ATG16L1 restrains macrophage NLRP3 activation and alveolar epithelial cell injury during septic lung injury
Yan Bai, Xinyu Zhan and Qing Zhu contributed equally to this work.
Abstract
Background
The lung is the organ most commonly affected by sepsis. Additionally, acute lung injury (ALI) resulting from sepsis is a major cause of death in intensive care units. Macrophages are essential for maintaining normal lung physiological functions and are implicated in various pulmonary diseases. An essential autophagy protein, autophagy-related protein 16-like 1 (ATG16L1), is crucial for the inflammatory activation of macrophages.
Methods
ATG16L1 expression was measured in lung from mice with sepsis. ALI was induced in myeloid ATG16L1-, NLRP3- and STING-deficient mice by intraperitoneal injection of lipopolysaccharide (LPS, 10 mg/kg). Using immunofluorescence and flow cytometry to assess the inflammatory status of LPS-treated bone marrow-derived macrophages (BMDMs). A co-culture system of BMDMs and MLE-12 cells was established in vitro.
Results
Myeloid ATG16L1-deficient mice exhibited exacerbated septic lung injury and a more intense inflammatory response following LPS treatment. Mechanistically, ATG16L1-deficient macrophages exhibited impaired LC3B lipidation, damaged mitochondria and reactive oxygen species (ROS) accumulation. These abnormalities led to the activation of NOD-like receptor family pyrin domain-containing protein 3 (NLRP3), subsequently enhancing proinflammatory response. Overactivated ATG16L1-deficient macrophages aggravated the damage to alveolar epithelial cells and enhanced the release of double-stranded DNA (dsDNA), thereby promoting STING activation and subsequent NLRP3 activation in macrophages, leading to positive feedback activation of macrophage NLRP3 signalling. Scavenging mitochondrial ROS or inhibiting STING activation effectively suppresses NLRP3 activation in macrophages and alleviates ALI. Furthermore, overexpression of myeloid ATG16L1 limits NLRP3 activation and reduces the severity of ALI.
Conclusions
Our findings reveal a new role for ATG16L1 in regulating macrophage NLRP3 feedback activation during sepsis, suggesting it as a potential therapeutic target for treating sepsis-induced ALI.
Key points
- Myeloid-specific ATG16L1 deficiency exacerbates sepsis-induced lung injury.
- ATG16L1-deficient macrophages exhibit impaired LC3B lipidation and ROS accumulation, leading to NLRP3 inflammasome activation.
- Uncontrolled inflammatory responses in ATG16L1-deficient macrophages aggravate alveolar epithelial cell damage.
- Alveolar epithelial cells release dsDNA, activating the cGAS-STING-NLRP3 signaling pathway, which subsequently triggers a positive feedback activation of NLRP3.
- Overexpression of ATG16L1 helps mitigate lung tissue inflammation, offering a novel therapeutic direction for sepsis-induced lung injury.
1 INTRODUCTION
Sepsis is a severe inflammatory response caused by infection, which can lead to multiple organ failure and death.1 In 2017, sepsis was estimated to cause 11 million deaths, accounting for nearly 20% of all global fatalities.2 Bacteria have been identified as the primary pathogens causing infection-induced sepsis.3 Lung is the most vulnerable organs during sepsis, with over 50% of patients with sepsis developing ALI.4 The primary characteristic of sepsis is immune dysregulation triggered by infection, which often manifests as excessive inflammation.5 During early stages of infection, various stimuli triggers the activation of pattern recognition receptors, prompting macrophages, dendritic cells and neutrophils to release inflammatory cytokines.6 In later stages, some patients exhibit persistent inflammation, immunosuppression and catabolic syndrome, which are characterised by prolonged hyperinflammation, immunosuppression and bone marrow dysfunction.7 Acute respiratory distress syndrome is a severe and progressive complication of sepsis, and has a high mortality rate.8
NLRP3 inflammasome is a well-studied supramolecular complex whose activation coordinates the cleavage of pro-caspase1 and secretes cytokines, subsequently triggering a robust immune response.9 Notably, the macrophage NLRP3 activation significantly contributes to mice ALI. The inhibition of NLRP3 can markedly alleviate lung injury.10, 11
Autophagy is a process of cellular self-digestion through which cells degrade and recycle damaged organelles and proteins, thereby maintaining cellular homeostasis,12 which facilitates the clearance of microorganisms and controls the inflammatory response by negatively regulating macrophage inflammasome activation.13, 14 ATG16L1 is essential for autophagy, aiding in autophagosome formation and maturation. It binds to ATG5 and recruits the ATG12‒ATG5 complex to the site of autophagosome formation.15 The ATG12‒ATG5‒ATG16L1 complex possesses E3 ligase activity, facilitating the recruitment of LC3 to the phagophore and activating the E2 ligase ATG3, which in turn mediates the conjugation of LC3 with phosphatidylethanolamine (PE).16, 17 Studies have indicated that the absence of ATG16L1 impairs the recruitment of the Atg12‒Atg5, leading to a failure in LC3 conjugation to PE.18 IL-22 stimulation of ATG16L1-deficient intestinal epithelial cells induces endoplasmic reticulum stress and necroptosis, subsequently activating STING signalling and inducing ileitis in vivo.19 Furthermore, ATG16L1-deficient macrophages exhibited defective autophagy and promoted the secretion of inflammatory cytokines upon LPS stimulation.18 However, the specific role of ATG16L1 in macrophages and its mechanisms of action in sepsis-induced lung injury remains poorly understood.
