Dysfunctional BTN3A together with deregulated immune checkpoints and type I/II IFN dictate defective interplay between pDCs and γδ T cells in melanoma patients, which impacts clinical outcomes
Abstract
Objectives
pDCs and γδ T cells emerge as potent immune players participating in the pathophysiology of cancers, yet still remaining enigmatic while harbouring a promising potential for clinical translations. Despite strategic and closed missions, crosstalk between pDCs and γδ T cells has not been deciphered yet in cancers, especially in melanoma where the long-term control of the tumor still remains a challenge.
Methods
This prompted us to explore the interplay between pDCs and γδ T cells in the context of melanoma, investigating the reciprocal features of pDCs or γδ T cells, the underlying molecular mechanisms and its impact on clinical outcomes.
Results
TLRL-activated pDCs from the blood and tumor infiltrate of melanoma patients displayed an impaired ability to activate, to modulate immune checkpoints and trigger the functionality of γδ T cells. Conversely, γδ T cells from the blood or tumor infiltrate of melanoma patients activated by PAg were defective in triggering pDCs’ activation and modulation of immune checkpoints, and failed to elicit the functionality of pDCs. Reversion of the dysfunctional cross-talks could be achieved by specific cytokine administration and immune checkpoint targeting. Strikingly, we revealed an increased expression of BTN3A on circulating and tumor-infiltrating pDCs and γδ T cells from melanoma patients, but stressed out the potential impairment of this molecule.
Conclusion
Our study uncovered that melanoma hijacked the bidirectional interplay between pDCs and γδ T cells to escape from immune control, and revealed BTN3A dysfunction. Such understanding will help harness and synergise the power of these potent immune cells to design new therapeutic approaches exploiting their antitumor potential while counteracting their skewing by tumors to improve patient outcomes.
Introduction
Interactions between several immune cells are crucial to initiate and orchestrate robust antitumor responses. The tumor microenvironment (TME) comprises various immune cells, including pDCs and γδ T cells, two potent immune players that emerge to participate in the pathophysiology of the disease, yet still remains enigmatic but harbours a promising potential for clinical translations. pDCs and γδ T cells are both orchestrators of immune responses1-5 and critical complementary players in cancer immunosurveillance.6-9 Following recognition of damaged-associated molecular patterns (DAMPs) or sensing of altered metabolism (phosphoantigens (PAgs)), pDCs and γδ T cells rapidly and massively produce type I or type II IFN, and interact with many immune cells, subsequently triggering and tuning immune responses. Their unique features combined with a high functional plasticity allow them to critically bridge innate and adaptive immunity.
pDCs are key players in the regulation of immunity.1, 3 TLR7/9 expression allows them to recognise pathogenic motifs (single-stranded RNA, unmethylated CpG-containing DNA) and subsequently drive activation and robust IFN-α production, thereby promoting innate and adaptive immune responses. Through their functional plasticity and ability to interact with different immune cells, pDCs can orientate immunity towards multiple profiles (immunity or tolerance) depending on the microenvironment.10, 11 pDCs play a crucial role in the initiation and orientation of antitumor immune responses, by their ability to induce antigen-specific adaptive responses12, 13 or by exerting a direct cytotoxic activity towards the tumor cells via TRAIL,14, 15 but are found to be subverted in many cancers and to contribute to the establishment of an immunosuppressive tumor microenvironment.16-19
γδ T cells are unconventional T cells displaying a critical role in immune responses against tumor cells4, 5, 20, 21 through their prompt activation, their ability to recognise tumor- and stress-associated molecules neglected by conventional αβ T cells in an MHC-unrestricted manner, their capacity to secrete immuno-stimulatory cytokines especially IFNγ and TNFα regulating and potentiating the effectiveness of other immune cells and their potential to exhibit cytotoxic activity through secretion of pro-apoptotic protease granzymes and pore-forming molecules perforin.5 Among the different γδ T-cell subsets, Vδ2+ cells, bearing the TCR Vγ9Vδ2, are predominant in peripheral blood and lymphoid tissues. Vδ2+ cells recognise pAg such as isopentenyl pyrophosphate (IPP), which accumulates intracellularly during dysregulated metabolism in many tumor cells, and (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP), which is produced by many pathogens. Aminobiphosphonates (ABPs) such as zoledronate (Zol), by inhibiting the IPP metabolising farnesyl diphosphate synthase (FPPS), cause the accumulation of IPP and consequently trigger the activation of Vγ9Vδ2 T cells. Vγ9Vδ2 T cells can sense PAg following interaction with cells expressing the butyrophilin-3 A1 (BTN3A1)/CD277 molecule.22, 23
pDCs and γδ T cells owe essential contribution to many types of protective immune responses, but may also drive pathogenic immune responses leading to immunopathologies.1, 3-5 In cancer, pDCs and γδ T cells have a pivotal role in triggering efficient antitumor immune responses, but the skewing of their potentialities may contribute to tumor escape from immunity, and establishment of an immunosuppressive tumor microenvironment.8, 24 pDCs25 and γδ T cells26 are found in multiple cancer types, especially in melanoma.16, 27, 28 Notably, although tumor-infiltrating pDCs have been associated with poor clinical outcome,16, 18, 29 tumor-infiltrating γδ T cells have been demonstrated to be the most favorable prognostic immune population among many cancer types.28, 30 In patients with melanoma, we have highlighted functional alterations of pDCs within the tumor microenvironment associated with impaired response to TLR7/9 activation and major modulations of the expression of some immune checkpoints (OX40L, ICOSL) leading to the induction of TH2-type and regulatory T-cell responses.16, 17 Besides, functional impairments of peripheral γδ T cells have been reported in melanoma patients.31-33 Tumors can subvert γδ T cells to exhibit tumor-promoting functions21, 34 and favor tumor progression,35 through polarisation towards Th2, Th17 or regulatory profile,36 recruitment of immunosuppressive myeloid cells37 and inhibition of antitumor immune responses.38 We and others previously highlighted that the features of circulating and tumor-infiltrating γδ T cells display critical perturbations with prognostic impact on clinical outcome28, 39: altered expression of NCR, KIR and immune checkpoints, impairment of cytotoxic activity and cytokine secretion. We identified NKp44, PD1, 41BB, TIM3 and LAG3 as crucial checkpoints allowing immune escape and tumor progression.
Based on their importance in contributing to immune-mediated control, both pDCs and γδ T cells have been already exploited as vectors or targets for immunotherapy of cancers,7, 40-44 especially in melanoma. Agonists of TLR7/TLR9, triggering high levels of type I IFN by pDCs while avoiding their impairment by the TME, are being clinically explored.45, 46 Tumor antigen-loaded pDCs properly activated can be vectors for immunotherapy and elicit favorable antitumor immune responses in patients upon vaccination.47, 48 Besides, it has been shown in patients with prostate cancer49 or melanoma50 that administration of aminobisphosphonates can lead to objective clinical responses, potentially through the indirect stimulation of Vδ2+ cells. The adoptive transfer of ex vivo expanded γδ T cells is achievable in patients51 and can inhibit melanoma tumor growth in SCID mice.52 These promising results on pDCs- and γδ T cell-based clinical translations sustain further investigations of these cells and their cross-talks.
