Clinical characteristics and risk factors for mortality among inpatients with COVID-19 in Wuhan, China
Fuyang Chen and Wenwu Sun contributed equally to this work.
Coronavirus disease 2019 (COVID-19) has become the worldwide pandemic. Currently, COVID-19, caused by the novel coronavirus, has had outbreaks in more than 213 countries and regions around the world and has caused many deaths. As of 24 April 2020, more than 2.62 million people had been diagnosed worldwide, of whom more than 180 000 died from the virus.1
This cohort included COVID-19 patients admitted to The Central Hospital of Wuhan on 1 January 2020 to 15 February 2020. Of the 660 participating inpatients with COVID-19, 82 died and 578 were discharged. We comprehensively identified the clinical characteristics at the time of admission in nonsurvivors and patients who recovered from it (Table 1), and we reported several novel risk factors of mortality in COVID-19 patients (Figure 1A). Furthermore, dynamic changes in these major markers during hospitalization and the survival outcomes under different conditions were tracked (Figure 1B–E).
| Characteristics | Total (n = 660) | Survivor (n = 578) | Non-survivor (n = 82) | P-value |
|---|---|---|---|---|
| Median (IQR) age, years | 55.0 (34.0-68.0) | 54.0 (37.0-66.0) | 71.0 (63.0-83.0) | <.0001 |
| ≤60 | 382 (57.9%) | 364 (63.0%) | 18 (22.0%) | <.0001 |
| >60 | 278 (42.1%) | 214 (37.0%) | 64 (78.0%) | |
| Sex | <.0001 | |||
| Female | 365 (55.3%) | 341 (59%) | 24 (29.3%) | |
| Male | 295 (44.7%) | 237 (41%) | 58 (70.7%) | |
| Comorbidity | 326 (49.4%) | 262 (45.3%) | 64 (78.0%) | <.0001 |
| Chronic obstructive lung disease | 43 (6.5%) | 32 (5.5%) | 11 (13.4%) | .007 |
| Hypertension | 230 (34.8%) | 177 (30.6%) | 53 (64.6%) | <.0001 |
| Diabetes | 114 (17.3%) | 93 (16.1%) | 21 (25.6%) | .033 |
| Myocardial infarction | 67 (10.2%) | 48 (8.3%) | 19 (23.2%) | <.0001 |
| Cerebral infarction | 52 (7.9%) | 29 (5.0%) | 23 (28.0%) | <.0001 |
| Kidney injury | 36 (5.5%) | 19 (3.3%) | 17 (20.7%) | <.0001 |
| Other | 38 (5.8%) | 28 (4.8%) | 10 (12.2%) | .015 |
| Symptoms at disease onset | ||||
| Fever | 524 (79.4%) | 455 (78.7) | 69 (84.1%) | .256 |
| Cough | 431 (65.3%) | 383 (58.5%) | 48 (66.3%) | .169 |
| Chest tightness | 259 (39.2%) | 225 (38.9%) | 34 (41.5%) | .660 |
