Macrophage C1q contributes to pulmonary fibrosis by disturbing the metabolism of alveolar epithelial cells
Dear Editor,
Idiopathic pulmonary fibrosis (IPF) is a devastating interstitial lung disease, driven primarily by damage and dysfunction of type-II alveolar epithelial cells (AECII). Since there is still no cure for IPF, new therapeutic approaches are desirable. In this study, we identified the complement component C1q as a source of disturbance of AECII metabolism.
When analyzing publicly available data,1 we found that C1q mRNA expression is upregulated in IPF patients (Figure S1). Therefore, we analyzed bronchoalveolar lavage fluids (BALF) of IPF patients for the presence of C1qA. Compared to controls, significantly increased levels of C1qA were present in BALF of IPF patients (Figure 1A). To investigate the role of C1q in fibrosis development, we used a virus-induced mouse model of IPF.2 First, we determined C1qA gene expression in lungs of both control (wild-type) and fibrosis-prone (interferon [IFN]-γR-/-) mice, analyzing uninfected mice and mice 14 days (acute inflammation phase), around 45 days (early fibrosis phase), and around 100 days (fibrosis phase) after infection with murine gammaherpesvirus 68 (MHV-68). C1qA was highly expressed in the acute inflammation phase in both mouse strains. It subsequently declined over time in wild-type mice whereas the decline was much less pronounced in IFN-γR-/- mice (Figure 1B). No differences were observed when comparing whole lung tissue protein levels (Figure 1C). However, mice with lung fibrosis (IFN-γR-/-) showed significantly elevated C1qA protein in BALF (Figure 1D). This was due to local production and secretion as in mice with increased C1qA in BALF (Figure S2A), C1qA in serum remained constant (Figure S2B). Higher C1qA gene expression in fibrotic mice was also observed in other fibrosis mouse models when analyzing publicly available data (Figure S3).
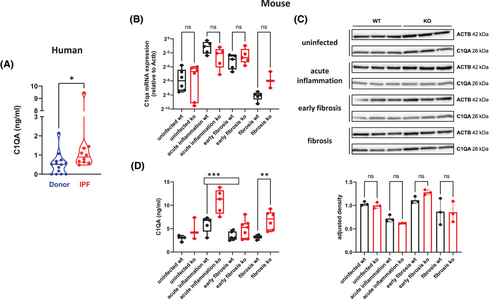
To determine the cellular source of C1q in the lung, we analyzed C1q gene expression in publicly available single-cell RNA sequencing data (Figure S4). In human lungs, macrophages are the main producers of C1qA, C1qB and C1qC. We immuno-stained sequential slices of murine lungs with the macrophage marker F4/80 and anti-C1qA (Figure S5A), and sequential slices of human lungs with anti-C1qA, the macrophage marker CD68 and the AECII marker proSPC (Figure S5B). In both cases, macrophages stained positive for C1qA. To further analyze C1q production by macrophages, we polarized MH-S cells (a murine alveolar macrophage cell line) into either M1 or M2 macrophages. Treatment with LPS + IFN-γ led to an increase in Nos2 expression, an M1 marker, while treatment with IL-4 resulted in increased Arg1 expression, an M2 marker (Figure S6A). Polarization into M2 macrophages did not increase cell-associated C1qA (Figure S6B) but significantly increased C1qA in the supernatant (Figure S6C).
We hypothesized that secreted C1qA might affect other cells in a paracrine fashion and focused on AECII. MLE-12 cells, a well-established murine AECII line, were treated with native human C1q that also reacts with murine cells.3 We observed a dose-dependent reduction in MTT (3-(4,5-dimethylthazolk-2-yl)-2,5-diphenyltetrazolium bromide)-assay activity (Figure 2A), suggesting that C1q induces cell death of AECII since the MTT-assay is commonly used as a measure of cell viability.4 Since we did not observe a corresponding increase of lactate dehydrogenase (LDH)-activity (an indicator of necrosis) in the supernatants of the treated cells (Figure 2B), we speculated that C1q induces non-necrotic cell death of AECII. As apoptosis of AECII is a main characteristic of IPF, we conducted an Apoptosis and Necrosis Assay. C1q did neither induce apoptosis (Figure S7A) nor necrosis (Figure S7B). We further confirmed the absence of apoptosis by both the Caspase-Glo 3/7 Assay (Figure S7C) and Western blot for cleaved PARP (Figure S7D). Next, we analyzed other known cell death pathways including ferroptosis, autophagy-dependent cell death, necroptosis, pyroptosis and parthanatos. We treated MLE-12 cells with C1q in the presence or absence of specific cell death inhibitors, and subsequently determined cell viability by MTT-assay, a strategy successfully applied by others.5, 6 None of the inhibitors was able to reverse the C1q-induced reduction in MTT-assay activity (Figure S8). To support these findings, we determined the gene expression of characteristic cell death-associated genes including Bax, Bcl-xl, Acsl4, RIPK3 and NLRP3 (Figure S9). We did not observe significant differences between control- or C1q-treated MLE-12 cells.