Despite strategic and close missions, cross-talks between pDCs and γδ T cells have not been deciphered yet in cancers. Available studies demonstrated bidirectional interactions between ex vivo generated monocyte-derived DCs (moDCs) and γδ T cells in healthy settings.53 Zol- or ABP-treated moDCs could trigger the expansion and activation of γδ T cells with effector and costimulatory activities.54-56 Besides, TLRL stimulated moDCs or pDCs trigger activation and IFNγ secretion by the Vγ9Vδ2 T-cell subset57, 58 that in turn promote the maturation of DCs and secretion of IL12p70,55, 59 underlying the potential cross-talk between PAMP-activated DCs and γδ T cells. Notably, we recently uncovered, in healthy settings, a potent bidirectional cross-talk between pDCs and γδ T cells through BTN3A, type I/II IFNs and immune checkpoints.60 Remarkably, a recent study revealed that activated pDCs preferentially attracted γδ T cells following their injection in the skin of melanoma patients,61 supporting the potential interplay between pDCs and γδ T cells in vivo in cancer patients.
pDCs and γδ T cells, even though exhibiting opposite clinical impacts, represent critical players in antitumor immunity, but the tumor hijacked them by exploiting their functional plasticity to favor tumor progression. Yet, interactions between these potent immune players have never been deciphered in cancer, especially in melanoma where the long-term control of the tumor in a majority of patients still remains a challenge, despite major improvements using immune checkpoint blockers. Such controversy prompted us to explore the interplay between pDCs and γδ T cells in the context of melanoma, investigating the phenotypic and functional features of pDCs or γδ T cells induced by the other partner purified from the blood or tumor infiltrate of melanoma patients, the underlying molecular mechanisms and its impact on clinical outcomes. We highlight, for the first time, crucial impairments of the bidirectional interactions between pDCs and γδ T cells in melanoma, deciphering the mechanisms underlying such cross-talk and clinical impact. Thus, melanoma hijacked the interplay between pDCs and γδ T cells to escape from immune control. Such understanding of the physiopathology of interplay between pDCs and γδ T cells will help harness and synergise the power of these potent immune cells to design new therapeutic approaches exploiting their antitumor potential while counteracting their skewing by tumors to improve patient outcomes.
Results
pDCs and γδ T cells interact within melanoma microenvironment in patients
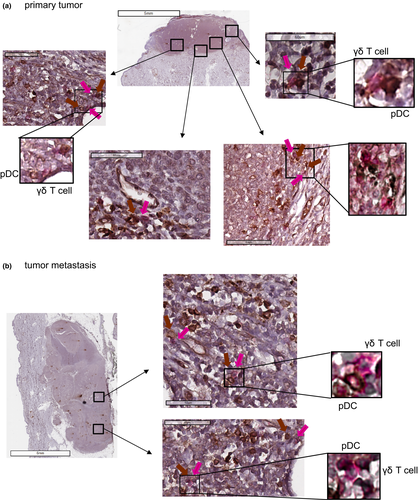
To first decipher whether there is an interplay between pDCs and γδ T cells in vivo, we assessed the localisation of these two immune players by immunohistochemistry on melanoma tumor sections. Closed contacts between pDCs and γδ T cells can be observed both in peri-tumoral area and intra-tumor bed of primary tumor (Figure 1a) and cutaneous metastasis (Figure 1b). These observations brought strong evidence that pDCs and γδ T cells co-exist and interact in the melanoma microenvironment in patients.
Circulating and tumor-infiltrating pDCs from melanoma patients are impaired in their ability to activate and modulate immune checkpoints on γδ T cells, in accordance with the clinical outcome of the patients
We then evaluated the ability of pDCs from the melanoma microenvironment to modulate the phenotype of γδ T cells. Purified pDCs from healthy donors’ blood, patients’ blood or tumor infiltrates were cocultured with γδ T cells purified from HDs’ blood in the absence or in the presence of TLR7-L (CL097) or TLR9L (CpGA), together with Zol or not, to promote accumulation of PAg and assess the potential synergistic effect between TLRL and Zol (Figure 2a). The use of whole γδ T cells allowed investigation of the impact of pDCs on both Tδ2+ and Tδ2- cell subsets. The analysis of the features of pDCs demonstrated that these cells upregulated costimulatory molecules (CD40, CD80 and CD86) and TRAIL as well as secreted IFN-α in response to TLR7-L and TLR9L stimulation but not Zol (Supplementary figure 1). As previously demonstrated,16 pDCs from blood and tumors of patients with melanoma displayed properties similar to pDCs from HDs, except that tumor-infiltrating pDCs appeared slightly impaired in their capacity to secrete IFN-α and IP10 in response to TLR9L stimulation, but their response to TLR7-L was not affected. The phenotypic features of γδ T cells including activation markers, immune checkpoints (ICP) and KIR/NCR expression of γδ T cells are depicted in Figure 2b–d, Supplementary figures 2 and 3. For all studied parameters, the levels on γδ T cells in the presence of unstimulated pDCs from HDs were similar to the level expressed by γδ T cells alone,28 attesting that in HD conditions, unstimulated pDCs do not modulate γδ T cells, except triggering a slight increase in CD69 expression (Supplementary figure 4). Although pDCs from HD blood trigger a strong upregulation of CD69 and CD25 markers on γδ T cells upon TLR7, TLR9 or Zol stimulation alone or in combination, pDCs from the blood and tumor infiltrate of patients were defective in activating γδ T cells in most cases (Figure 2b, Supplementary figures 2a, b and 3a), both Tδ2+ and Tδ2- subsets (Supplementary figure 3b). Interestingly, circulating pDCs from patients were still able to trigger Tδ2+ activation in the presence of Zol at levels similar to the HD group. Even though γδ T cells from HDs and patients can be directly activated by IFNα, IFNα has only a slight impact during cocultures of pDCs and γδ T cells (Supplementary figure 5).
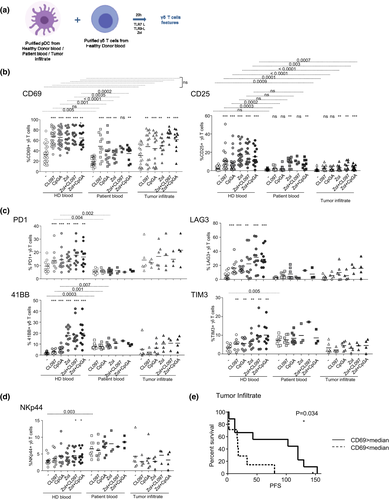
To gain further insight into the modulations induced by pDCs from melanoma patients, we analysed the expression of a panel of immune checkpoints on γδ T cells. 41BB (CD137), PD1 (CD279), TIM3 (CD366) and LAG3 (CD223) expressions were significantly induced on whole γδ T and Tδ2+ cells, and although at a lower level on Tδ2- subsets, in the presence of TLRL-/Zol-stimulated pDCs in the HD group (Figure 2c, Supplementary figures 2c and 3c). This ICP profile was not further triggered by pDCs from melanoma patients in the presence of TLRL or Zol compared to the pDC and γδ T-cell coculture in the absence of stimulation (condition) (Figure 2c, Supplementary figures 2c and 3c). However, unstimulated circulating pDCs from patients drove levels of 4-1BB and TIM3 on γδ T cells to be higher than those of HDs who were not further improved upon stimulation. Interestingly, among the KIR/NCR molecules, we observed that NKp44 (CD336) was induced on γδ T cells by circulating pDCs from melanoma patients in the absence of additional ex vivo stimulation (Figure 2d), whereas the expression of NKG2D and NKp30 remained similar between the groups (Supplementary figure 3d). The heat map based on ICP or KIR/NCR expression illustrates the differential profile of γδ T cells triggered by pDCs from blood and tumor infiltrate of melanoma patients compared to HDs (Supplementary figure 3e and f). To assess the clinical relevance of our findings, we performed correlations between the features of γδ T cells cultured with circulating or tumor-infiltrating pDCs and clinical outcomes of the corresponding patients (Supplementary table 4a and b, respectively). Circulating or tumor-infiltrating pDCs able to trigger high proportions of CD69-, TIM3- and LAG3-expressing γδ T cells (in unstimulated or TLRL conditions) were linked with a better clinical outcome (Figure 2e), whereas high proportions of NKp44-expressing γδ T cells might be associated with a worse clinical outcome (Supplementary table 4). Taken together, these results highlighted that circulating and tumor-infiltrating pDCs harboured an altered capacity to properly activate γδ T cells, and skewed their ICP and NCR profile.