| Thoracalgia | 28 (4.2%) | 28 (4.8%) | 0 (0%) | .081 |
| Myalgia | 155 (23.5%) | 139 (24.0%) | 16 (19.5%) | .364 |
| Fatigue | 237 (23.5%) | 206 (23.5%) | 31 (23.5%) | .702 |
| Diarrhea | 53 (8.0%) | 47 (8.1%) | 6 (7.3%) | .800 |
| Headache | 42 (6.4%) | 38 (6.6%) | 4 (4.9%) | .556 |
| APACHEII | 2.5 (1.0-5.0) | 2.0 (1.0-4.0) | 10.0 (8.0-14.0) | <.0001 |
| SOFA | 1.0 (0.0-3.0) | 1.0 (0.0-2.0) | 5.0 (3.0-6.0) | <.0001 |
| CURB-65 | 0.0 (0.0-1.0) | 0.0 (0.0-1.0) | 2.0 (1.0-2.0) | <.0001 |
| 0-1 | 549/642 (85.5%) | 519/566 (91.7%) | 30/76 (39.5%) | <.0001 |
| 2 | 72/642 (11.2%) | 41/566 (7.2%) | 31/76 (40.8%) | |
| 3-5 | 21/642 (3.3%) | 6/566 (1.1%) | 15/7 (19.7%) | |
| Median (IQR) time from onset of symptom to hospital admission, days | 7.0 (3.0-10.0) | 7.0 (4.0-10.0) | 6.0 (2.0-9.0) | .960 |
| Evaluation of computed tomography imaging on hospital admission | <.0001 | |||
| Normal | 345/643 (53.6%) | 319/567 (56.3%) | 26/76 (34.2%) | |
| Medium | 147/643 (22.9%) | 138/567 (24.3%) | 9/76 (11.8%) | |
| Severe | 151/643 (23.5%) | 110/567 (19.4%) | 41/76 (54.0%) | |
| Progression of imaging | <.0001 | |||
| None | 307/579 (53.0%) | 305/548 (55.7%) | 2/31 (6.5%) | |
| Yes | 272/579 (47.0%) | 243/548 (44.3%) | 29/31 (93.5%) | |
| Laboratory Findings | ||||
| White blood cell count, ×10⁹ per L | 4.9 (3.8-6.6) | 4.8 (3.8-6.2) | 6.3 (4.0-9.7) | .001 |
| <4 | 193/656 (29.4%) | 173/576 (30.0%) | 20/80 (25.0%) | <.0001 |
| 4-10 | 419/656 (63.9%) | 376/576 (65.3%) | 43/80 (53.7%) | |
| >10 | 44/656 (6.7%) | 27/576 (4.7%) | 17/80 (21.3%) | |
| Neutrophil count, ×10⁹ per L | 3.4 (2.3-4.9) | 3.3 (2.2-4.5) | 4.7 (3.1-8.7) | <.0001 |
| <1.8 | 99/656 (15.1%) | 89/576 (15.4%) | 10/80 (12.5%) | <.0001 |
| 1.8-6.3 | 467/565 (71.2%) | 425/576 (73.8%) | 42/80 (52.5%) | |
| >6.3 | 90/565 (13.7%) | 62/576 (10.8%) | 28/80 (35.0%) | |
| Lymphocyte count, ×10⁹per L | 1.0 (0.7-1.4) | 1.0 (0.7-1.4) | 0.6 (0.4-0.9) | <.0001 |
| ≥0.8 | 430/656 (65.5%) | 403/576 (70.0%) | 27/80 (33.7%) | <.0001 |
| <0.8 | 226/656 (34.5%) | 173/576 (30.0%) | 53/80 (66.3%) | |
| CD4+/CD8+ ratio | 1.5 (1.1-2.4) | 1.6 (1.1-2.4) | 1.5 (0.9-2.4) | .463 |
| <0.95 | 27/154 (17.5%) | 22/132 (16.7%) | 5/22 (22.7%) | .742 |
| 0.95-2.1 | 80/154 (51.9%) | 70/132 (53.0%) | 10/22 (45.5%) | |
| >2.1 | 47/154 (30.6%) | 40/132 (30.3%) | 7/22 (31.8%) | |
