A novel method to standardise serum IgA measurements shows an increased prevalence of IgA deficiency in young children with recurrent respiratory tract infections
Abstract
Objectives
While physicians are often confronted with immunoglobulin A (IgA) deficiency in children with recurrent infections, the clinical relevance of this finding is unclear. Large-scale studies examining the significance of IgA deficiency in children are hampered by differences in techniques for measuring IgA and the physiological increase of IgA with age. Both result in a variety of reference values used for diagnosing IgA deficiency. We propose a new laboratory-independent method to accurately compare IgA measurements in children of varying ages.
Methods
We present a method to standardise IgA values for age and laboratory differences. We applied this method to a multicentre case–control study of children under the age of seven suffering from recurrent respiratory tract infections (rRTI, cases) and children who had IgA measured as part of coeliac disease screening (controls). We defined IgA deficiency as serum IgA measurements < 2.5% for age-specific reference values.
Results
We developed reference values for IgA for seven age groups and five different laboratory assays. Using these reference values, IgA measurements from 417 cases and 224 controls were standardised to compare groups. In children aged 2 years and older, IgA deficiency was observed in 2.9% (7/242) of cases and 0% (0/189) of controls (P = 0.02).
Conclusion
We present a method to compare IgA values in cohorts that vary in age and laboratory assay. This way, we showed that IgA deficiency was more prevalent in children with rRTI compared with controls. This implicates that IgA deficiency may be a clinically relevant condition, even in young children.
Introduction
Immunoglobulin A (IgA) is the most abundantly produced antibody in humans and is mainly found in mucosal secretions, where it forms a first line of defence against invading pathogens. IgA deficiency is the most common immunodeficiency in children with prevalence rates ranging from 1:170 to 1:400.1, 2
A distinction can be made between partial and complete IgA deficiencies.3 Partial IgA deficiency is used for patients who have a serum IgA level between 0.07 g L−1 and two standard deviations below the population average level normalised for age with normal serum immunoglobulin G (IgG) and immunoglobulin M (IgM) levels, in whom other causes of hypogammaglobinaemia have been excluded and who have a normal IgG response to vaccination.3, 4 The term complete IgA deficiency is used for patients who meet the criteria of partial IgA deficiency, except have a serum IgA level below 0.07 g L−1. Both definitions exclude children younger than 4 years since in this age group, low IgA levels are often transient.
IgA deficiency is often regarded a mild condition, with textbooks and reviews mentioning that up to 90% of IgA-deficient individuals are asymptomatic.4-8 Defining a patient as asymptomatic can be arbitrary as was shown by a cohort study with a 20-year follow-up. This study included 159 IgA-deficient individuals who were initially classified as asymptomatic, but appeared to suffer significantly more often from respiratory infections (60% versus 33%) and autoimmune diseases (23% versus 5%) during follow-up compared with controls.9 In large population-based cohorts, both complete and partially IgA-deficient children indeed had an increased risk of infections and food intolerance, compared with age-matched controls.1, 10-12 This indicates that both complete and partial IgA deficiencies in children can be clinically relevant.
In the diagnostic work-up of children with recurrent respiratory tract infections (rRTI), a common condition in young children, physicians are often confronted with low IgA levels. Most studies investigating IgA deficiency in children only include patients with complete IgA deficiency and exclude children younger than 4 years to comply with official definitions.3 Therefore, little is known about the disease burden in IgA-deficient children under the age of 4 years, which hampers the clinical interpretation of this finding. Another reason for the relative absence of studies describing (partial) IgA deficiency in young children is the lack of universal reference values. Developing IgA reference values for young children requires standardisation between laboratory assays. Also, to determine age-specific reference values, serum samples from large cohorts of healthy children of different ages are needed, which poses ethical challenges. As a result, many sets of reference values exist, and therefore, many different definitions of partial IgA deficiency in children have been published.13-16 This limits comparability between studies performed in different settings.