pDCs from the blood and tumor infiltrate of melanoma patients exhibit a deficient ability to trigger the functionality of γδ T cells, in relation with the clinical outcome of the patients
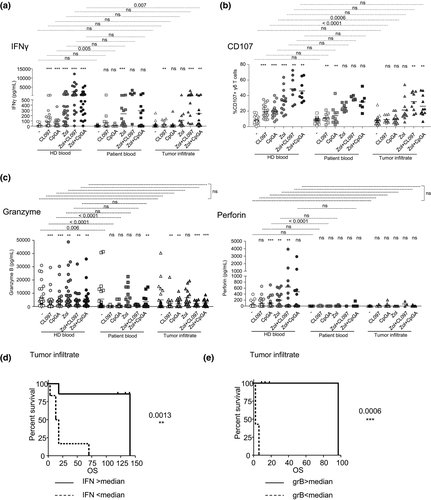
We further examined the ability of pDCs from melanoma patients to trigger the functional features of γδ T cells by evaluating the secretion of cytokines and cytotoxic properties of γδ T cells upon cocultures. TLRL and/or Zol-treated pDCs elicited productions of IFNγ and TNFα in cocultures in healthy context, which were not upregulated in the presence of pDCs from blood or tumor infiltrates of melanoma patients (Figure 3a, Supplementary figure 6a). Besides, TGFβ, IL4, IL10, IL13 and IL17 secretions were not impacted by melanoma patients’ pDCs (Supplementary figure 6a). We then explored the cytotoxic potential of γδ T cells towards melanoma tumor cells by assessing membrane-bound CD107a/b and perforin and granzyme B secretions (Figure 3b and c, Supplementary figure 6b–d). Notably, although the cytotoxic abilities of γδ T cells were strongly triggered by activated HD’s pDCs, circulating or tumor-infiltrating pDCs from melanoma patients induced significantly lower expression of CD107 (Figure 3b, Supplementary figure 6c) and weaker granzyme B and perforin secretions (Figure 3c). Such impairment in the melanoma groups was mostly observed in TLRL conditions, as the presence of Zol ensured a certain level of cytotoxicity of the γδ T cells compared to unstimulated conditions, even though lower than the HD group, which was attributable to the Tδ2+ subset (Supplementary figure 6d). Remarkably, higher secretions of IFNγ (Figure 3d) and TNFα (Supplementary figure 6e), and greater grB secretion (Figure 2e, Supplementary figure 6f) in cocultures with pDCs from blood and/or tumor infiltrate of melanoma patients in the presence of Zol together or not with TLR9L were associated with better PFS and/or OS of the patients (Supplementary table 4). Altogether, these data showed for the first time that melanoma impaired the capacity of TLRL-/Zol-stimulated pDCs to trigger a potent Th1-oriented cytotoxic response of γδ T cells. The heat map bringing together all the features of γδ T cells (activation, cytokine secretion, cytotoxicity) (Supplementary figure 7) illustrated the dysfunctional cross-talk between pDCs and γδ T cells triggered by pDCs from blood and tumor infiltrate of melanoma patients. Importantly, we further highlighted that, in autologous settings, pDCs and γδ T cells from HDs cross-activated each other upon appropriate stimulation, while pDCs and γδ T cells from the blood or tumor infiltrate of melanoma patients failed to appropriately trigger the activation and functionality of the other partner (Supplementary figure 8), excluding any pitfalls due to the allogeneic settings. Furthermore, as age and gender were documented to influence γδ T cell functionality, we re-examined the features of the bidirectional cross-talks between pDCs and γδ T cells by comparing young and elderly individuals, as well as male and female individuals for each group (Supplementary figure 9). Within each group, we observed similar results between young and elderly individuals when separated by the median age of each group (Supplementary figure 9a), and between males and females (Supplementary figure 9c). We observed a dysfunctional pDC and γδ T cell cross-talk both in young and old melanoma patients compared to age-matched HDs (Supplementary figure 9b), and in both male and female melanoma patients compared to sex-matched HDs (Supplementary figure 9d).
γδ T cells from blood or tumor infiltrate of melanoma patients were defective in triggering activation of pDCs and modulation of immune checkpoints, in line with the clinical outcomes
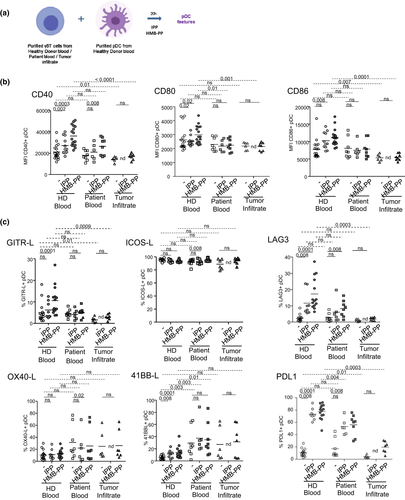
We next assessed the ability of γδ T cells from the melanoma microenvironment to modulate the activation status and phenotype of pDCs. Purified whole γδ T cells from HDs’ blood, patients’ blood or tumor infiltrate were cocultured with pDCs purified from HDs’ blood in the absence or presence of PAg (IPP or HMB-PP) to promote the activation of γδ T cells (Figure 4a). The phenotypic features of pDCs including activation markers and immune checkpoint profile were then depicted (Figures 4b, c, Supplementary figure 10a–g). For all studied parameters, levels on pDCs in the presence of unstimulated γδ T cells from HD were similar to the one expressed by pDCs alone,60 attesting that in HD conditions, unstimulated γδ T cells do not modulate pDCs. Although γδ T cells from HDs’ blood induced a marked upregulation of the activation markers CD40, CD80 and CD86 on pDCs in the presence of IPP or HMB-PP, circulating and tumor-infiltrating γδ T cells from patients were strongly impaired to activate pDCs (Figure 4b, Supplementary figure 10a–c). Notably, we also observed that higher CD86 expression induced by tumor-derived γδ T cells in the presence of HMB-PP might be associated with better OS (Supplementary table 5). To further decipher the impact of γδ T cells from melanoma patients on pDCs, we analysed the expression of a panel of immune checkpoints on pDCs (Figure 4c, Supplementary figure 10d and e). We observed that γδ T cells from HDs elicited the expression of GITR-L, LAG3, 4-1BBL, PDL1, PDL2 and OX40 on pDCs in the presence of IPP or HMB-PP stimulation (Figure 4c, Supplementary figure 10e). Such modulations were triggered to a lesser extent by circulating γδ T cells from patients or totally abrogated when tumor-infiltrating γδ T cells were used, except for 4-1BBL, PDL2 and OX40/OX40L in which an expression was higher when using circulating γδ T cells from patients compared to HDs. In addition, in unstimulated conditions, γδ T cells from blood and tumor infiltrate of patients elicited lower levels of ICOSL than HDs. The heat map based on ICP expression upon HMB-PP stimulation illustrated the differential profile of pDCs triggered by γδ T cells from blood and tumor infiltrate of melanoma patients compared to HDs (Supplementary figure 10f). Next, by performing Euclidian distance-based hierarchical clustering, each group was located in distinct areas of PCA analyses (based on PC1 and PC2), thus allowing intra-groups clustering based on the ICP profile of pDCs despite low group sizes (Supplementary figure 10g). Interestingly, by assessing the interrelations between ICPs triggered by HMB-PP-stimulated γδ T cells on pDCs through correlation analyses (Supplementary figure 11), we found that the cross-regulations between ICP elicited by γδ T cells from HDs’ blood were modified when considering γδ T cells from the blood or tumor infiltrate of melanoma patients. For example, ICOSL and 4-1BBL, that tended to negatively correlate with all other ICPs in HDs, showed mostly positive correlations in the blood and tumor infiltrate of melanoma patients. All these observations suggested that circulating and tumor-infiltrating γδ T cells from patients have an altered ability to properly activate pDCs and skewed their ICP profile.