| CD4+ T cells, % | 40.0 (20.0-60.0) | 50.0 (30.0-70.0) | 20.0 (20.0-30.0) | <.0001 |
| <30 | 50/154 (32.5%) | 33/132 (25.0%) | 17/22 (77.3%) | <.0001 |
| 30-46 | 34/154 (22.1%) | 32/132 (24.2%) | 2/22 (9.1%) | |
| >46 | 70/154 (45.4%) | 67/132 (50.8%) | 3/22 (13.6%) | |
| CD8+ T cells, % | 30.0 (20.0-40.0) | 30.0 (20.0-40.0) | 10.0 (10.0-30.0) | .001 |
| <20 | 58/154 (37.7%) | 43/132 (32.6%) | 15/22 (68.2%) | .006 |
| 20-33 | 43/154 (27.9%) | 40/132 (30.3%) | 3/22 (13.6%) | |
| >33 | 53/154 (34.4%) | 49/132 (37.1%) | 4/22 (18.2%) | |
| Eosinophilic granulocyte count, × 10⁹per L | 0.01 (0.00-0.04) | 0.01 (0.00-0.04) | 0.00 (0.00-0.02) | .021 |
| <0.02 | 411/846 (63.4%) | 356/574 (62.0%) | 55/74 (74.3%) | .039 |
| 0.02-0.52 | 237/648 (36.6%) | 218/574 (38.0%) | 19/74 (25.7%) | |
| Basophilic granulocyte count, ×10⁹per L | 0.01 (0.01-0.02) | 0.01 (0.01-0.02) | 0.01 (0.01-0.02) | .824 |
| 0-0.06 | 641/647 (99.1%) | 568/573 (99.1%) | 73/74 (98.6%) | 1.000 |
| >0.06 | 6/647 (0.9%) | 5/573 (0.9%) | 1/74 (1.4%) | |
| Hemoglobin, g/dL | 128.0 (118.0-139.0) | 128.0 (120.0-140.0) | 122.0 (106.3-137.8) | .002 |
| Platelet count, × 10⁹ per L | 178.0 (135.3-225.8) | 182.0 (143.0-231.8) | 130.0 (106.8-183.8) | <.0001 |
| <125 | 118/656 (18.0%) | 84/576 (14.6%) | 34/80 (42.5%) | <.0001 |
| 125-350 | 507/656 (77.3%) | 461/576 (80.0%) | 46/80 (57.5%) | |
| >350 | 31/656 (4.7%) | 31/576 (5.4%) | 0/80 (0%) | |
| C-reactive protein, mg/dL | 1.9 (0.5-4.6) | 1.5 (0.4-4.1) | 5.4 (2.7-8.7) | <.0001 |
| 0-0.6 | 190/640 (29.7%) | 186/564 (33.0%) | 4/76 (5.3%) | <.0001 |
| >0.6 | 450/640 (70.3%) | 378/564 (67.0%) | 72/76 (94.7%) | |
| Procalcitonin, ng/mL | 0.1 (0.0-0.1) | 0.1 (0.0-0.1) | 0.2 (0.1-0.5) | <.0001 |
| <0.1 | 444/600 (74.0%) | 421/531 (79.3%) | 23/69 (33.3%) | <.0001 |
| ≥0.1 | 156/600 (26.0%) | 110/531 (20.7%) | 46/69 (66.7%) | |
| PaO2/FiO2, mmHg | 346.0 (243.0-510.0) | 402.0 (276.0-520.0) | 142.0 (92.5-225.8) | <.0001 |
| <100 | 25/647 (3.9%) | 4/567 (0.7%) | 21/80 (26.2%) | <.0001 |
| 100-300 | 224/647 (34.6%) | 176/567 (31.0%) | 48/80 (60.0%) | |
| >300 | 398/647 (61.5%) | 387/567 (68.3%) | 11/80 (13.8%) | |
| Lactate, mmol/L | 1.2 (0.8-1.9) | 1.2 (0.8-1.8) | 2.1 (1.4-2.8) | <.0001 |
| ≥1.5 | 259/644 (40.2%) | 202/565 (35.8%) | 57/79 (72.2%) | <.0001 |
| Blood urea nitrogen, mmol/L | 4.2 (3.3-5.6) | 4.0 (3.2-5.2) | 6.5 (4.8-9.9) | <.0001 |