In a large multicentre case–control study, we standardised serum IgA measurements for different ages and between-hospital variation in assays to determine the clinical relevance of low IgA levels in young children with rRTIs.
Results
Standardisation of IgA measurements
We developed a method that enabled age and laboratory assay independent comparison of IgA measurements. For the total hospital cohort, 16 410 paediatric serum IgA measurements were available to identify reference intervals (Supplementary table 1). No reference values could be calculated for the Beckman Coulter Immage 800 and the BindingSite Optilite in children up to 1 year because the groups contained less than 30 measurements.
Next, serum IgA measurements of all children in the case–control study were converted into percentiles based on age and assay-specific reference values. This way, we were able to standardise IgA measurements and correct for age and assay differences before comparing IgA measurements between cases and controls (Figure 1).

Validation of standardisation method in a separate cohort
To confirm the validity of the conversion of IgA measurements into percentiles, we measured serum IgA from 55 children on three different platforms. The IgA measurements were converted to percentiles and compared with Deming regression analysis (Supplementary figure 1). There were no significant differences between platforms. The R2 of the results between all analyzers was 0.95 or higher. We conclude that our standardisation method can be used to group IgA values determined with different techniques for children of different ages.
IgA deficiency prevalence in a case–control study according to existing and standardised reference values
A total of 944 children were identified for potential inclusion in the case–control study (Figure 2). Subsequently, 134 children were excluded because coeliac disease could not be excluded, 87 because of an IgG (subclass) deficiency and 82 because an exclusion diagnosis was present. Hence, 417 cases and 224 controls remained in the final case–control study. Cases were significantly younger than controls [median age 2.3 years (interquartile range, IQR 1.4–3.6 years) and 4.4 years (IQR 2.8–5.6 years), respectively, P < 0.01). Sex did not significantly differ between cases and controls.
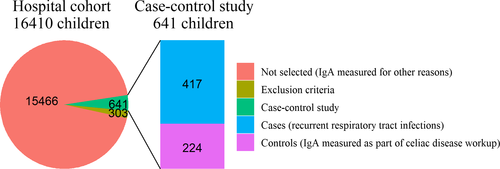
When sets of reference values as currently used in clinical practice were used to define the prevalence of IgA deficiency in cases and controls, we saw a wide variation in prevalence rates (Table 1). This emphasises the difficulty in assessing the clinical relevance of IgA deficiency when different cut-off values are used.
| Reference values | Prevalence of IgA deficiency | P-value | |
|---|---|---|---|
| Casesa | Controlsa | ||
| Mayo Clinics 13 | 8.9% (37/417) | 4.5% (10/224) | 0.06 |
| CALIPER study14 | 1.9% (8/417) | 2.2% (5/224) | 1.00 |
| Sanquin Dutch blood bank15 | 9.1% (38/417) | 11.2% (25/224) | 0.49 |
| Garcia-Prat et al.16 | 2.4% (10/417) | 3.1% (7/224) | 0.77 |
- a Cases were children suffering from recurrent respiratory tract infections, and controls were children screened for coeliac disease in whom coeliac disease was subsequently excluded.
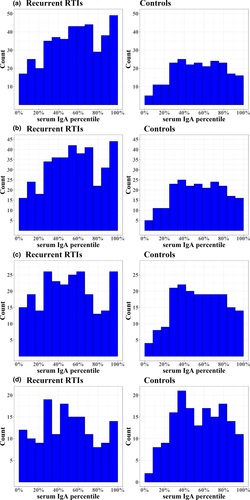
When using the new standardised reference values to define IgA values in cases and controls, we observed that in the total cohort, serum IgA levels did not differ between children with rRTI and controls (Table 2). However, after stratification by age, cases had a borderline significantly lower IgA percentile compared with controls in children aged 2 years and older [median 0.51 (IQR 0.30–0.71) versus median 0.55 (IQR 0.37–0.75), respectively (P = 0.07)]. In children aged 3 years and older, the difference between cases and controls was even more pronounced, with a significantly lower IgA percentile in cases compared with controls older [median 0.49 (IQR 0.28–0.70) versus median 0.56 (IQR 0.37–0.75), respectively (P = 0.04)]. Also, the distribution of IgA percentiles in both these age subgroups showed an overabundance of low percentiles, with significantly more cases having serum IgA percentiles below 25% compared with controls [Figure 3, for children above the age of 2 years, 20% (48/242) of cases versus 11% (21/189) of controls (P = 0.02), and for children above the age of 3 years, 21% (31/151) of cases versus 12% (19/161) of controls (P = 0.05)].