γδ T cells from blood or tumor infiltrate of melanoma patients failed to elicit the functionality of pDCs
To go deeper into the characterisation of interplay between γδ T cells and pDCs, and further decipher the impact of deregulated γδ T cells on pDCs, we then studied the capacity of γδ T cells from melanoma microenvironment to modulate the functional properties of pDCs. Notably, the ability of PAg-activated γδ T cells to induce IFNα, IP10 and TNFα secretions by pDCs observed in a healthy context was strongly impaired with circulating or tumor-infiltrating γδ T cells of melanoma patients (Figure 5a). Furthermore, the triggering of TRAIL expression on pDCs by PAg-activated γδ T cells observed in a healthy context was totally abrogated when using circulating or tumor-infiltrating γδ T cells from melanoma patients (Figure 5b, Supplementary figure 12). We then assessed, following the coculture with unstimulated or PAg-activated γδ T cells, the ability of pDCs to respond to a subsequent TLR9L (CpGA) stimulation. Following coculture with unstimulated or PAg-activated γδ T cells from HDs’ blood, pDCs upregulated at least one of the activation markers (CD40 and/or CD80), TRAIL expression and IFNα secretion upon CpGA stimulation compared to the control condition (−) (Figure 5c and d, white bars). Remarkably, upon coculture with circulating γδ T cells from melanoma patients, pDCs retained their ability to respond to TLR9L stimulation as revealed for most of the studied parameters, except for basal IP10 secretion following pre-culture with the PAg-activated γδ T cells, which was abrogated (Figure 5c and d, light grey bars). Even though upon coculture with unstimulated and/or PAg-activated tumor-infiltrating γδ T cells from melanoma patients, pDCs displayed an altered ability to express CD40 and secrete IFNα/IP10 in control conditions (−), as well as to upregulate CD80 and secrete IP10 in response to CpGA stimulation, their potential to secrete IFNα in response to TLR9L stimulation remained intact compared to the HD group (Figure 5c and d, dark grey bars), suggesting that pDCs are still able to respond to a TLRL stimulation, despite their deregulation by γδ T cells. Thus, all these observations revealed that circulating or tumor-infiltrating γδ T cells from melanoma patients severely failed to directly trigger the functionality of pDCs, yet sparing their capacity to respond to a subsequent TLR9L stimulation. Importantly, we re-examined the features of the bidirectional cross-talks between γδ T cells and pDCs by comparing young and elderly individuals, as well as male and female individuals for each group (Supplementary figure 13). We observed similar results between young and elderly individuals when separated by the median age of each group (Supplementary figure 13a), and between males and females (Supplementary figure 13c), except a slight diminished level of CD40 on pDCs from female melanoma patients compared to males. We observed a dysfunctional γδ T cell and pDC cross-talk in both young and old melanoma patients compared to age-matched HDs (Supplementary figure 13b), and in both male and female melanoma patients compared to sex-matched HDs (Supplementary figure 13d).

The phenotypic and functional features of γδ T cells triggered by pDCs allowed clustering of patients, and highlighted perturbed interrelations between the capacities of pDCs dictated by melanoma
To have a global view of the features of pDCs driven by γδ T cells from melanoma patients compared to controls, we executed Euclidian distance based hierarchical clustering and ran PCA analyses. The heat map based on the phenotypic (activation status, ICP profile) and functional (TRAIL expression, cytokine secretion) parameters of pDCs elicited upon coculture with PAg-stimulated γδ T cells illustrated the distinct patterns of the features of pDCs depending on the source of γδ T cells (Supplementary figure 14a). Furthermore, despite limited size, each group was located in distinct areas of PCA analyses (based on PC1 and PC2), thus allowing intra-groups clustering when considering the potentialities of pDCs driven by γδ T cells (Supplementary figure 14b). In addition, to assess the interrelations between the features elicited on pDCs, we performed correlation analyses between the parameters for each group. The graphical Spearman correlation matrix revealed that the profile of positive and negative interrelations between the parameters observed in HDs was modified in blood and tumor infiltrate of patients (Supplementary figure 15). Thus, we uncovered that the profile of pDCs dictated by γδ T cells allowed patients’ clustering and that melanoma may drive perturbed regulations between the capacities of pDCs.
Reversion of the dysfunctional bidirectional cross-talks between pDCs and γδ T cells in melanoma patients by cytokine administration and immune checkpoint blocking
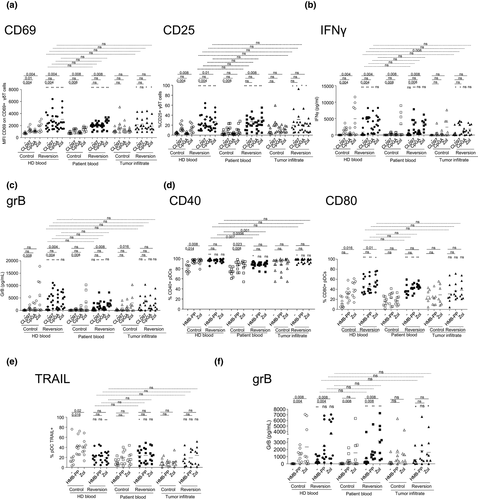
We next explored potential strategies to restore proper bidirectional interplay between pDCs and γδ T cells in the context of melanoma. We previously uncovered the pathophysiological features of both pDCs16, 17 and γδ T cells28 in melanoma patients together with the molecular mechanisms of the bidirectional interplay between pDCs and γδ T cells,60 bringing us tracks and clues on potential defects to fill, and skewed parameters to block. For the interplay between pDCs and γδ T cells (Figure 6a–c), molecules to block were selected based on their over expression by pDCs from melanoma patients compared to HDs, on the expression of the corresponding receptors/ligands on γδ T cells, whereas factors to restore were picked based on their defective secretion by pDCs from melanoma patients compared to HDs upon TLR7/9 triggering. Hence, possible membrane candidates to counteract are 41BB-L, OX40L, ICOSL, PD1 and GITR, and potential soluble candidates required are IFNα and TNFα. Notably, blocking interactions between 41BB/4-1BB-L, OX40/OX40L, ICOS/ICOSL, PD1/PDL1, GITR/GITR-L, and adding rhIFNα and rhTNFα can restore patients’ pDCs ability to properly activate γδ T cells (Figure 6a, Supplementary figure 16a), especially Tδ2+ cells (Supplementary figure 16b) with appropriate cytokine secretion (Figure 6b) and cytotoxic properties (Figure 6c). For the interplay between γδ T cells and pDCs (Figure 6d–f), molecules to block were selected based on their over expression by γδ T cells from melanoma patients compared to HDs, the expression of the corresponding receptors/ligands on pDCs, and factors to restore were picked based on their defective secretion by γδ T cells from melanoma patients compared to HDs upon PAg stimulation. Hence, possible membrane candidates to block were 41BB, OX40, TIM3 and LAG3, and potential soluble candidates to add were IFNγ and TNFα. We observed that the blocking of 41BB, OX40, TIM3, LAG3 and addition of rhIFNγ and rhTNFα can restore the ability of patients’ γδ T cells to properly activate pDCs (Figure 6d) and trigger their cytotoxicity (Figure 6e and f) in most cases. The defects in crosstalks beween pDCs and γδ T cells and vice versa observed in melanoma patients compared to HDs (white symbols) were reversed by the reversion mixture to levels comparable to the ones in HDs. Even though the reversion mix potentiates also the cross-talks in HDs, it allows overcoming the dysfunctions in melanoma patients. Importantly, the reversion was also effective on purified cell subsets, which exclude the potential influence of other immune cells in the outcome of modulation of pDC-dependent γδ T cells or of γδ T cell-dependent pDCs (Supplementary figure 17). Thus, the restoration of efficient bidirectional interplay between pDCs and γδ T cells in melanoma patients can be achieved through specific cytokine administration and immune checkpoint targeting, opening new opportunities to potentiate these crucial immune players towards effective antitumor immunity.