| <2.9 | 84/653 (12.9%) | 82/575 (14.2%) | 2/78 (2.5%) | <.0001 |
| 2.9-8.2 | 521/653 (79.8%) | 469/575 (81.6%) | 52/78 (66.7%) | |
| >8.2 | 48/653 (7.3%) | 24/575 (4.2%) | 24/78 (30.8%) | |
| Creatinine, μmol/L | 65.1 (52.0-79.5) | 63.3 (51.3-77.2) | 78.3 (60.0-116.4) | <.0001 |
| <57 | 227/653 (34.8%) | 213/575 (37.0%) | 14/78 (18.0%) | <.0001 |
| 57-111 | 389/653 (59.6%) | 341/575 (59.3%) | 48/78 (61.5%) | |
| >111 | 37/653 (5.6%) | 21/575 (3.7%) | 16/78 (20.5%) | |
| Total bilirubin, μmol/L | 8.7 (6.4-12.0) | 8.8 (6.5-11.6) | 8.4 (5.6-14.1) | .504 |
| ALT, U/L | 20.0 (13.2-32.5) | 19.7 (13.2-33.0) | 21.0 (13.5-30.4) | .937 |
| >50 | 67/648 (10.3%) | 64/574 (11.1%) | 3/74 (4.1%) | .059 |
| FIB, g/L | 3.0 (2.5-3.5) | 2.9 (2.5-3.4) | 3.5 (3.0-3.8) | <.0001 |
| <2 | 21/625 (3.4%) | 20/550 (3.6%) | 1/75 (1.3%) | .001 |
| 2-4 | 542/625 (86.7%) | 484/550 (88.0%) | 58/75 (77.3%) | |
| >4 | 62/625 (9.9%) | 46/550 (8.4%) | 16/75 (21.4%) | |
| D-dimer, μg/L | 0.6 (0.3-1.4) | 0.5 (0.3-1.1) | 1.4 (0.7-5.2) | <.0001 |
| ≤1 | 435/632 (68.8%) | 408/563 (72.5%) | 27/69 (39.1%) | <.0001 |
| >1 | 197/632 (31.2%) | 155/563 (27.5%) | 42/69 (60.9%) | |
| Lactate dehydrogenase, U/L | 191.0 (153.0-254.3) | 185.5 (150.0-237.0) | 292.0 (211.8-474.8) | <.0001 |
| >245 | 161/598 (26.9%) | 116/524 (22.1%) | 45/74 (60.8%) | <.0001 |
| Creatine kinase, U/L | 75.5 (47.0-133.3) | 73.0 (45.8-122.0) | 144.3 (65.8-256.2) | <.0001 |
| >190 | 88/598 (14.7%) | 60/524 (11.5%) | 28/74 (37.8%) | <.0001 |
| CKMB, U/L | 8.0 (6.0-11.9) | 7.4 (6.0-11.0) | 11.1 (7.8-17.0) | <.0001 |
| >24 | 25/608 (4.1%) | 13/533 (2.4%) | 12/75 (16.0%) | <.0001 |
| IL-6, pg/mL | 4.7 (2.3-19.0) | 3.9 (2.2-9.6) | 43.8 (20.1-62.6) | <.0001 |
| HDL-C, mmol/L | 1.0 (0.8-1.2) | 1.0 (0.8-1.2) | 0.9 (0.7-1.1) | .009 |
| LDL-C, mmol/L | 2.2 (1.7-2.7) | 2.2 (1.7-2.7) | 2.0 (1.5-2.7) | .113 |
| Total triglyceride, mmol/L | 1.0 (0.8-1.4) | 1.0 (0.8-1.4) | 1.1 (0.7-1.4) | .881 |
| Total cholesterol, mmol/L | 3.8 (3.2-4.4) | 3.8 (3.2-4.5) | 3.4 (2.9-4.2) | .042 |
| Nonesterified fatty acid, mmol/L | 0.5 (0.3-0.6) | 0.5 (0.3-0.6) | 0.6 (0.4-0.7) | .040 |
| HbA1C, % | 7.4 (6.5-8.8) | 7.3 (6.4-9.1) | 7.4 (6.9-8.0) | .912 |
| >6 | 63/75 (84.0%) | 50/61 (82.0%) | 13/14 (92.9%) | .550 |
| Glucose, mmol/L | 5.7 (5.0-7.4) | 5.6 (4.9-7.0) | 7.1 (5.7-9.0) | <.0001 |