Finally, we examined the prevalence of IgA deficiency in the case-control study. Again, no difference in IgA deficiency prevalence rate was observed between cases and controls for the entire cohort (1.9% (8/416) versus 0.5% (1/212), respectively (P = 0.17), Table 2). However, in children aged two years and older, a significantly higher prevalence rate was seen in cases (2.9% (7/242)) compared with controls (0.0% (0/189), P = 0.02). Once again, in children aged three years and older this difference was even more pronounced (4.0% (6/151) of cases versus 0.0% (0/189) of controls, P = 0.01).
| Median IgA percentile [IQR] | Prevalence of IgA deficiency | |||||
|---|---|---|---|---|---|---|
| Casesa | Controlsa | P-value | Casesa | Controlsa | P-value | |
| Entire cohortb | 0.57 [0.35–0.79] | 0.55 [0.37–0.75] | 0.60 | 1.9% (8/416) | 0.5% (1/221) | 0.17 |
| Children ≥ 1 year | 0.55 [0.34–0.75] | 0.55 [0.37–0.75] | 0.97 | 2.1% (8/382) | 0.5% (1/220) | 0.17 |
| Children ≥ 2 years | 0.51 [0.30–0.71] | 0.55 [0.37–0.75] | 0.07 | 2.9% (7/242) | 0.0% (0/189) | 0.02 |
| Children ≥ 3 years | 0.49 [0.28–0.70] | 0.56 [0.37–0.75] | 0.04 | 4.0% (6/151) | 0.0% (0/189) | 0.01 |
- IQR, Interquartile range.
- a After exclusion of four children under the age of 1 year in whom no IgA could be converted due to lack of reference values, the entire case–control cohort consisted of 637 children aged 6 months to 7 years.
- b Cases (n = 416) were children suffering from recurrent respiratory tract infections, and controls (n = 221) were children screened for coeliac disease in whom coeliac disease was subsequently excluded.
Discussion
In the present study, we present a method to standardise serum IgA measurements for children between different ages, hospitals and laboratory methods. After applying this technique, we found that IgA deficiency was significantly more prevalent in children aged 2 years and older suffering from rRTI compared with controls. This difference was even more pronounced in children aged 3 years and up, suggesting that in young children age should not be seen as a strict cut-off value to diagnose a potentially clinically relevant IgA deficiency. Rather, as indicated by our results, the clinical relevance of a slow immunological maturation and associated IgA deficiency increases with age, starting in early childhood.
The major strength of this study is the age and laboratory assay-independent comparison of IgA measurements. In addition to IgA, this method could also be applied to many other laboratory markers to standardise and compare values between hospitals in both clinical care and research. Moreover, standardisation enabled the comparison of IgA values between children in different age groups.
At birth, IgA is virtually absent, after which it steadily increases throughout childhood.17, 18 We observed that the lower limit of the reference interval increased with age, which is in line with clinically used reference values.13-16 We found that the cut-off for complete IgA deficiency (IgA < 0.07 g L−1) fell within the normal range in children up to 24 months. The fact that no difference in serum IgA and prevalence of IgA deficiency was observed in children younger than 24 months in our case–control study is likely a result of these naturally low IgA levels. In accordance with our reference intervals, several other studies found lower limits below 0.07 g L−1 for children younger than 2 years.14, 16 This indicates that IgA deficiency in children up to 2 years is likely a physiological phenomenon of a still developing immune system.