Herein, the critical role of ATG16L1 in controlling lung tissue inflammation has been confirmed. Mechanistically, our study revealed that ATG16L1-deficient macrophages are involved in the regulation of molecules such as NLRP3 and STING, resulting in an uncontrolled inflammatory response and subsequent macrophage activation in the lung tissues.
2 MATERIALS AND METHODS
2.1 Mice studies
Wild-type (WT), FloxP-atg16l1 (ATG16L1fl/fl), myeloid-specific atg16l1-knockout (ATG16L1mKO), myeloid-specific atg16l1-overexpressing-knockin (ATG16L1mKI), FloxP-nlrp3 (NLRP3fl/fl) and myeloid-specific nlrp3-knockout (NLRP3mKO) C57BL/6 male mice were engineered by GemPharmatech Co. FloxP-Tmem173 (STINGfl/fl) and myeloide-specific Tmem173-knockout (STINGmKO) male mice on a C57BL/6 background were engineered by Shanghai Model Organisms Center. The ATG16L1fl/fl, NLRP3fl/fl and STINGfl/fl mice served as WT controls. All mice were housed in a standard, specific pathogen-free environment. Animal procedures complied with the legal and ethical guidelines set forth in the protocol approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (IACUC-2402019).
Mice were intraperitoneally injected with LPS (10 mg/kg; Sigma‒Aldrich) to establish the model of sepsis-induced ALI. The sham group was injected intraperitoneally with an equal amount of phosphate buffer saline (PBS). A dose of LPS (20 mg/kg) was administered through intraperitoneal injection for the survival studies.
The selective NLRP3 inhibitor MCC950 (10 mg/kg; MedChemExpress) was administered intraperitoneally 4 h before the LPS injection to inhibit the NLRP3 inflammasome. To reduce mitochondrial reactive oxygen species (ROS) production, Mitotempo (20 mg/kg, Sigma), a mitochondria-specific antioxidant, was given via intraperitoneal injection. To inhibit anti-8-hydroxydeoxyguanosine (8-OHdG), mice received an intraperitoneal injection of anti-8-OHdG antibody (10 mg/kg; GeneTex) 2 h before the LPS injection, while the control group was administered immunoglobulin G (5 mg/kg; R&D Systems).
2.2 BMDMs culture and treatments
As previously mentioned, BMDMs were isolated from the mice and cultured in Dulbecco's Modified Eagle Medium(DMEM) medium containing colony-stimulating factor (CSF).20 LPS (100 ng/mL) stimulated BMDMs for 12 h. In in vitro experiments, BMDMs were pretreated with Mitotempo (1 mM; MedChemExpress), MCC950 (10 µM; MedChemExpress), 3-MA (5 Mm; MedChemExpress) and C-176 (20 µM; MedChemExpress) 2 h before LPS stimulation.
2.3 NLRP3 siRNA and STING-siRNA in vivo and in vitro transfection
STING-siRNA (Santa Cruz Biotechnology) was dissolved in an in vivo transfection reagent and intratracheally administered to mice 48 h prior to LPS treatment. The control group was administered a negative control siRNA using the same vector.
For in vitro transfection of BMDMs, targeting siRNAs for NLRP3 and STING, along with a negative control siRNA (Santa Cruz Biotechnology), were dissolved in OPTI-MEM medium. Prior to transfection, BMDMs were pre-incubated in six-well plates with antibiotic-free medium for 24 h. Transfection was carried out using Lipofectamine 2000 (Invitrogen) and serum-free medium upon reaching 60%–80% confluency. After 6 h, fresh medium with 10% fetal bovine serum (FBS) replaced the transfection medium. The BMDMs were then incubated at 37°C in a humidified atmosphere with 5% CO2. BMDMs and supernatants were then collected for subsequent Western blot and quantitative real-time qPCR (RT-qPCR) analyses.
2.4 Histopathology
Lung tissues sections (4 µm thick) were stained with haematoxylin and eosin (H&E). Observe the samples using an optical microscope (Carl Zeiss). Lung tissue damage is assessed by evaluating the degree of haemorrhage, neutrophil infiltration, hyaline membrane formation, persistent debris in the airspaces and septal thickening.21
2.5 BMDMs and lung tissues immunofluorescence staining
BMDMs were fixed using 4% paraformaldehyde for 15 min. The membranes were treated with. 3% Triton X-100 for 10 min to permeabilise, followed by a 60-min blocking step using goat serum. The lung tissue sections were deparaffinised and underwent antigen retrieval, followed by blocking with goat serum for 60 min. Stained BMDMs and lung tissues were observed and imaged on a laser confocal microscope with 10×, 20× and 40× objective lenses (Zeiss LSM 780, Carl Zeiss). Antibodies used are provided in Table S1.