Circulating and tumor-infiltrating pDCs and γδ T cells from melanoma patients displayed higher level of BTN3A that were revealed to be nonfunctional
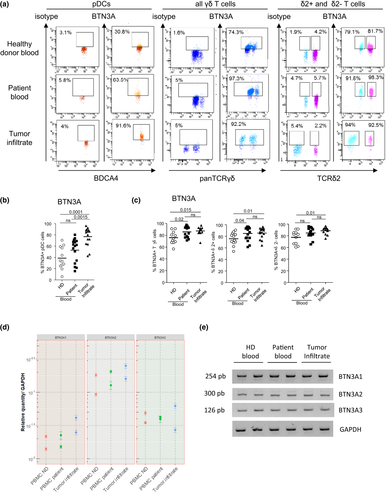
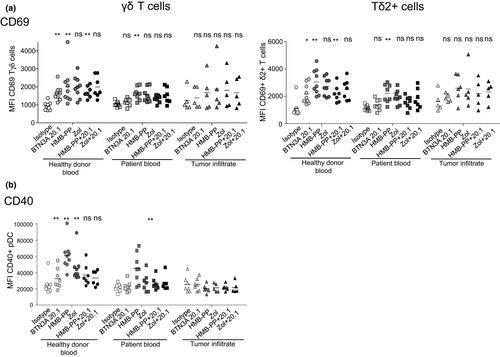
As we previously demonstrated the key role of BTN3A during interplay between pDCs and γδ T cells, we then assessed whether the dysfunctional cross-talks highlighted here in the context of melanoma could be due to an altered expression of BTN3A by pDCs or γδ T cells. We evaluated the expression of BTN3A on pDCs and γδ T cells from HDs’ blood, patients’ blood and tumor infiltrate by flow cytometry (Figure 7a). Unexpectedly, we observed an increased expression of BTN3A on tumor-infiltrating pDCs compared to pDCs from the HD group (Figure 7b), as well as a higher expression of BTN3A on γδ T cells from the blood and tumor of melanoma patients compared to the blood of HDs, especially on the Tδ2+ subset (Figure 7c), that was linked with a better clinical outcome (Supplementary table 6). We then investigated whether melanoma patients displayed the expression of the three isoforms of BTN3A and their relative expression level. As BTN3A1, BTN3A2 and BTN3A3 could not be distinguished based on their extracellular part, we performed RT-qPCR on PBMC from the different groups to analyse the relative expression of the isoforms (Figure 7d and e). BTN3A2 was the greatest expressed isoform in all groups (Figure 7d), and BTN3A1 and BTN3A2 tended to be more expressed in tumor infiltrate compared to HDs’ blood (Figure 7d). As the BTN3A1/A3 isoforms, which are sensitive to PAg, do not seem to be defective in melanoma patients, we then evaluated the functionality of BTN3A using an agonistic antibody (clone 20.1). Notably, although anti-BTN3A 20.1 Abs triggered an upregulation of CD69 expression on γδ T cells especially on the Tδ2+ subset, and of CD40 on pDCs within PBMC of HDs, we observed no impact of this agonistic Ab on γδ T cells (Figure 8a) and pDCs (Figure 8b) within PBMC or tumor infiltrate of melanoma patients, suggesting a functional impairment of BTN3A in melanoma patients. The combined use of agonistic anti-BTN3A 20.1 Abs together with HMB-PP or Zol do not improve the response observed with HMB-PP or Zol alone. Thus, our investigations pointed out for the first time a higher expression of BTN3A on circulating and tumor-infiltrating pDCs and γδ T cells from melanoma patients with adequate BTN3A1/A2/A3 isoforms expressed, but stressed out its potential functional impairment.
Discussion
pDCs and γδ T cells harboured unique properties and functional plasticity that steer their attractiveness for immunotherapy. Although being investigated individually, their interplay has not been extensively explored especially in melanoma where these potent immune players are involved in the pathophysiology of the disease, and represent targets and vectors for immunotherapies. Here, we provide a detailed investigation of the bidirectional cross-talks between pDCs and γδ T cells in both the blood and tumor microenvironment, elucidating the phenotypic and functional features driven by the other partner, in relationship with the clinical outcome of the patients (see Supplementary figure 18, Graphical summary of the findings). Our study, even though performed with rather low group sizes, brings better understanding of the physiopathology of the interplay between pDCs and γδ T cells in melanoma, which will help design new therapeutic approaches exploiting their potential to improve patient outcomes.
In this study, we demonstrated severe alterations of the bidirectional cross-talk between pDCs and γδ T cells in melanoma. Although healthy TLRL-activated pDCs drove a potent activation and functionality of γδ T cells, pDCs from blood and tumor infiltrate of melanoma patients displayed an impaired ability to activate and modulate immune checkpoints on γδ T cells, and exhibited a deficient ability to trigger the functionality of γδ T cells. Conversely, healthy γδ T cells activated by PAg triggered phenotypic changes in pDCs and elicited their functional activities, but γδ T cells from blood or tumor infiltrate of melanoma patients were defective in triggering the activation of pDCs and modulation of immune checkpoints, and failed to elicit their functionality. These dysfunctional bidirectional cross-talks between pDCs and γδ T cells were independent of the age and gender of the patients, but observed in both young and elderly patients, as well as in both male and female patients. Nevertheless, for the cross-talk between γδ T cells and pDCs, due to the high discrepancies in age between HDs and patients, we cannot exclude age-related effects in the differential outcome of the cross-talks. Circulating pDCs or γδ T cells were almost totally unable to trigger the activation and functionality of their partner when purified from stage III/IV patients, whereas still efficient when purified from stage I/II patients. Tumor-infiltrating pDCs or γδ T cells were also more strongly impaired when isolated from stage IV patients compared to stage III patients. Thus, we revealed that melanoma hijacked the interplay between pDCs and γδ T cells to escape from immune control, and highlighted that circulating and tumor-infiltrating pDCs and γδ T cells are promising potential biomarkers of clinical evolution.
By exploring potential strategies to restore the bidirectional interplay between pDCs and γδ T cells in the context of melanoma, we demonstrated that the reversion of the dysfunctional bidirectional cross-talks could be achieved by specific cytokine administration and immune checkpoint targeting, opening new opportunities to potentiate these crucial immune players towards effective antitumor immunity and further modulate and orientate the outcomes of these cross-talks. Indeed, we identified critical immune checkpoint molecules governing the interplay between pDCs and γδ T cells: OX40/OX40L, ICOS/ICOSL, PD1/PDL1, 41BB/4-1BB-L, GITR/GITR-L, TIM3 and LAG3, which are all targets of the current and next generation immune checkpoint therapies.62 Deciphering the precise role of each molecule in the reversion of the dysfunctional cross-talks would be very pertinent to assess, but unfortunately it is not feasible. It could be anticipated that each molecule may be important to partially bypass the dysfunctions observed at the level of pDCs and γδ T cells in melanoma patients, but acting simultaneously on several pathways would be needed to fully restore or enhance efficient cross-talks. Thus, pDCs, γδ T cells and their inter-relationships are likely to be affected by immune checkpoint blocker therapies, supporting their study in the context of such immunotherapies.
We and others previously depicted specific features of γδ T cells in the context of melanoma28, 39 and further uncovered here that some of them may be driven by melanoma-skewed pDCs, as they are recapitulated on healthy γδ T cells cocultured with circulating- or tumor-infiltrating pDCs from melanoma patients. The reported accumulation of FoxP3+ regulatory γδ T cells within melanoma tumors could be linked to the higher IL10 production by γδ T cells upon coculture with pDCs. The impaired ability of γδ T cells to exhibit activation molecules, secrete cytokines in response to stimulation and their altered antitumor cytotoxic potential may be driven by pDCs. Furthermore, we also previously highlighted higher proportions of circulating γδ T cells expressing 4-1BB and TIM3 in melanoma patients. Here, we showed that pDCs from the blood of melanoma patients drove increased expression of 4-1BB and TIM3 on healthy γδ T cells. Interestingly, we previously identified high proportions of NKp44+ circulating γδ T cells, which were predictive of poor clinical outcomes, and highlighted here that circulating pDCs from melanoma patients triggered NKp44 expression on γδ T cells, also linked to an early relapse. Notably, splice variants of human NCR encoding isoforms with inhibitory functions can be triggered in some tissue microenvironments by specific ligands and influence the clinical outcomes in many immuno-pathological contexts.63, 64 Interestingly, it has been shown that the inhibitory isoform of NKp44 can cross-link proliferating cell nuclear antigen (PCNA) expressed by many tumor types, and the blocking of NKp44-PCNA interactions inhibited tumor growth in mouse models.65 Hence, NCR can be considered as novel innate immune checkpoints, offering an exciting therapeutic target to manipulate in cancer.