The prevalence of IgA deficiency was 1:35 (7/242) for children aged 2 years and older and 1:25 (6/151) for children 3 years and older in symptomatic children, while none of the control children in these age groups had IgA deficiency. This is in line with other studies reporting that prevalence rates of IgA deficiency in healthy children range from 1:170 to 1:400, while rates in children referred for rRTI range from 1:4 to 1:65.19-21 In contrast to most studies investigating children with rRTI, we included a control group that enabled us to compare the prevalence of IgA deficiency in children with and without rRTI.
The clinical relevance of low levels of IgA in children, especially those with rRTI, is highlighted by a longitudinal case–control study conducted in Croatia. This study included 95 complete and partial IgA-deficient children aged 4–18 years and 67 healthy age-matched controls. Significantly lower pulmonary function (peak expiratory flow and maximal expiratory flow at 50% of the forced vital capacity) was observed for both partial and complete IgA-deficient children compared with healthy controls.12 Furthermore, bronchiectasis as a sequela of recurrent pneumonia is described in cohort studies in up to 40% of IgA-deficient children.22, 23 Early diagnosis of IgA deficiency with adequate prevention and treatment of rRTI may reduce the number of infections and thereby the risk of these long-term complications.24 We therefore recommend IgA levels to be measured in all children with rRTI aged 2 years and above.
A limitation of our study was that no full patient records could be investigated for the case–control study. We therefore applied very strict inclusion and exclusion criteria, which significantly diminished the number of eligible children. This approach ensured that observed differences in serum IgA and IgA-deficient prevalence between cases and controls were associated with the clinical presentation. However, the prevalence rates we present in this study are likely an underrepresentation of the actual prevalence rate of IgA deficiency, since this immunological defect is often accompanied by IgG (subclass) deficiency, allergies and/or autoimmune phenomena. Further, because of incomplete clinical information we could have missed other conditions associated with low IgA levels in the cases. Hence, our results may not be generalisable to other cohorts. Finally, the diagnosis codes used to identify children with rRTI in the case group represent a wide spectrum of rRTI disease severity. We were therefore unable to determine whether lower IgA levels were associated with more severe rRTI disease burden.
Because we were unable to collect additional information on genetics and/or family history, we could not differentiate between different types of IgA deficiency. The aetiology of IgA deficiency represents a heterogeneous group of abnormalities. Intrinsic B-cell defects, T cell, cytokine network abnormalities and environmental factors (including the microbiome composition) have all been associated with IgA deficiency.25-29 While no clear inheritance pattern had been described, several monogenic mutations have been associated with IgA deficiency.30 Also, multiple HLA and non-HLA genes have been described to be associated with disease phenotypes and development.31-33 Especially, loci of the 8.1 haplotype, such as HLA-DQB and HLA-DRB, are strongly associated with IgA deficiency, as shown in a genome-wide association study (GWAS) meta-analysis of 1635 IgA-deficient patients.34 Multiple auto-immune diseases, including diabetes mellitus, systemic lupus erythematosus, dermatitis herpetiformis and myasthenia gravis, are also reportedly associated with the 8.1 haplotype, suggesting a genetic link between IgA deficiency and autoimmunity.34-37
Conclusion
In conclusion, we emphasise that increased awareness is needed among clinicians that IgA deficiency in young children can be a clinically relevant condition. Serum IgA measurements could be part of the routine diagnostic evaluation of children suffering from rRTI aged 2 years and up. For accurate interpretation and comparison of IgA levels, especially when measured between different centres and/or cohorts, our presented method can be used. Applying this method to identify partial IgA deficiency opens doors for future large multicentre prospective cohort studies including (young) partially IgA-deficient children. These studies are needed to determine the exact risk of infections, allergies and auto-immune phenomena in the full spectrum of IgA deficiency. Long-term follow-up of these patients could further help identify partial and complete IgA deficient patients at risk of developing long-term complications such as pulmonary damage. This way, the measurement of serum IgA levels with our presented method in all young children with rRTI can ensure early diagnosis of IgA deficiency, which could greatly improve the quality of life and outcome of children with IgA deficiency.