2.6 Western blot
BMDMs and lung tissue samples were utilised to extract cellular proteins using RIPA buffer containing protease and phosphatase inhibitors. The supernatant from BMDMs was collected, and proteins were precipitated with methanol and chloroform to isolate the supernatant proteins.22 The extracted proteins were separated by SDS/PAGE gels (8%, 10% or 15% [vol/vol]). Antibodies used are provided in Table S2. The ImageJ software was used to perform quantitative and semi-quantitative analyses of the Western blot results.
2.7 Assessment of mitochondrial membrane potential (MMP) and ROS levels
In healthy mitochondria, JC-10 aggregates in the mitochondrial matrix to form polymers, emitting intense red fluorescence (Ex = 540 nm and Em = 590 nm). In damaged mitochondria, a reduction or loss of membrane potential causes JC-10 to exist as a monomer in the plasma of stem cells, producing green fluorescence (Ex = 490 nm and Em = 525 nm). MMP was assessed using the JC-10 kit (Maokang). The measurement of ROS levels in BMDMs and lung tissues was conducted using 2,7-dichlorodihydrofluorescein diacetate (Yeasen). The measurement was conducted via flow cytometry and immunofluorescence staining.
2.8 Measurement of malondialdehyde (MDA), superoxide dismutase (SOD) and the glutathione/glutathione oxidized (GSH/GSSG) levels
Lung tissues were washed with PBS, and the tissue was minced and homogenised by sonication. The tissue was further homogenised and centrifuged at 10 000 × g for 10 min to obtain the supernatant. The concentrations of MDA, GSH, GSSG and SOD were determined according to the manufacturer's instructions (Nanjing Jiancheng).
2.9 Real-time qPCR
TRIZOL reagent (Invitrogen) was used to isolate total RNA from lung tissues and BMDMs. The RNA was subsequently converted into cDNA with the assistance of the Takara reverse transcriptase kit (Takara). RT-qPCR analysis was conducted using the SYBR RT-PCR kit (Takara). Quantitative PCR was repeated four times. The PCR primer sequences are provided in Table S3. Expression levels of individual genes were normalised to β-actin as an internal control.
2.10 Quantification of 8-OHdG levels
8-OHdG in mice serum and co-culture systems MLE-12 supernatant were determined using an 8-hydroxydeoxyguanosine Assay Kit (Nanjing Jiancheng).
2.11 Transmission electron microscopy
BMDMs treated with LPS were fixed using electron microscopy fixative (G1102-100ML, Servicebio). Processed macrophages were analysed using transmission electron microscopy. Imaging was performed using an electron microscope (HITACHI). The images were captured at magnifications of 2.5K× and 7K×.
2.12 Establish a co-culture system of BMDMs and MLE-12 cells
Mouse alveolar cells (MLE-12, ATCC) are cultured in the basolateral chamber of a six-well transwell system, and BMDMs are placed in the apical chamber. After co-culturing in the system for 24 h, the supernatant from the MLE-12 cells is collected, and the cells are separated for analysis.
2.13 Lactate dehydrogenase activity assay
To assess MLE-12 cell death, the level of lactate dehydrogenase (LDH) in the supernatant of co-cultured MLE-12 cells was measured using a kit in accordance with the manufacturer's protocols (Nanjing Jiancheng).
2.14 Statistical analysis
Statistical analysis was conducted using the GraphPad Prism software (version 9.5.1, GraphPad Software Inc.). The Shapiro‒Wilk test was used to assess the normality of the data. Continuous variables with a normal distribution were analysed using either the Student's unpaired t-test or ANOVA with post hoc Tukey's test. For continuous data that are not normally distributed, the Kruskal‒Wallis rank-sum test is recommended for group comparisons. A *p ≤. 05 (two-tailed) was regarded as statistically significant.