BTN3A harbours an emergent yet growing role in the field of tumor immunity, and our study brings crucial insights regarding BTN3A function on immune cells from tumor microenvironment. We previously uncovered that human pDCs express BTN3A molecules,60 which are mandatory for the proper cross-talk with γδ T cells.60 We now reveal, for the first time, an increased expression of BTN3A on circulating and tumor-infiltrating pDCs and γδ T cells of melanoma patients, yet paradoxically associated with a defective interplay between these two immune cells, stressing out its potential functional impairment. Such observations have been made using antibodies that recognise the extracellular part of BTN3A, therefore unable to distinguish the isoforms of BTN3A. It remains to be determined which isoform is overexpressed on pDCs and γδ T cells in melanoma, even though RT-PCR analyses of the three BTN3A1/A2/A3 isoforms seem to suggest an increased expression of BTN3A2 in melanoma patients compared to HDs. In addition, mutations within the intracellular part of BTN3A1 affect 5–10% of melanoma patients and may prevent the proper sensing of PAg by BTN3A1. Thus, pDCs and γδ T cells from melanoma microenvironment may be deficient for PAg sensing despite displaying high level of BTN3A, and thus not well equipped to favor PAg-dependent cellular interplay. It has been demonstrated that intracellular IPP, accumulated following mevalonate pathway inhibition through Zol treatment, will either provoke a conformational change of BTN3A1/A3 or be released by DCs through the ATP-binding cassette transporter A1 (ABCA1) in cooperation with apolipoprotein A-I (apo-I) and BTN3A1,66 mediating the activation of γδ T cells. Regulation of BTN3A1 stability together with protein trafficking and expression of the transporter ABCA1 within tumor microenvironment may be crucial to trigger optimal cross-talks between pDCs and γδ T cells. Moreover, it has been demonstrated that BTN3A1 is essential for type I IFN signalling upon nucleic acid sensing.67 In addition, the recent discovery that BTN2A1 plays a critical role in PAg recognition by γδ T cells by binding the γδ TCR in conjunction with BTN3A168 sustain even more the rationale to study deeply the BTN family of molecules both on tumor cells and immune infiltrating cells to elucidate the mechanism of BTN3A dysfunction in melanoma. Besides, it has been highlighted that the polymorphism of genes coding for BTNs is associated with pathologies in humans69 especially cancers. A positive association between BTN3A2 expression, higher T-cell infiltration and better prognosis has been pointed out in ovarian cancer70 and breast cancer,71 suggesting that BTN3A2 expression in tumor microenvironment modulates the density and nature of immune infiltration. BTN3A2 is the most expressed isoform on tumor cells (solid tumors or haematological tumors) as shown in colon cancer72 and pancreatic adenocarcinoma.73 Its overexpression is strongly associated with worse clinical outcome73: BTN3A2 lacks the B30.2 intracellular domain, therefore behaving as a potential decoy receptor that favors immune escape by avoiding recognition of tumor by γδ T cells. Outstandingly, circulating levels of soluble forms of BTN3A1 and pan-BTN3As have a strong prognostic significance in patients with pancreatic adenocarcinoma, high levels being associated with short survivals,73, 74 potentially by preventing Vγ9Vδ2 T cells to exhibit cytotoxic activities towards tumor cells. In the light of our data, it would be interesting to further explore the expression of BTN3A isoforms in melanoma, both on tumor cells and on immune infiltrating cells, together with the circulating levels of soluble forms of BTN3A, and assess their prognostic impact on clinical outcomes as well as their influence on the nature and density of immune infiltration. Thus, BTN3A, by owning a compulsory role in the bidirectional cross-talks between pDCs and γδ T cells, appears to be crucial in the regulation of immune responses towards tumor cells. The different isoforms of BTN3 molecules exhibit stimulatory or inhibitory activities, therefore behaving as immune checkpoints, which could be targeted to potentiate the antitumor activity of γδ T cells, and are promising to design or optimise immunotherapeutic strategies.75
By highlighting closed contacts between pDCs and γδ T cells within melanoma microenvironment in patients, we brought evidence for the first time that these two immune players directly interact in vivo. Most of the studies were performed ex vivo, but there is in vivo evidence that the cross-talk between DCs, especially pDCs, and γδ T cells is involved in many pathophysiological conditions. Both pDCs and γδ T cells infiltrate tumors and could meet within tumor microenvironment. Remarkably, a recent study revealed that activated pDCs preferentially attracted γδ T cells following their injection in the skin of melanoma patients,61 supporting the potential interplay between pDCs and γδ T cells in vivo in cancer patients. Activated human pDCs secreted high levels of chemokines that preferentially attract γδ T cells, especially the CXCR3 ligands CXCL9 (MIG), CXCL10 (IP10) and CXCL11 (I-TAC) as well as CCL2 (MCP1), CCL3 (MIP1α), CCL4 (MIP1β) and CCL5 (RANTES). Furthermore, granulysin secreted by activated Vδ2+ T cells induce chemotaxis or fugetaxis of DCs depending on their maturation status.76 Notably, the cross-talk between DCs and γδ T cells can be exploited by pathogens for immune evasion: moDCs infected by HIV can inhibit Vγ9Vδ2 T-cell functions (proliferation, cytokine production).77 Nevertheless, cross-talk between pDCs and γδ T cells could also initiate or boost immune responses to pathogens. During West Nile Virus infection, γδ T cells promote the maturation of DCs and subsequent T-cell priming,78 and BCG-infected DCs can prime and expand cytotoxic γδ T cells.79 The interplay between DCs and γδ T cells is also central in host–microbiota interactions as well as IL17-driven inflammatory diseases, as microbiota-activated CD103+ DCs can elicit γδ T17.80 Besides, activated γδ T cells promote DC maturation and exacerbate the development of autoimmune disease in a mouse model.81 In addition, DCs activated by ABP can empower γδ T cells with antitumor immunity82 through elicitation of TAA-specific CD8 T-cell responses.
Even though pDCs and γδ T cells could be hijacked by melanoma, they displayed features that positively correlate with clinical outcomes,39, 83, 84 revealing their crucial antitumor potential and prompting their use as vectors or targets for cancer immunotherapy. Anti-cancer therapies based on the exploitation of the potential of pDCs40 or γδ T cells42, 44 are emerging, especially in melanoma.85, 86 Protective antitumor responses can be achieved upon mobilisation of pDCs by administration of TLRL or direct use of pDCs as vectors for vaccination.40, 47, 87 Besides, successful clinical trials aiming at stimulating Vδ2+ cells with ABP (zoledronate, pamidronate) or at adoptively transferring ex vivo expanded γδ T cells proved that γδ T cell-based therapeutic approaches are promising to fight tumors and improve patient outcomes. Yet, the impacts of pDC-based immunotherapies on γδ T cells as well as the effect of strategies based on γδ T cells on pDCs have not been investigated. Our findings highlighted the possibility that ABP may exert a therapeutic activity by exploiting the interactions between pDCs and γδ T cells. Indeed, the interplay between pDCs and γδ T cells may participate in the favorable immune effects of Zol in cancer88: ABP used as adjuvants for the treatment of malignant osteolytic bone disease could promote the antitumor functions of γδ T cells through pDCs. Furthermore, Zol can induce the expression of BTN3A1 within the tumor microenvironment as described in colorectal cancer, hence stimulating antitumor γδ T cells and sensitising tumor cells to cytotoxicity mediated by γδ T cells.89 The interplay between pDCs and γδ T cells could be exploited, influencing pDCs through the impact on γδ T cells, and affecting γδ T cells by modulation of pDCs. Our findings also provide rationale to simultaneously target pDCs and γδ T cells using both TLR agonists and ABP, in order to combine the engagement of both cell types and synergise their potencies. Thus, the expanded knowledge on interactions between pDCs and γδ T cells paves the way to design novel immunotherapies harnessing their potential.