Methods
IgA reference values from a large hospital cohort
We determined paediatric reference values based on a large hospital cohort including children up to 7 years with IgA measured between 1 January 2005 and 1 July 2019. Children were included in two Dutch hospitals: the Wilhelmina Children's Hospital (part of UMC Utrecht) and the St Antonius Hospital. In the 14-year study period, the participating laboratories used five analyzers (Siemens BNII, BindingSite SPA+, Beckman Coulter AU5811, Beckman Coulter Immage 800 and BindingSite Optilite) for nephelometric or turbidimetric assay to measure serum IgA concentrations. We defined seven age categories: 0–1, 1–2, 2–3, 3–4, 4–5, 5–6 and 6–7 years. A minimum of 30 IgA measurements per age*analyzer group were used to identify a distribution for reference values.38 IgA values follow a gamma-distribution, with a high-density peak in low values and a large tail to the right. With the Mixtools package, we identified different gamma-distributions of IgA results for each age*analyzer group within a mixed hospital population, as described by Concordet et al.39 On the basis of the shape and location of the distributions, we identified the ‘healthy’ and diseased hospital population. Reference values per group were determined by identifying the lower 2.5% and the upper 97.5% of the healthy distribution. This analysis was done four times with different set.seed() function settings. The mean lower and upper values of these four reference values were used for the final reference value per age*analyzer group to correct for minor differences within the random sequence of the Mixtools package.
Standardisation of IgA measurements
Next, we used these reference values to standardise the IgA measurements. IgA results were converted into ‘heuristic percentiles’, which represent a location within a reference population distribution.40 The heuristic percentile per case–control study subject was based on the location of their IgA measurement within the distribution matching age and analyzer method as described above. This normalisation was performed per five laboratory procedures and per seven age groups. This standardisation allowed us to subsequently analyse individuals in all (7 × 5) 35 different groups and compare standardised values between subgroups.
Validation of the standardisation method in a separate cohort
To confirm the validity of the standardisation method, we used 55 paediatric samples collected as part of a prospective cohort study [Deficiency IgA Microbiome Respiratory tract infections (DIMER) study]. This study included children up to 7 years suffering from rRTI undergoing immunological screening. Exclusion criteria were (1) primary immunodeficiencies requiring immunoglobulin substitution, (2) secondary immunodeficiencies and/or (3) major congenital anomalies. Serum samples from 55 children of different ages and measured on two different analyzers (BindingSite SPA+ and Beckman Coulter AU5811) were used to re-measure IgA. Per child, IgA results from different analyzers were converted to percentiles as described above and compared to determine the validity of our standardisation method.
The case–control study
We investigated IgA deficiency in young children suffering from rRTI and in a control group of children in whom serum IgA was determined for coeliac disease screening, but coeliac disease was excluded. We included children aged 6 months to 7 years, with IgA measured between 01-01-2005 and 01-07-2019 in the Wilhelmina Children's Hospital or the St Antonius Hospital. Children were identified by extracting age, diagnosis and laboratory results from electronic patient files in a pseudonymous fashion. Diagnoses were registered in a coded way, and these codes were used to determine whether a child was eligible for the case–control study. Children were included as cases when they had an IgA measurement and a diagnosis of upper and/or lower rRTI. Children with an IgA measurement and abdominal pain and/or a clinical suspicion of coeliac disease were included in the coeliac screening control group.