3 RESULTS
3.1 Myeloid-specific ATG16L1 deficiency exacerbates sepsis-induced lung injury
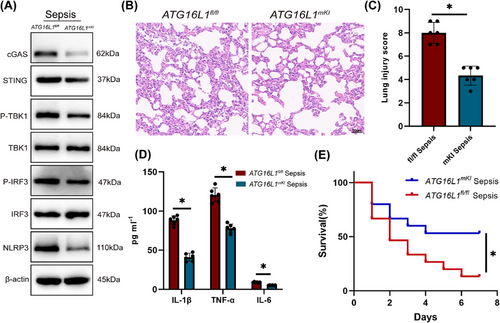
Initially, ATG16L1 levels in the lungs of normal and septic mice were examined. Following LPS treatment, the protein levels of ATG16L1 in lungs increased (Figure 1A). Then, the alveolar macrophages and neutrophils were isolated from the BALF following methods previously established by our research group.23 Flow cytometry revealed an increase in ATG16L1 levels in alveolar macrophages, while no significant changes were observed in neutrophils (Figure 1B,C). Furthermore, immunofluorescence co-localisation analysis confirmed that ATG16L1 was upregulated in lung macrophages during sepsis (Figure 1D). We constructed mice with myeloid ATG16L1 deficiency (Figure S1A). Following LPS treatment, lung tissue H&E staining indicated increased infiltration of inflammatory cells and aggravated damage to the lungs of ATG16L1mKO mice (Figures 1E and S1D). Compared with the ATG16L1fl/fl group, the ATG16L1mKO group had a lower 7-day survival rate (Figure 1F). Additionally, a significant decrease ZO-1, Occludin and Claudin 3 levels was observed in the lung tissues of ATG16L1-deficient mice, indicating more severe disruption of the lung tissue barrier structure (Figure 1G,H). The concentrations and mRNA of IL-1β, TNF-α and IL-6 significantly increased in ATG16L1mKO mice (Figures and S1E). These results suggest that ATG16L1-deficient mice exhibit a stronger inflammatory response and more severe lung tissue damage following LPS challenge.

3.2 ATG16L1 deficiency promotes macrophage NLRP3 inflammasome activation to aggravate ALI
Activation of the macrophages NLRP3 results in the release of inflammatory cytokines, which can cause damage to epithelial and endothelial cells.21 To further explore the role of NLRP3 in ALI, myeloid-specific NLRP3-knockout mice were generated (Figure S1B). We confirmed that deletion of myeloid NLRP3 alleviated inflammation in lung tissues and reduced inflammatory cytokine levels in BALF (Figures 2A,B and S2A). To examine the impact of ATG16L1 on NLRP3 activation, a sepsis model was established, NLRP3 and cleaved-caspase1 significantly elevated in ATG16L1-deficient mice (Figure 2C). BMDMs stimulated with LPS and ATP markedly enhanced NLRP3 activation (Figures 2D and S2B). The cytokines IL-1β, TNF-α and IL-6 and their mRNA expression in BMDMs supernatants were significantly elevated (Figures 2E and S2C). To investigate the interaction between ATG16L1 and the NLRP3 inflammasome, NLRP3 siRNA was utilised to knock down NLRP3 in BMDMs. NLRP3 siRNA effectively reduced NLRP3 expression in BMDMs and decreased cl-caspase1 activation (Figure 2F). Furthermore, the secretion of inflammatory factors was significantly reduced in the supernatant of BMDMs treated with NLRP3 siRNA (Figure 2G). MCC950, an NLRP3 inhibitor, effectively suppressed NLRP3 activation in ATG16L1-knockout BMDMs (Figure S2D). The cytokines IL-1β, TNF-α and IL-6 in BMDMs supernatants were significantly elevated (Figure S2E). MCC950 significantly alleviated lung tissues inflammation and reduced lung injury (Figure S2F,G). These results suggest that ATG16L1 deficiency amplifies the inflammatory response through the activation of NLRP3.

3.3 Deletion of ATG16L1 increases macrophage ROS accumulation by inhibiting autophagy
ROS are crucial in activating the NLRP3 inflammasome.24 Disruption of mitochondrial homeostasis results in the generation of significant levels of ROS.25 Immunofluorescence revealed increased ROS in the lung tissues of ATG16L1-deficient mice compared with ATG16L1fl/fl mice (Figure 3A). The findings revealed that myeloid ATG16L1-deficient mice exhibited elevated MDA levels in the lung tissue and reduced levels of GSH/GSSG and SOD (Figure 3B‒D). Furthermore, in vitro, MMP significant decrease in ATG16L1-deficient macrophages stimulated with LPS, which suggests mitochondrial damage and dysfunction (Figures 3E and S2A). Additionally, a significant increase in total cellular ROS and mitochondrial ROS levels was observed (Figure S2B‒D). Autophagy is crucial for clearing damaged mitochondria.26 When autophagy is defective, damaged mitochondria accumulate, hindering the scavenging of free radicals.25 Following LPS stimulation, normal BMDMs demonstrated autophagy activation, whereas ATG16L1-deficient macrophages exhibited impaired LC3B lipidation and autophagy (Figure 3F,G). Electron microscopy revealed that ATG16L1-deficient BMDMs accumulated damaged mitochondria and exhibited reduced autophagosome formation (Figure 3H). ROS regulatory factors, including FOXO3a, Nrf2 and P53, were examined. Following LPS treatment of BMDMs, the expression of FOXO3a and P53 was upregulated, and Nrf2 levels were downregulated. However, no significant differences were observed between BMDMs with or without ATG16L1 deletion (Figure S3E). We hypothesised that the lack of noticeable differences in macrophages with ATG16L1 deletion may be attributed to the complexity of the regulatory interactions between various signalling pathways. Furthermore, we overexpressed ATG16L1 in BMDMs (Figure S1A). Following LPS stimulation, the production of ROS and of the NLRP3 signalling pathway in ATG16L1mKI BMDMs were reduced, and the secretion of IL-1β, TNF-α and IL-6 in the BMDMs supernatant was also decreased (Figures 3I,J and S3F). 3-MA, an autophagy inhibitor, abrogated the effects of ATG16L1 (Figure S3G,H). These results demonstrated that ATG16L1 regulates mitochondrial oxidative stress in macrophages and reduces ROS production through autophagy.
