Cannabinoid receptor 2 facilitates the Schwann cells-dependent peripheral nerve regeneration
Heng Xu, Lu Wen, and Yi Luo contributed equally to this study.
Dear Editor:
Here, we demonstrated that cannabinoid receptor 2 (CB2) plays a pivotal role in Schwann cells (SCs) by promoting the remyelination process following peripheral nerve injury (PNI). Selective activation of CB2 shows potential as a therapeutic approach to enhance nerve repair in these injuries.
Schwann cells form Büngner bands to guide axonal regeneration during PNI. However, the slow pace of axonal growth (∼1–3 mm/day) often leads to the degradation of these structures within 8 weeks, thereby disrupting the regenerative process.1, 2 CB2 activation has been shown to promote remyelination and enhance nerve regeneration by modulating inflammatory responses and supporting cellular processes essential for nerve repair.3, 4 However, the cellular mechanisms of CB2 function in remyelination remain unclear. This prompted the hypothesis that selective activation of CB2 could maintain Schwann cell function and create a more conducive environment for axonal regeneration.
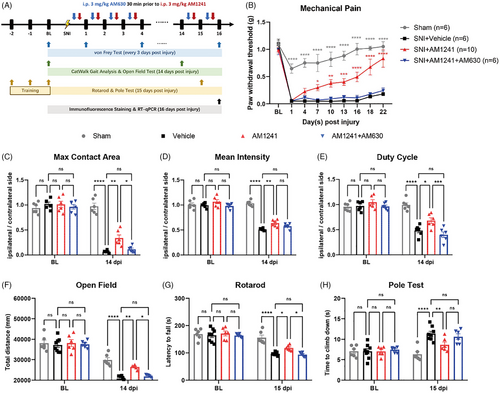
To investigate this, we employed a mouse model of crush injury and treated the mice with delta-9-tetrahydrocannabinol (Δ9-THC, a partial agonist of both CB1 and CB2 receptors, for 16 consecutive days; Figure S1A). Interestingly, Δ9-THC treatment resulted in significantly increased growth rate of injured axons and improved behaviour in mice, including reflexive mechanical allodynia and walking parameters (Figure S1B–K). To exclude the possibility that Δ9-THC exerts its effect via CB1 activation, we used CB2-specific agonists. Although the agonist (GW842166X, EC50: ∼63 nM) did not improve mechanical pain (Figure S2A), the more efficient agonist, AM1241 (EC50: ∼3.4 nM), showed therapeutic efficacy. As expected, compared with vehicle treatment, daily administration of AM1241 significantly improved mechanical allodynia, gait abnormalities and motor function in mice. These improvements were further abolished when AM1241 was co-administered with the CB2 antagonist AM630 (Figure 1 and Figure S2B). Consistent with these behavioural data, immunofluorescence staining showed that while nerves in the control group began to extend along their original growth direction, this change appeared to be more pronounced after AM1241 treatment (Figure S3A–C). We also examined the mRNA expression of genes related to myelination in the sciatic nerve tissues using real-time quantitative PCR (RT-qPCR). In the presence of AM1241, pro-myelination-related genes including Sox10, Egr2 and Tprv4 were significantly upregulated, whereas AM630 blocked CB2-dependent gene upregulation. Trpv4 has been proved to delay the re-myelination after nerve injury.5, 6 Interestingly, the expression of the SCs dedifferentiation-related genes Sox2 and c-Jun was not affected (Figure S3D). Moreover, Aniline Blue staining and electron microscopy demonstrated that CB2 stimulation significantly increased myelin thickness, an effect abolished by the CB2 antagonist AM630 (Figure S4). Supporting this, immunofluorescence staining and RT-qPCR analyses of Mpz and Mbp showed consistent changes (Figure S5). Collectively, these findings suggest that pharmacological activation of CB2 enhances the promyelination process in Schwann cells, actively promoting nerve regeneration.