| ≤6.1 | 381/653 (58.3%) | 358/575 (62.3%) | 23/78 (29.5%) | <.0001 |
| >6.1 | 272/653 (41.7%) | 217/575 (37.7%) | 55/78 (70.5%) | |
| Treatments | ||||
| Quinotone | 420 (63.6%) | 368 (63.7%) | 52 (63.4%) | .964 |
| Cephalosporins | 293 (44.4%) | 241 (41.7%) | 52 (63.4%) | <.0001 |
| Ribavirin | 370 (56.1%) | 314 (54.3%) | 56 (68.3%) | .017 |
| Oseltamivir | 336 (50.9%) | 295 (51.0%) | 41 (50.0%) | .860 |
| Arbidol | 135 (20.5%) | 130 (22.5%) | 5 (6.1%) | .001 |
| Kaletra | 29 (4.4%) | 28 (4.8%) | 1 (1.2%) | .226 |
| Early corticosteroids | 184 (27.9%) | 154 (26.6%) | 30 (36.6%) | .060 |
| Early intravenous immunoglobulin | 160 (24.2%) | 143 (24.7%) | 17 (20.7%) | .428 |
| Thymosin | 127 (19.2%) | 110 (19.2%) | 17 (20.7%) | .715 |
| Heparin | 213 (32.2%) | 169 (29.2%) | 44 (53.7%) | <.0001 |
| Median (IQR) time from hospital admission to high-flow nasal cannula oxygen therapy, days | 4.0 (1.0-10.0) | 4.0 (1.0-7.0) | 4.0 (1.0-13.0) | .369 |
| Mechanical ventilation | 104 (15.8%) | 44 (7.6%) | 60 (74.1%) | <.0001 |
| Cause of death | ||||
| Respiratory failure | 63 (76.9%) | 0 | 63 (76.9%) | |
| Pulmonary embolism and respiratory failure | 1 (1.2%) | 0 | 1 (1.2%) | |
| Pulmonary infection and respiratory failure | 1 (1.2%) | 0 | 1 (1.2%) | |
| Heart failure | 2 (2.4%) | 0 | 2 (2.4%) | |
| Cardiac arrest | 6 (7.3%) | 0 | 6 (7.3%) | |
| Other | 9 (11.0) | 0 | 9 (11.0) |
- Note. P-values were calculated by Mann-Whitney U test, χ² test, or Fisher's exact test; bold if statistically significant, P < .05.
- Abbreviations: ALT, alanine aminotransferase; APACHEII, Acute Physiology and Chronic Health Evaluation; CKMB, creatine kinase isoenzyme-MB; FIB, fibrinogen; HbA1C, hemoglobin A1C; HDL-C, high-density lipid cholesterol; IL-6, interleukin-6; IQR, interquartile range; LDL-C, low-density lipid cholesterol; SOFA, Sequential Organ Failure Assessment.
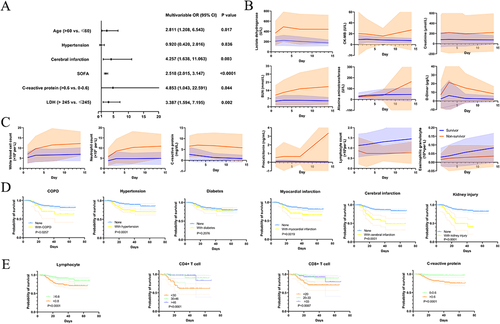
Consistent with the previous study, advanced age and high SOFA scores on admission were crucial risk factors for COVID-19 patient mortality.2 Intriguingly, according to our multivariable logistic regression analysis, we discovered that a history of cerebral infarction, C-reactive protein (CRP) levels >0.6 mg/dL, and lactate dehydrogenase levels >245 U/L on admission significantly elevated the odds of in-hospital death (Figure 1A).