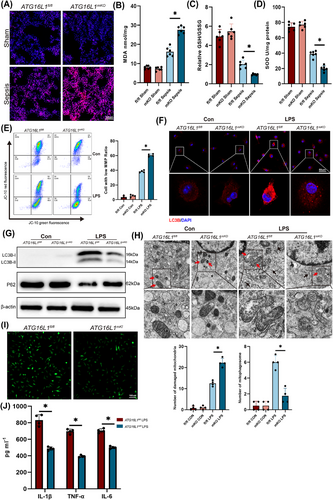
Mitotempo, a mitochondrial-targeted ROS scavenger, notably decreases ROS levels in ALI (Figure S4A). By scavenging ROS, it significantly inhibits NLRP3 activation in lung tissues and BMDMs (Figure S4B). Furthermore, Mitotempo significantly alleviated lung tissues inflammation, reduced BALF cytokines and the inflammatory cytokine mRNA (Figure S4C‒F). In vitro, Mitotempo reduces ROS levels and prevents NLRP3 activation in LPS-treated BMDMs, significantly decreased cytokines in the supernatant of LPS-treated BMDMs and downregulated their mRNA expression (Figure S5A‒E). In summary, these results indicate that ROS accumulation in ATG16L1-deficient macrophages mediates NLRP3 activation, thereby promoting macrophage inflammatory responses and exacerbating ALI.
3.4 ATG16L1-deficient macrophages release inflammatory cytokines to damage alveolar epithelial cells after LPS treatment
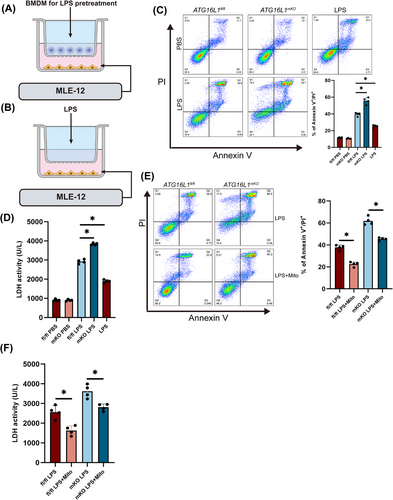
Upon LPS stimulation, macrophages secrete inflammatory cytokines that mediate lung tissue damage.27 A co-culture system of macrophages and alveolar epithelial cells was established to investigate the effects of inflammation-activated macrophages on alveolar epithelial cells (Figure 4A). Considering that LPS in the co-culture system could potentially affect the activity of alveolar epithelial cells, MLE-12 cells were treated with LPS alone to exclude the impact of LPS on alveolar epithelial cells (Figure 4B). MLE-12 cells co-cultured with ATG16L1-deficient BMDMs showed increased apoptosis and necrosis (Figure 4C). Additionally, elevated LDH levels in the supernatant of MLE-12 cells indicated alveolar epithelial cell damage (Figure 4D). After Mitotempo treatment, reduced damage to alveolar epithelial cells was observed (Figure 4E,F). These results suggest that ATG16L1-deficient macrophages exacerbate alveolar epithelial cell damage through an amplified inflammatory response, thereby aggravating tissue injury.
3.5 Inflammatory injury of alveolar epithelial cells enhances macrophage NLRP3 activation via double-stranded DNA‒STING signalling
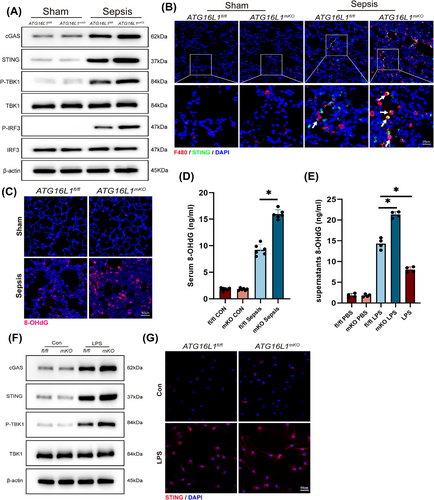
Macrophage STING activation is critical in the development of ALI caused by sepsis.28, 29 We observed a significant activation of the cGAS‒STING of ATG16L1-deficient mice lung following LPS stimulation (Figure 5A). Immunofluorescence co-localisation revealed increased macrophage infiltration accompanied by STING activation in macrophages (Figure 5B). cGAS recognises dsDNA and facilitates the translocation of STING to the Golgi apparatus.30 The 8-OHdG can be used to detect oxidative stress-induced damage to nuclear and mitochondrial DNA. Levels of 8-OHdG in the lung tissue and serum of ATG16L1-deficient mice were markedly elevated after LPS treatment (Figure 5C,D). 8-OHdG levels were significantly increased in the supernatant of MLE-12 cells cultured with ATG16L1mKO BMDMs compared to ATG16L1fl/fl (Figure 5E). To explore the activation of macrophage STING by dsDNA released from alveolar epithelial cells. The supernatants of co-cultured MLE-12 cells were collected and used as conditioned medium (MLE-12-CM) to stimulate BMDMs, resulting in activation of STING in BMDMs (Figure 5F,G).