Altogether, our study uncovered that melanoma hijacked the bidirectional interplay between pDCs and γδ T cells to escape from immune control. Yet, pDCs and γδ T cells have the potential to exhibit and trigger potent antitumor responses to successfully drive favorable clinical outcomes. A better understanding of the functional plasticity of pDCs and γδ T cells will help designing new therapeutic approaches exploiting the potential and interplay of these potent immune players while counteracting their skewing by tumor cells. Both pDCs and γδ T cells harbour critical roles in the induction of immune responses and orientation. Their unique and crucial properties together with their functional plasticity render them very attractive both as targets and vectors for cancer immunotherapy. The understanding of the interplay between pDCs and γδ T cells will help harness their power against cancer to improve cancer immunotherapies and patient outcomes. Our findings pave the way to manipulate these potent and promising cell partners to design innovative immunotherapeutic strategies.
Methods
Samples from patients with melanoma and healthy donors
Blood and tumor (primary tumor, cutaneous metastases and lymph node metastases) samples not needed for pathological investigations were obtained from 65 and 48 melanoma patients, respectively, stage I-IV. Patients were staged according to the American Joint Committee on Cancer (AJCC) staging system for melanoma 2009 (V7). Clinical features are shown in Supplementary table 1 (blood samples) and table 2 (tumor samples). Progression free survival (PFS) and overall survival (OS) were calculated both from diagnosis and sampling times. Blood samples were also obtained from 82 healthy volunteers (HDs). PBMCs were purified by Ficoll-Hypaque density-gradient centrifugation (Eurobio). Tumor samples were mechanically dilacerated and digested with 2 mg mL-1 collagenase-D (Roche) 20 U mL-1 DNase (Sigma). The resulting tumor-infiltrating cell suspensions were filtered and washed. The study was conducted in accordance with the principles expressed in the Declaration of Helsinki. For samples used for the cross-talk between pDC and γδ T cells median age was 49 years for HDs, 46 years for patients’ blood and 65 years for tumor infiltrates. Distribution of gender (male/female) was 0.94 for HDs, 1.1 for patients’ blood and 0.58 for tumor infiltrates. For samples used for the γδ T cell and pDCs cross-talk, median age was 21 years for HDs, 61 years for patients’ blood and 65 years for tumor infiltrates. Distribution of gender (male/female) was 2 for HDs, 1.5 for patients’ blood and 1.25 for tumor infiltrates. For samples used for the BTN3A investigation, median age was 52 years for HDs, 50 years for patients’ blood and 67 years for tumor infiltrates. Distribution of gender (male/female) was 1.37 for HDs, 0.83 for patients’ blood and 0.58 for tumor infiltrates. All procedures were approved by the Ethics committee of Grenoble University Hospital (CHUGA) and the French Blood Agency’s Institutional Review Board Committee (IRB), and declared under the reference #DC-2008–787 (EFS) and collection AC-2017-2949 (CHUGA). Patients gave the written informed consent, and their records were de-identified prior to the analysis.
Immunohistochemistry
Immunochemistry staining was performed on 3-µm fixed paraffin-embedded tissue sections using a Benchmark ULTRA autostainer (Roche/Ventana Medical Systems, Tucson, AZ, USA). After deparaffinisation, antigen retrieving was carried out with Cell Conditioning 1 (CC1) standard antigen retrieval buffer (Tris/Borate/EDTA pH 8.4) for 64 min. Detection was done in two steps. First, CD123 mouse monoclonal antibody (clone 7G3, BD Biosciences) was incubated at 37°C for 1 h, at a 1:100 dilution, and revelation was achieved in brown by using ‘UltraView Universal DAB detection kit’ (Ventana Medical System/Roche). Second, after washing steps, pre-diluted TCR delta mouse monoclonal antibody (clone BSB-127, BioSB) was incubated at 37°C for 1 h and revelation was achieved in red by using the ‘UltraView Universal Alcaline Phosphatase RED detection kit’ (Ventana Medical System/Roche). Nuclear counterstain was done with haematoxylin for 12 min.
Purification of pDCs and γδ T cells
pDCs and γδ T cells were purified using the EasySep Human pDC enrichment kit and the EasySep Human γδ T-cell enrichment kit (StemCell), respectively, according to the manufacturer’s instructions. The purity obtained was systematically above 90.5% for pDCs and 95% for γδ T cells. Samples from all groups were treated similarly, and cell viability was systematically assessed.
Tumor cell lines
Human melanoma lines COLO829 (ATCC Cat# CRL-1974, RRID:CVCL_1137) and A375 (ATCC Cat# CRL-7904, RRID:CVCL_0132) were purchased from ATCC (LGC-Standards).
Cultures were performed in RPMI1640-Glutamax (Invitrogen) supplemented with 1% non-essential amino acids, 1 mm sodium pyruvate (Sigma), 100 µg mL−1 gentamycin and 10% foetal calf serum (FCS) (Invitrogen). All cell lines were obtained from ATCC and tested negative for mycoplasma contamination. Cell lines were authenticated by phenotypic analysis and tumor antigen expression by RT-PCR and flow cytometry.
pDCs–γδ T cells coculture experiments
Purified pDCs and γδ T cells or whole PBMCs/tumor infiltrates (as indicated on the Figures) were resuspended, respectively, at 2 × 106 mL−1 or 250 000 cells per 50 µL in complete RPMI1640 10% FCS, and cocultured in a 1:1 ratio 20 h at 37°C, 5% CO2 (1 × 106 mL−1 final for each cell subset). Cocultures were performed in the absence or the presence of TLR7-L (CL097, 1 μg mL−1), TLR9L (CpGA, 1.5 μm) (Invivogen) and/or zoledronate (10 μm) (Novartis) to activate pDCs, as well as IPP (80 μm) or HMB-PP (200 nm) (Sigma) together with IL2 (0.1 UI mL−1) (Peprotech) and/or zoledronate (10 μm) to activate γδ T cells. In some experiments, whole PBMC and CD45+ tumor infiltrates, or purified pDCs or γδ T cells were first pre-incubated 20 min with blocking antibodies (functional grade quality, no azide/low endotoxin (NA/LE)) alone or in different mixtures before adding the other cell partner. Cultures were performed in the presence of the following blocking antibodies (functional grade quality) and/or mixture of human recombinant cytokines: anti-IFNAR2 Abs (pbl assay) or mixture of anti-41BB (antibodies online) (1 μg mL−1), anti-OX40L, anti-PD1 (R&D Systems), anti-ICOSL (Invitrogen), anti-GITR (Thermo Fisher) (all at 10 μg mL−1), recombinant human (rh)IFNαA and IFNα2b each at 2000 UI mL−1, and rhTNFα at 50 pg mL−1 (BioTechne) to assess impact on the features of pDC-triggered γδ T cells; mixture of anti-41BB (antibodies online) (at 1 μg mL−1), anti-OX40L (R&D Systems), anti-TIM3 (Biolegend), anti-LAG3 (Adipogen) (all at 10 μg mL−1), rhIFNγ at 10 ng mL−1 and rhTNFα at 10 ng mL−1 (BioTechne) to assess impact on the features of γδ T cell-triggered pDCs. Controls were performed using mouse IgG and/or Goat IgG control isotypes (31–41 μg mL−1 according to the corresponding amount of specific antibodies mixed together) (Thermo Fisher). In some experiments, purified γδ T cells from HDs’ blood, patients’ blood or tumor infiltrates were cultured directly with recombinant human (rh)IFNαA and IFNα2b each at 1000, 2000 or 10 000 UI mL−1 (BioTechne). The activation status of γδ T cells (CD69 expression) was assessed 20 h later by flow cytometry.