Our findings show that the history of cerebral infarction, an independent risk factor for inpatient death, caused a significantly poorer outcome for COVID-19 patients. The possible reasons are as follows. First, cerebral infarction may occur mainly in elderly people, and older age was a crucial predictor for COVID-19 mortality.2 Second, when cerebral infarction impacts respiratory muscles and respiratory centers or causes hemiplegia, it may cause pulmonary conditions to deteriorate.3 Third, hypercoagulability is associated with cerebral infarction, and cerebral infarction patients may have preexisting hypercoagulability.4 Additionally, we observed higher D-dimer in nonsurvivors, suggesting that COVID-19 patients with cerebral infarction may have an aggravating hypercoagulable state, which could contribute to severe dysfunction. Therefore, cerebral infarction patients should recognize that there is a high possibility of severe illness in the event of viral infection and should follow strict precautions.
In nonsurvivors, baseline myocardial injury markers such as lactate dehydrogenase and creatine kinase-MB (CKMB) were elevated; CKMB increased rapidly from day 7 after admission, and lactate dehydrogenase was highest on day 3 after admission. Levels of kidney injury markers such as creatinine were clearly greater in death group, and blood urea nitrogen increased throughout the clinical course. Baseline alanine aminotransferase (ALT) was not quite different in the two groups, but the ALT of nonsurvivors increased rapidly from day 7 and was visibly higher on day 14. D-dimer was elevated in nonsurvivors compared to survivors, but no difference on day 14. As reported, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) interacted with ACE2 receptors, which are expressed in in most organs of human; on this basis, it is reasonable to hypothesize the involvement and impairment of multiple organs.5
The number of white blood cell and neutrophil and CRP levels of die patients were greater than discharged patients throughout the hospital stay, and procalcitonin augmented rapidly begin with day 7, becoming notably larger in die patients by day 14. For nonsurvivors, lymphocyte counts decreased from admission to day 3 and remained low level. Compared with nonsurvivor, eosinophilic granulocyte counts were dramatically lower in surviving group by day 14. Patients with decreased CD4+ T cells (<30%) had dramatically worse outcomes than those with normal CD4+ T cell levels. Similarly, a reduced proportion of CD8+ T cells (<20%) was associated with poor outcomes. Furthermore, patients with higher C-reactive protein had significantly lower survival than those with normal C-reactive protein levels (Figure 1E).
The direct cytopathic effect of the virus and its ability to escape from the host's immune response are considered the main factors of viral disease progression and can even threaten life.6 Consistent with changes in complete blood counts in the previous study,2 our study found that the white blood count and neutrophil counts of the nonsurvivor group during hospitalization were significantly higher and their lymphocyte counts were significantly lower than those of the survivor group, indicating that the nonsurviving patients suffered more serious bacterial or fungal infections during hospitalization. Previous studies have shown that the proportions and absolute counts of lymphocyte populations in severe COVID-19 patients are reduced, especially CD4+ T cells and CD8+ T cells.7 Another study also showed that SARS-CoV-2 infection could cause a decrease in the abundance and function of peripheral blood T cells and NK cells.8 Consistent with previous research, the present study showed that quantity of CD4+ T cell, lymphocyte, and CD8+ T cell in nonsurviving group was less than that in surviving patients. More interestingly, we found that these three variables were prognostic factors in COVID-19 patients, helping to predict the severity of their condition. These results may have some limitations: bacterial infection or repeated infections in COVID-19 patients will also affect immune indicators. Nonetheless, our findings and those of other researchers indicate that SARS-CoV-2 infection may mainly affect T cells in the lymph, causing cytokine storms in the body; these severe reactions would reduce the body's immunity and trigger multiple organ failure, ultimately leading to the death of the patient.
In conclusion, advanced age, increased SOFA scores a, history of cerebral infarction, CRP larger than 0.6 mg/dL, and lactate dehydrogenase greater than 245 U/L at admission were independent risk factors for in-hospital death in COVID-19. Moreover, these data highlight the greater multiple organ involvement and greater changes in inflammation and immunity markers among nonsurvivors during hospitalization. More intensive attention should be paid to patients with these risk factors, in case of rapid deterioration and bad prognosis.
ACKNOWLEDGMENT
The letter is granted by the National Natural Science Foundation of China (No. 81471781).
DISCLOSURE
The authors declare no conflict of interest.