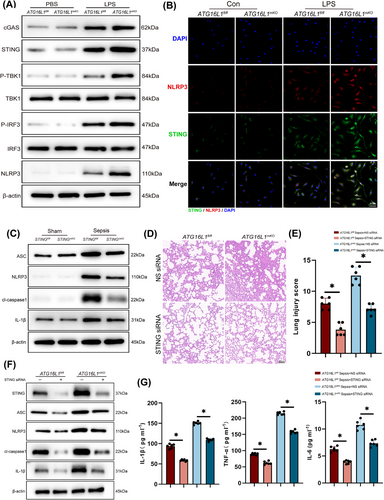
Previous researches have confirmed that the STING regulates the NLRP3 inflammasome activating.31, 32 Activation of the BMDMs STING‒NLRP3 was observed after MLE-12 CM stimulation (Figure 6A,B). Subsequently, myeloid-specific STING-deficient mice were generated (Figure S1C), and reduced NLRP3 activation was observed in the lung tissue of STING-deficient mice after sepsis induction (Figure 6C). BMDMs extracted from the bone marrow of STINGfl/fl and STINGmKO mice showed reduced NLRP3 activation upon LPS stimulation, as STING was depleted (Figure S6A). To further investigate the regulatory role of STING in the modulation of NLRP3, siRNA was constructed to knock down STING levels in mice and BMDMs. The experimental results demonstrated that in vivo knockdown of STING alleviated lung tissue inflammation, activation of the NLRP3 inflammasome pathway and reduced levels of inflammatory cytokines in BALF (Figure 6D‒G). In vitro experiments, STING siRNA effectively knocked down STING in BMDMs and reduced the activation of the NLRP3 inflammasome pathway and the secretion of inflammatory factors (Figure S6B‒D). Furthermore, C-176, a targeted STING inhibitor, inhibited STING activation in macrophages and reduced NLRP3 activation (Figure S6E,F). BMDMs treated with C-176 showed a significantly reduced production of inflammatory cytokines (Figure S6G,H). In conclusion, our experimental results indicate that ATG16L1 deficiency leads to the accumulation of dsDNA in the lung tissue, subsequently activating the STING signalling pathway in macrophages. STING promotes NLRP3 activation, forming a positive feedback loop that amplifies the inflammatory response.
3.6 Clearance of dsDNA suppresses macrophage STING‒NLRP3 activation to protect lungs against septic injury
To explore whether blocking the STING‒NLRP3 pathway would be beneficial for controlling lung inflammation, mice with sepsis were pretreated with anti-8-OHdG antibodies. Our results showed that anti-8-OHdG antibody treatment reduced STING‒NLRP3 activation in the lung tissue (Figure 7A). Similarly, anti-8-OHdG treatment of BMDMs decreased STING‒NLRP3 activation (Figure S7A). Additionally, anti-8-OHdG treatment significantly alleviated lung tissue inflammation (Figure 7B,C). ZO-1, Occludin and Claudin 3 levels in lung tissues were upregulated after anti-8-OHdG treatment, indicating an improvement in lung barrier structure (Figure 7D,E). Furthermore, the concentrations of inflammatory factors in BALF significantly reduced after clearing dsDNA (Figures 7F and S7B). In summary, our study suggests that clearing damaged DNA in vivo can reduce lung tissues inflammation.
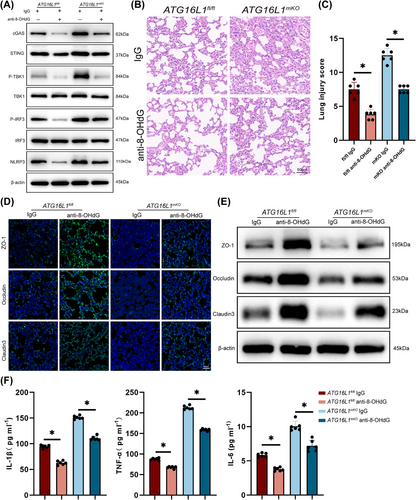
3.7 Myeloid-ATG16L1 overexpression alleviates septic lung injury
To verify the protective role of ATG16L1 against sepsis-induced lung injury, ATG16L1 overexpression mice were generated (Figure S1A). Compared to ATG16L1fl/fl mice, the overexpression of ATG16L1 notably inhibited the cGAS‒STING‒NLRP3 signalling pathway in lung tissue subjected to LPS treatment (Figure 8A). Additionally, ATG16L1 overexpression markedly reduced lung inflammation in septic mice (Figure 8B‒D), leading to an improved 7-day survival rate (Figure 8E). In summary, ATG16L1 overexpression provides significant benefits against sepsis-induced lung injury.
