that there is a complex relationship between endothelial cell dysfunction and pathological angiogenesis in Moyamoya disease. In this study, to demonstrate the genetic and functional characteristics of EC in MMD patients, human brain microvascular endothelial cells were cultured with the serum of patients with Moyamoya disease as the cell model for in vitro experiments. Adachi et al. reported the influence of STAB1 on the tube-forming ability of endothelial cell (EC) through a tube formation assay.6 We found that overexpressed STAB1 promoted the tube formation and migration capabilities of EC (Figures S2 and 1D, F, and H). Therefore, in the pathogenesis of MMD, overexpressed STAB1 may disrupt vascular homeostasis by acting on EC. STAB1 mediates immune cell adhesion to the vascular wall and directed chemotaxis.7 Furthermore, the GSEA results suggest that among the pathways enriched by STAB1, there are several related to immunity, such as Antigen processing and presentation, Intestinal immune network for IgA production (Figure S4). The result of immune infiltration analysis suggests that STAB1 is correlated with various immune cells, of which Treg and CD56brightNK cells are more significant than other (Figure 3A and B).
Regulation T cell (Treg cell) are a unique cell lineage within the adaptive immune microenvironment that has been reported to impact on angiogenesis.8 Weng et al. found that the proportion of Treg cells in the peripheral blood of patients with MMD was significantly higher than the other groups.9 We also discovered an increased number of Treg cells in MMD, which might be associated with ischemia and hypoxia induced by vascular stenosis. In our preliminary direct-contact co-culture, Tregs appeared to enhance endothelial-cell proliferation (Figure 2D). In MMD, EC may recruit Tregs due to hypoxia, which reach the hypoxic area and affect angiogenesis by regulating EC.
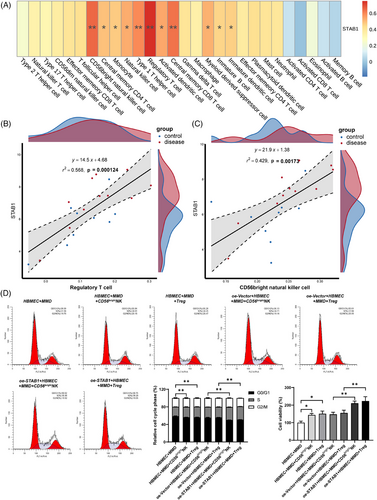
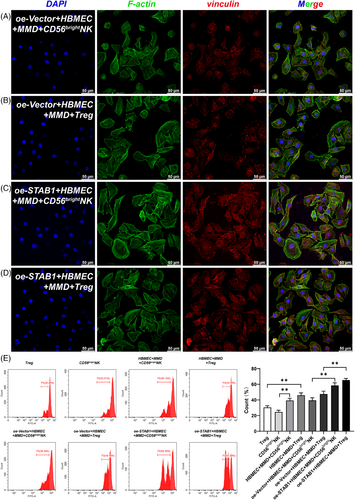
CD56bright nature killer cell (CD56brightNK cell) is a subset of nature killer cells. Li et al. reported the cytotoxic effect of NK cells around the lesion on cerebrovascular endothelial cells in intracerebral haemorrhage.10 In our study, an interaction between CD56 brightNK cell and the abnormally enlarged cytoskeleton of ECs was likewise observed (Figures 3A and D and 4, S5, and S6). In MMD, it is possible that the enlarged cytoskeleton of endothelial cells induces the proliferative promotion effect of CD56brightNK cell on endothelial cells. STAB1 mediates immune cell adhesion to EC for immune response and inflammation.7 Overexpression of STAB1 in EC may attract CD56 brightNK and Treg cells to the area for regulating angiogenesis. Interestingly, this indicates that there may be a positive interaction between EC, CD56 brightNK and Treg cell. This positive interaction may accelerate and amplify the vascular stenosis and collateral proliferation, as well as the inflammation in MMD.
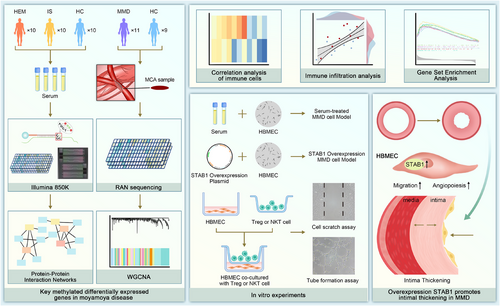
While we mitigated this limitation by validating differentially methylated genes in independent arterial transcriptome datasets and conducting functional analyses in endothelial cells, the lock-specific methylation changes within the vessel wall still need to be confirmed by expanding the sample size and in vivo studies. In vitro experiments, co-culture experiments lack transwell control and cytokine secretion profiles.
In conclusion, our study provides valuable implications for the DNA methylation in MMD, pushing the boundaries of our knowledge of the unclear pathogenesis.
AUTHOR CONTRIBUTIONS
SH, YJ, and YL conceived and designed the experiments. SH, ZY, CX, LR, YT, and JZ performed experiments. YJ and YL contributed reagents, materials, and analytical tools. SH, ZY, YJ, and YL wrote the manuscript.
ACKNOWLEDGEMENTS
The authors thank the patients with Moyamoya disease and controls who agreed to participate in the study.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
FUNDING INFORMATION
This study was supported by National High Level Hospital Clinical Research Funding (2023-PUMCH-E-011), CAMS Innovation Fund for Medical Sciences (CIFMS) (2023-I2M-C&T-B-048). The above funds provide the testing and processing costs, data collection, analysis and interpretation of this experiment.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee of Peking Union Medical College Hospital, Beijing, China (I-24PJ1573). All participants signed informed consent forms.
Open Research
DATA AVAILABILITY STATEMENT
Any information presented in the current study are available from the corresponding author on reasonable request.