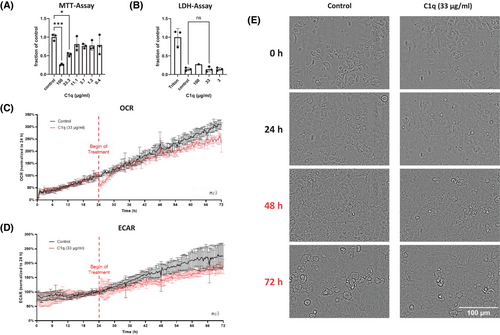
Since our data suggested that the reduction in formazan formation (MTT-assay) upon C1q treatment is not due to AECII death, we performed multiparametric monitoring of cellular metabolic responses, continuously measuring oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) and simultaneously performing periodic microscopic imaging using the CYRIS FLOX analysis platform (INCYTON GmbH, Planegg, Germany). Treatment of MLE-12 cells with a moderate dose of C1q (33 µg/mL) resulted in a significant reduction of both OCR (Figure 2C) and ECAR (Figure 2D) (C1q vs. control: p < 0.05; Mann-Whitney test). In contrast, the morphological integrity of the cells was not affected (Figure 2E).
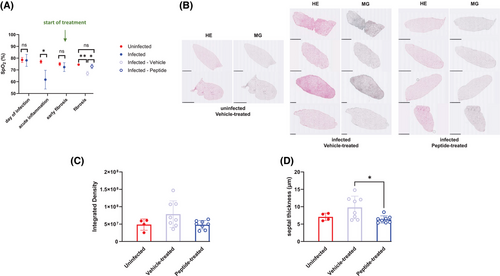
Finally, we applied a C1q inhibitory peptide7 that has already been successfully used in vivo in a mouse model of chronic hepatitis8 to investigate fibrosis development in the presence or absence of the peptide. IFN-γR-/- mice were left uninfected or were infected with MHV-68. From day 44 (= early fibrosis phase) after infection, the infected mice were either treated i.p. with 2J peptide (2 mg/kg) or with vehicle control twice a week as described by others8 until day 85 (= fibrotic phase) after infection. Treatment with the C1q inhibitory peptide significantly improved peripheral oxygen saturation (SpO2) (Figure 3A), and considerably prevented fibrosis development as determined by Hematoxylin/Eosin- and Masson-Goldner trichrome-staining of lung sections (Figure 3B,C; Figure S10) and by measurement of mean septal thickness (an indicator of fibrosis) (Figure 3D).
In summary, we identified C1q, produced by macrophages, as an inducer of a metabolic disorder of AECII, emphasizing the importance of macrophage—epithelial cross-talk for the development of pulmonary fibrosis, and propose C1q as a potential new therapeutic target.
AUTHOR CONTRIBUTIONS
Fenja Prüfer performed experiments and analyzed and interpreted the data. Beatrix Steer performed experiments. Eva Kaufmann performed experiments and analyzed and interpreted the data. Peter Wolf performed experiments and analyzed and interpreted the data. Barbara Adler discussed and interpreted the data, and reviewed and revised the final manuscript. Martina Korfei performed experiments and analyzed and interpreted the data. Andreas Günther discussed and interpreted the data. Melanie Königshoff discussed and interpreted the data. Heiko Adler supervised the project, designed the experiments, analyzed the data and wrote and edited the paper.
ACKNOWLEDGEMENTS
We are grateful to the members of our animal facilities for expert technical assistance. We also thank the members of our groups for helpful discussions. We gratefully acknowledge the provision of human BAL and tissue samples and clinical data from the CPC-M bioArchive and its partners at the Hospital of the Ludwig-Maximilians-University Munich, the Asklepios Biobank Gauting and the Ludwig-Maximilians-University Munich. We thank the patients and their families for their support.
Open access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
Peter Wolf is an employee of the company INCYTON GmbH (Germany), which developed and distributes the utilized analysis platform CYRIS FLOX. All other authors declare no conflict of interest.
FUNDING INFORMATION
This work was funded by the FöFoLe-program of the LMU Munich as well as by the German Center for Lung Research (DZL).
ETHICS STATEMENT
The study was approved by the local ethics committee of the Ludwig-Maximilians-University Munich, Germany (Projects 333-10, 455-12).
PATIENT CONSENT STATEMENT
Written informed consent was obtained for all study participants. The animal study protocol was approved by the government of Upper Bavaria and performed in compliance with the German Animal Welfare Act.
Open Research
DATA AVAILABILITY STATEMENT
The data presented in this study are available upon request from the corresponding author.