4 DISCUSSION
An analysis of 30 606 inflammatory bowel disease (IBD) patients revealed that the ATG16L1 variant rs2241880 is a persistent risk factor for the development of IBD and may influence patient prognosis.33 Autophagy mediated by ATG16L1 in microglia ameliorates central nervous system inflammation and alleviates inflammatory responses and clinical symptoms of experimental autoimmune encephalomyelitis.34 Nevertheless, the function of macrophage ATG16L1 in regulating lung inflammation and the NLRP3 inflammasome activating remains to be elucidated. Here, we found that myeloid ATG16L1-knockout mice were observed to exhibit exacerbated lung inflammation, reduced survival rates and increased activation of the NLRP3 inflammasome. Further investigation indicated that loss of ATG16L1 impaired autophagy and activated the NLRP3 inflammasome by accumulating of mitochondrial ROS, which triggered macrophage inflammatory activation. Secretion of inflammatory cytokines by activated macrophages damaged alveolar epithelial cells and releases dsDNA. The dsDNA released by damaged alveolar epithelial cells activates the STING‒NLRP3 pathway, thereby creating a positive feedback loop for NLRP3 activation. The positive feedback activation of NLRP3 triggers an uncontrollable inflammatory response, thereby exacerbating sepsis-induced ALI.
The immune system is pivotal in the onset of sepsis. Macrophages, which are the predominant immune cells in lung tissue, preserving lung homeostasis and protecting against pathogenic invasion.35 During ALI, macrophages and their secreted exosomes activate neutrophils and promote their secretion of inflammatory factors, thereby exacerbating ALI.36 TNF-α, released by macrophage cells, induces Granulocyte-macrophage colony-stimulating factor (GM-CSF) expression in the alveolar epithelium. GM-CSF maintains the alveolar barrier function by promoting the proliferation of alveolar epithelial cells.37 The NLRP3 inflammasome is an important sensors in the innate immune and expressed in myeloid immune cells.38 In COVID-19 patients, the activation of inflammasomes in lung macrophages leads to the release of IL-1 and IL-18, which may cause excessive lung inflammation.39 However, the released inflammatory factors may also aid in combating viral invasion. LPS treatment activates the NLRP3 inflammasome in alveolar macrophages, mediates inflammatory response, and plays a significant role in ALI.11, 40 Potassium efflux, increased cytosolic calcium levels, and ROS accumulation effectively activate the NLRP3 inflammasome.24 ROS are one of the earliest discovered activators of the NLRP3 inflammasome. ROS production promotes the relocation of NLRP3 to the mitochondria-associated ER membrane and recruits ASC, thereby facilitating NLRP3 inflammasome activation.41 Our study confirmed that upon LPS stimulation of ATG16L1-deficient macrophages, cytosolic ROS levels significantly increased and the assembly of the NLRP3 inflammasome was enhanced. Scavenging ROS effectively prevents NLRP3 inflammasome activation and mitigates inflammatory damage in lung tissue.
The balance between ROS production and elimination is crucial to avoid cellular damage and maintain homeostasis.42 FOXO3a, Nrf2 and P53 play a crucial role in regulating ROS production. Mitochondrial ROS can activate Nrf2, whereas excessive ROS suppresses Nrf2.43 At physiological levels, P53 transactivates antioxidant genes to control ROS levels, whereas at elevated levels, P53 facilitates ROS generation.44 In adipocytes deficient in autophagy proteins ATG3 and ATG16L1, the accumulation of dysfunctional mitochondria increases, resulting in increased activation of Nrf2 and Keap1.45 In ATG5-knockout mice with impaired autophagy, P62 accumulation increases and Nrf2 activation plays a critical role in APAP-induced acute liver injury.46 Mitochondria are the most important and typical sources of ROS in macrophages during antibacterial responses.47 When mitochondrial homeostasis is disrupted, mitochondria generate and release large amounts of ROS, ultimately activating NLRP3 inflammasome.48 Following the LPS stimulation of macrophages, mitophagy plays a major role in ROS elimination, inflammation inhibition and pyroptosis suppression.11 ROS activates autophagy, which degrades and recycles damaged intracellular macromolecules and dysfunctional organelles.49 ATG16L1, a key molecule that promotes LC3 lipidation, has become the focus of IBD research in recent years.50 After Salmonella enterica serovar Typhimurium infection in the small intestine and cecum, ATG16L1-mediated autophagy promotes Salmonella clearance, thereby reducing intestinal inflammation.51 ATG16L1 also prevents necroptosis in intestinal epithelial cells. ATG16L1 deficiency disrupts mitochondrial homeostasis and enhances endoplasmic reticulum stress.52 LPS stimulation of ATG16L1-deficient macrophages induces the production of substantial amounts of the inflammatory cytokines.18 Furthermore, autophagy regulates activation of inflammasomes, and defects in autophagy lead to impaired degradation of NLRP3.53 However, the regulatory mechanism of ATG16L1 on NLRP3 and its role in sepsis remain unclear. Our study confirmed that upon LPS stimulation of ATG16L1-deficient macrophages, cytosolic ROS levels significantly increased, thereby promoting NLRP3 inflammasome activation. These results underscore the role of ATG16L1-mediated autophagy in the regulation of the NLRP3 inflammasome.