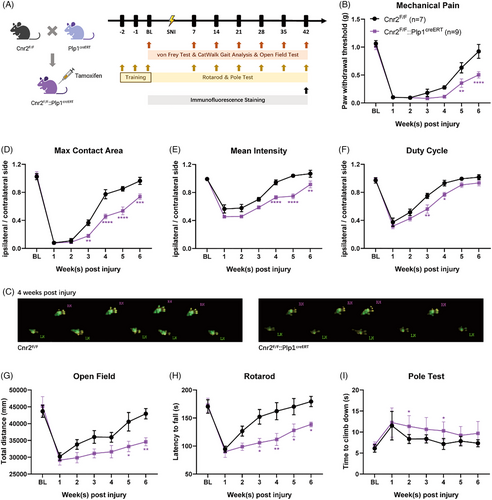
As CB2 is widely distributed in the periphery, we investigated the specific role of SC-expressing CB2 in nerve repair. To test this hypothesis, we crossed Cnr2 (the gene encoding CB2) floxed mice with mice expressing Cre recombinase under the control of the Plp1 promoter to generate mice lacking Cnr2 in SCs. After tamoxifen treatment, an sciatic nerve injury (SNI) model was established in Cnr2F/F::Plp1creERT mice and their littermates (Figure 2A). Interestingly, behavioural tests showed that the paw withdrawal threshold of Cnr2F/F mice returned to normal levels approximately 10 weeks after surgery, whereas Cnr2F/F::Plp1creERT mice were still experiencing SNI-induced pain (Figure 2B). Gait analysis (Figure 2C–F) and motor function tests (Figure 2G–I) also confirmed the delayed functional recovery after genetic ablation of CB2 in SCs. Moreover, Cnr2F/F::Plp1creERT mice showed lower fluorescence intensity of AQP1, MPZ, MBP and sporadically distributed TUJ1 signals when compared with that in the Cnr2F/F mice (Figures S6 and S7). These data suggest that SC-expressed CB2 is essential for peripheral nerve regeneration.
To clarify the role of SC-expressing CB2, we first confirmed that Cnr2 is substantially expressed in SCs by RT-qPCR, whereas Cnr1 showed much lower expression (Figure 3A). As a result, pharmacological activation of CB2 with AM1241 upregulated the expression of myelination-related factors, which was reversed by the co-application of AM630 (Figure 3B). We then determined the CB2 function using in vitro calcium imaging on cultured primary SCs isolated from newborn mice. Interestingly, perfusion with AM1241 elicited robust calcium influx in SCs, which were nearly abolished by the co-application of the CB2 antagonist AM630 (Figure 3C–E). Moreover, the Ki67 positive signal also significantly increased upon AM1241 stimulation, and the proportion of Ki67+/SOX10+ cells was reduced by pre-incubation with AM630 (Figure 3F,G). Consistently, the number of viable cells determined using the cell counting kit-8 showed that the activation of CB2 promoted cell proliferation (Figure 3H). Taken together, these results suggest that CB2 functionally regulates SCs proliferation, and the activation of SCs-expressing CB2 may accelerate injured peripheral nerve re-myelination.

Resident macrophages facilitate axonal sprouting into the distal stump by clearing debris,7 and macrophage-expressed CB2 may play a potential role in this process and promote peripheral nerve repair.8 To investigate whether CB2 expressed in macrophages contributes to this process, we genetically ablated CB2 from SCs by crossing the Cnr2F/F with CX3CR1creERT mice (Figure S8A). After the induction of SNI model, behavioural tests revealed no significant differences between the Cnr2F/F:: CX3CR1creERT mice and control littermates (Figure S8B–I), suggesting that resident macrophage-expressed CB2 may be not involved in peripheral nerve repair.
SCs are critical for forming bands of Büngner, which serve as essential structural and molecular guides for axonal regeneration following PNI.9, 10 Enhancing the activity of SCs could substantially improve the efficiency of nerve regeneration. In this study, we demonstrated that pharmacological activation of CB2 markedly enhanced SC proliferation and promyelination, while genetic ablation of SC-specific CB2 aggravated pain and motor dysfunction in the SNI model (Figure S9). These findings highlight the therapeutic potential of targeting SC-expressed CB2 to promote remyelination and improve recovery outcomes after nerve injury.
AUTHOR CONTRIBUTIONS
Heng Xu designed research, performed research and wrote the paper. Lu Wen performed research, analyzed data and wrote the paper. Yi Luo performed research and analyzed data. Jiaying Zhou performed research. Sheng Yao performed research and contributed new reagents. Wei Ding contributed new reagents or analytic tools. Jing Feng designed research, contributed new reagents or analytic tools and wrote the paper.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (grant no. 32241003, 82171214, and U24A20783), Shanghai Municipal Natural Science Foundation (grant no. 23ZR1474500), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant No. XDB1060000), State Key Laboratory of Chemical Biology, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai Rising-Star Program (24QB2701200) and Shanghai “Rising Stars of Medical Talents” Youth Development Program (SHWSRS(2023)_26).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.