Restimulation of pDCs
After coculture with γδ T cells, the ability of pDCs to respond to a subsequent TLRL stimulation was assessed following the first coculture. In this case, the γδ T cells–pDCs cocultures were harvested upon 20 h, washed and counted, and pDCs were resuspended at 1 × 106 mL−1 and further cultured 24 h in the absence or presence of TLR7-L (CL097, 1 μg mL−1) or TLR9L (CpGA, 1.5 μm) (Invivogen).
Phenotypic analysis
The phenotypic features of pDCs and γδ T cells were assessed as indicated either at the basal state or upon 20-h cocultures, and after the subsequent restimulation for pDCs. Cell suspensions were stained with anti-human antibodies and their isotype-matched controls in PBS 2% FCS. pDCs were defined as CD45+ HLA-DR+ BDCA4+. γδ T cells were identified as CD45+ CD3+ panTCRγδ+ and further divided into δ2+ and δ2− subsets.
The activation status of the cells was determined using anti-CD40, anti-CD80 (Beckman), anti-CD86 (BD) Abs for pDCs and anti-CD69 (BD), anti-CD25 (eBiosciences) antibodies for γδ T cells. The expression profile of immune checkpoints was analysed using anti-OX40, anti-OX40L, anti-ICOS, anti-41BB, anti-41BB-L, anti-PD1, anti-PDL1, anti-PDL2 (BD), anti-ICOSL, anti-TIM3, anti-CTLA4, anti-LAG3 (eBiosciences) Abs; activating and inhibitory NKR are depicted using NKG2D (BD), NKp30, NKp44 (Beckman Coulter Cat# IM3710, RRID:AB_2857937) (Beckman) Abs. TRAIL expression was evaluated on pDCs using anti-TRAIL Abs (BD Biosciences Cat# 564243, RRID:AB_2738696). The expression of BTN3A was assessed on pDCs and γδ T cells at the basal state using anti-BTN3A Abs (clone BT3.1, Miltenyi Biotec Cat# 130-108-222, RRID:AB_2656908). Suspensions were submitted to flow cytometry analysis using a FACS CantoII and BD FACSDiva Software, RRID:SCR_001456 (BD). 50 000 to 200 000 cells (pDCs or γδ T cells) per sample were acquired. We carefully defined alived cells by excluding the cell debris and dead cells on the FSC-A/SSC-A dot plot. We further gated on CD45high cells before defining the cells of interest. We analysed either the percentage of positive cells or the mean fluorescence intensity (MFI) of the positive cells as indicated. To ensure quality control during the study, we performed a systematic standardisation of the fluorescence intensities using a cytometer set-up and tracking beads (CST) (BD).
Cytotoxic activity
The cytotoxic activity of γδ T cells was evaluated by a CD107 degranulation assay and perforin measurement upon coculture with target cells. After cocultures of pDCs and T cells, the cells were washed, γδ T cells were counted and further cocultured with melanoma tumor cells (COLO829, A375) in a 20:1 ratio for 5 h. Anti-human CD107a/b Abs (BD) were added at the start of the coculture together with GolgiSTOP (BD) for the last 4 h. The cells were then labelled with CD45, CD3, panTCRγδ and TCRδ2 Abs (BD) before flow cytometry analysis. Perforin secretion was evaluated in the coculture supernatants using Human Perforin (PRF1) ELISA kit (AbCam).
Dosage of soluble factors
Human soluble IFNγ, TNFα, IFNα, IP10, IL4, IL10, IL17-A, TGFβ and granzyme B production were measured in the coculture supernatants using a Cytometric Bead Array assay (CBA, BD) and FCAPArray software (BD).
Quantification of the isoforms of BTN3A by RT-qPCR
PBMC from HDs’ or patients’ blood, and CD45+ cells isolated from tumor infiltrates (StemCell) were directly lysed in RLT Buffer from the Qiagen RNeasy Plus mini kit, and homogenised with QIAshredder (Qiagen). RNA was extracted with Qiagen kit RNeasy Plus mini kit according to the manufacturer’s instructions and quantified on a NanoDrop spectrophotometer. 1µg of RNA was used to synthesise cDNA using the iScript reverse transcription kit (Bio-Rad) according to the manufacturer’s instructions (5 min at 25°C, 20 min at 46°C, 1 min at 95°C). qPCR was performed using the iTaq universal SYBR Green supermix (Bio-Rad) on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad) with the following protocol: 95°C 30 s, [95°C 5 s, 60°C 20 s] (45 cycles), 72°C 10 min. Analysis was done with the CFX Manager software (Bio-Rad). The primers designed and used for the qPCR analysis are shown in Supplementary table 3 (synthesised by Eurogentec). Amplification products were run on a 5% agarose gel to verify the size of amplicons and the absence of non-specific products. The specificity of amplification for BTN3A1 is due to the forward primer, while the reverse primer for BTN3A2 binds to a unique BTN3A2 region. Both forward and reverse primers contribute to the specific amplification of BTN3A3.
Functionality of BTN3A
The functionality of BTN3A on γδ T cells and pDCs was assessed within PBMCs from HDs or melanoma patients, and within tumor infiltrates. PBMCs or tumor infiltrates were incubated with anti-BTN3A agonist antibody (clone 20.1) or anti-BTN3A blocking antibody (clone 103.2) (Creative Biolabs, Cat# PABL-414 and Cat# PABL-415, respectively) at 10 μg mL−1, or with mouse IgG control isotype (10 μg mL−1), in the presence or not of HMB-PP (200 nm) (Sigma) or zoledronate (10 μm) (Novartis). Activation of γδ T cells (CD69 and CD25 expression), pDCs (CD40 expression) and cytokine secretion (IFNγ, TNFα) was assessed upon 20 h of culture. Analyses were done by flow cytometry using a FACS CantoII and FACSDiva Software (BD).
Statistical analysis
Raw data were analysed in a blinded way. Statistical analyses were performed using GraphPad Prism software, RRID:SCR_002798 using the Wilcoxon matched t-test and the Mann–Whitney unpaired non-parametric test combined with Bonferroni correction and the Log-rank test. Survival analyses (Cox regression, Kaplan–Meier), correlation matrix, heatmaps and principal component analysis (PCA) were performed using the survival, GGally, gplots, ggplot2, RRID:SCR_014601, ggbiplot, MissMDA and FactoMineR, RRID:SCR_014602 packages of the R i386 software version 3.6.2.
Acknowledgments
We thank Dr Dominique Legrand, Yannick Bouvier and her/his staff at EFS Auvergne Rhone-Alpes for providing healthy volunteers’ blood samples and associated information. We acknowledge the surgeons and Dr Nicole Pinel from the anatomo-pathology Department from CHU Grenoble Alpes for providing tumor samples. We thank Dr Nathalie Bendriss-Vermare and Dr Myriam Capone for stimulating discussions and precious advice. We are grateful to all the volunteers and patients who agreed to participate in this study.
Conflict of interest
The authors report no conflict of interest.
Author contributions
Pauline Girard: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing-original draft; Writing-review & editing. Eleonora Sosa Cuevas: Conceptualization; Data curation; Formal analysis; Methodology; Writing-review & editing. Benedicte Ponsard: Data curation; Formal analysis; Methodology; Writing-review & editing. Stephane Mouret: Data curation; Formal analysis; Investigation; Resources; Writing-review & editing. Hugo Gil: Data curation; Formal analysis; Methodology; Resources; Validation; Writing-review & editing. Edwige Col: Data curation; Formal analysis; Methodology; Resources; Validation; Writing-review & editing. Florence De Fraipont: Formal analysis; Investigation; Resources; Writing-review & editing. Nathalie Sturm: Data curation; Formal analysis; Methodology; Resources; Validation; Writing-review & editing. Julie Charles: Conceptualization; Formal analysis; Resources; Supervision; Writing-review & editing. Olivier Manches: Data curation; Formal analysis; Investigation; Methodology; Writing-review & editing. Laurence Chaperot: Funding acquisition; Project administration; Supervision; Writing-review & editing. Caroline Aspord: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Writing-original draft; Writing-review & editing.