As a DAMP, DNA has strong immunogenicity. The cGAS‒STING is the primary pathway recognised intracellular DNA recognition.54 Upon binding of dsDNA to cGAS, the catalytic activity of cGAS is activated through an allosteric mechanism, resulting in the production of 2,3-cGAMP, which subsequently activates the downstream STING pathway. STING activation triggers TANK-binding kinase 1 (TBK1) and induces the transcription of interferon-stimulated genes involved in antibacterial and antitumor responses.55-57 Activated neutrophils release neutrophil extracellular traps, which activate the pulmonary cGAS‒STING pathway, thereby exacerbating LPS-induced lung inflammation.58 In the later stages, STING is transported to the lysosome for degradation, with the blockade of STING degradation playing an important role in anti-tumour responses.59 In dendritic cells and fibroblasts, autophagy involving ATG5, ATG9, ATG12 and ATG16L1 contributes to STING degradation.60 Furthermore, cGAS‒STING can activate autophagy independently of TBK1 activation and the INF mechanism.61 STING promotes lysosomal activity through the activation of transcription factor EB, thereby resisting pathogen invasion.62 Mechanistically, STING activation directly promotes the binding of lipidated LC3B to the autophagosome membrane via the WD40 domain of ATG16L.63 This suggests that the interaction between ATG16L1 and STING may influence various diseases, including inflammation, cancer and Alzheimer's disease.
Our previous study demonstrated that STING-mediated NLRP3 activation aggravated liver ischaemia‒reperfusion injury in ageing macrophages.31 Loss of myeloid XBP1 impairs BNIP3-mediated mitophagy and promotes macrophage cGAS/STING/NLRP3 activation through leakage of mtDNA into the cytosol, thereby exacerbating liver fibrosis.64 In sepsis-induced ALI, LPS increased the transcription factor c-Myc, which activated the STING‒NLRP3.28 Our previous research found that the knockout of ATG16L1 inhibits lipophagy in macrophages, having a key role in the progression of metabolic dysfunction-associated steatohepatitis.65 In this context, activated macrophages were observed to interact with alveolar epithelial cells, thereby triggering the STING‒NLRP3 axis, resulting in excessive activation of NLRP3 and causing an uncontrollable inflammatory response.
Several drugs target ATG16L1/NLRP3/STING and play a crucial role in controlling inflammatory responses. Tocilizumab and Ustekinumab significantly upregulates ATG16L1 levels, alleviating intestinal inflammation.66 Norwogonin inhibits the NLRP3/nuclear factor-κB pathway, thereby decreasing the release of inflammatory factors and alleviating LPS-induced ALI.67 Clioquinol directly inhibits NLRP3 inflammasome activation, reduces macrophage inflammatory factor production, and alleviates experimental sepsis, colitis and acute peritonitis.68 Gelsevirine and Glycyrrhiza uralensis improve sepsis by inhibiting the cGAS‒STING signalling pathway in microglia and macrophages.69, 70
In summary, this study revealed that ATG16L1 regulates macrophage NLRP3 activation during sepsis and interacts with alveolar epithelial cells to form a positive feedback activation loop during sepsis-induced lung injury. However, this study has certain limitations. Owing to the lack of clinical samples, the role and mechanisms of ATG16L1 in humans require further investigation. Furthermore, it remains to be explored whether ATG16L1-mediated autophagy influences the clearance of dsDNA derived from alveolar epithelial cells.
AUTHOR CONTRIBUTIONS
Yan Bai, Xinyu Zhan and Qing Zhu performed experiments. Xingyue Ji, Yingying Lu, Yiyun Gao, Fei Li and Zhu Guan analysed the data. Haoming Zhou and Zhuqing Rao designed the research and wrote the manuscript. All authors reviewed the manuscript.
ACKNOWLEDGEMENTS
We would like to thank the Core Facility of the First Affiliated Hospital of Nanjing Medical University for its help in the experiment. This work was supported by the National Natural Science Foundation of China (grant numbers 81901628 and 882370668) and the Natural Science Foundation of Jiangsu Province (grant number BK20240053).
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
ETHICS STATEMENT
Animal procedures complied with the legal and ethical guidelines set forth in the protocol approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (IACUC-2402019).
Open Research
DATA AVAILABILITY STATEMENT
All data supporting the findings of this study are available within the paper.