After initial screening, all subjects were evaluated for exclusion criteria. Laboratory results from cases were used to exclude patients with an IgG or IgG1-3 subclass deficiency as defined by Dutch reference values.15 Controls with evidence of coeliac disease, that is anti-tissue transglutaminase IgA/IgG and/or anti-gliadin IgA/IgG above cut-off, and/or positive anti-deamidated gliadin peptide IgA/IgG and/or the presence of anti-endomysium IgA/IgG, were excluded. Controls in whom IgG (subclasses) and/or IgM were determined were also excluded, since this could indicate that the physician suspected that the child suffered from an underlying immune disorder. Other exclusion criteria were diagnoses that indicated an underlying structural condition that could cause rRTI or a diagnosis for which the treatment was associated with secondary IgA deficiency (JIA, IBD, epilepsy, rheumatoid arthritis, diabetes mellitus, thyroid diseases, nephrotic syndrome, Wilson's disease, hypertension and liver disease).41-46
To highlight the effect of varying reference values used in different countries and studies, we applied several reference intervals to the case–control cohort to determine the prevalence of IgA deficiency according to a variety of definitions. Four sets of published and commonly used reference intervals per age group in young children were selected: Mayo Clinics, CALIPER study, the Sanquin Dutch blood bank and reference values described by Garcia-Prat et al.13-16
Finally, we examined the prevalence of IgA deficiency in the case–control cohort based on our standardisation method. We defined IgA deficiency as having an IgA level below 2.5% for the standardised reference values determined as described above. This definition meets the requirements for partial IgA deficiency as mentioned earlier.3
Ethics approval
The UMC Utrecht, Division of Pediatrics and the hospital board of the St Antonius Hospital reviewed and approved the determination of paediatric reference values and the case–control study.
Ethical approval for the DIMER study, part of the national Primary Immunodeficiency study, was received from the Medical Ethical Committee of the Erasmus Medical Center in Rotterdam, the Netherlands, and the UMC Utrecht in Utrecht, the Netherlands (METC:NL40331.078). The legal guardians of all participating children signed informed consent to participate in the study. This study and all work related to this study have been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Statistical analysis
For validation of the standardisation method, we compared IgA percentiles with Deming regression. Deming regression is similar to linear regression, except the error for both the x- and the y-variable are measured instead of only the y-variable. After standardisation, the non-parametric Mann–Whitney U-test was used to compare age and technique corrected IgA values between cases and controls. Prevalence rates of IgA deficiency were compared between groups using the Chi-square test/Fisher's exact test, as appropriate. Statistical significance was defined as a P-value below 0.05. All statistical analyses were performed in R version 4.0.3 (R-Studio 1.3).
Code availability
All R scripts are available from https://gitlab.com/mkoenen2/IMPRESS.
Acknowledgments
We thank Martine van Engelen and Dayana Martins Gueth for their help in the coordination and inclusion of children in the prospective clinical cohort study. We thank Bob Meek for his help in data collection and Hans de Graaf for his assistance in the laboratory. This work was supported by the Wilhelmina Children's Hospital Research Fund and the Fellowship clinical research talent from the UMC Utrecht to LV. Further financial support was obtained from the ‘Christine Bader Stichting Irene KinderZiekenhuis’.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Mischa H Koenen: Conceptualization; Data curation; Formal analysis; Data interpretation; Validation; Visualization; Writing-original draft; Writing-review & editing. Madeleen Bosma: Conceptualization; Data curation; Data interpretation; Writing-original draft. Udo A Roorda: Data curation; Data interpretation. Fabiënne MY Wopereis: Conceptualization; Data curation; Data interpretation; Writing-original draft. Anja Roos: Data curation; Data interpretation; Writing-original draft. Erhard van der Vries: Data interpretation; Writing-original draft, Supervision. Debby Bogaert: Data interpretation; Writing-original draft, Supervision. Elisabeth AM Sanders: Data interpretation; Writing-original draft. Marianne Boes: Data interpretation; Writing-original draft, Supervision. Jojanneke Heidema: Data interpretation; Writing-original draft. Joris M van Montfrans: Data interpretation; Writing-original draft. Walter AF Balemans: Conceptualization; Data interpretation; Writing-original draft, Supervision. Thijs C van Holten: Data curation; Formal analysis; Data interpretation; Validation; Writing-original draft; Supervision. Lilly M Verhagen: Conceptualization; Data curation; Formal analysis; Validation; Data interpretation; Funding acquisition; Writing-original draft; Writing-review & editing; Supervision.




