Milk exosomes: Harnessing nature's duality for cancer therapy
Abstract
Milk exosomes, extracellular nanovesicles that are naturally present in milk, have gained significant attention in cancer research for their potential to revolutionize cancer treatment strategies. They possess a specific set of characteristics that make them promising nanoscale vehicles for targeted drug delivery systems. Their inherent biocompatibility, coupled with their ability to effectively encapsulate and transport therapeutic agents directly into tumor cells, suggests the possibility of developing novel cancer therapies, potentially minimizing side effects associated with conventional therapies. However, recent studies have shown that milk exosomes have a dual nature, which is not entirely positive. Although they show potential in delivering anticancer therapeutics, evidence suggests they may also, under certain conditions, contribute to cancer progression. This paradoxical nature necessitates a better understanding of how they interact and work in different stages of cancer. Further investigation is crucial to understand the factors influencing their behaviour and to develop strategies that can maximize their therapeutic prospects while mitigating potential risks associated with their use in cancer treatment.
Extracellular vesicles (EVs) based nanomedicine development, a cutting-edge era for cancer therapeutics. Exosomes are the most popular EVs subpopulation in cancer therapeutic devolvement.1, 2 Milk exosomes naturally secreted by epithelial cells of mammary gland.3 Their significant potential as a natural, biocompatible, and promising nanoscale delivery platform for anti-cancer therapeutics and targeted drug delivery, making them an effective tool for cancer therapy.4, 5 Milk exosomes (mExo) components are found to be highly heterogeneous, with the potential to carry diverse cargoes of proteins, lipids, and nucleic acids (DNA, RNA).6, 7 Readily available and scalable source from mammalian milk, natural bio-compatibility and low immunogenicity.3 mExo have the ability to accumulate in tumour tissues through the expression of specific surface ligands that facilitate tumour homing and cellular internalization via endocytosis, marked by the preferential accumulation of milk exosomes in tumour tissues compared to normal tissues.4, 8 Their stability in the gastrointestinal tract and protection of cargo from degradation caused by digestive enzymes, enable easier and effective oral delivery.3 Human breast milk-derived exosomes have been utilized to deliver gene-modifying agents, such as small interfering RNAs and CRISPR-Cas9 systems, to lung cancer cells. These exosome-encapsulated gene-modifying agents can silence the expression of oncogenes or induce the expression of tumour suppressor genes, thereby inhibiting the proliferation and survival of lung cancer cells by targeting the key oncogenic drivers and signalling pathways critical for cancer progression.8 Human breast milk-derived exosomes have been utilized as a cancer vaccine for breast cancer.9 These exosomes are loaded with tumour-associated antigens, such as HER2, which is overexpressed in a subset of aggressive breast cancers. The exosome-based vaccine elicits a robust anti-tumour immune response by presenting the antigens to immune cells, stimulating the activation of cytotoxic T cells and the production of anti-tumour antibodies.9 Bovine (Bos taurus) milk-derived exosomes have been utilized as a therapeutic approach for delivering anti-cancer drugs, such as doxorubicin, to target breast cancer cells. These exosomes can effectively cross biological barriers and demonstrate inherent biocompatibility, making them an ideal carrier for cytotoxic agents. The exosome-mediated delivery of doxorubicin has been shown to inhibit the proliferation of breast cancer cells by arresting their cell cycle and inducing apoptosis.4, 10 Similarly, Bovine milk-derived exosomes are engineered to carry specific microRNAs, such as miRNA-125b and miRNA-let-7, that can target and downregulate oncogenic pathways in prostate cancer cells. These exosomes serve as an effective delivery vehicle, protecting the microRNAs from degradation and facilitating their uptake by the target cells. The exosome-delivered microRNAs have been shown to target key regulators of prostate cancer progression, including the androgen receptor and the Ras/MAPK signalling pathway.6, 8 Bovine milk-derived exosomes utilized as a therapeutic approach for delivering anti-angiogenic agents, such as endostatin, to target colorectal cancer.4 Bovine milk-derived exosomes inhibit the tumour's blood supply and oxygen/nutrient delivery, the exosome-delivered anti-angiogenic agents effectively starve the cancer cells, thereby impeding the progression and metastasis of colorectal cancer.4 Camelid(dromedaries) milk exosomes can serve as a delivery vehicle for nanoparticle-encapsulated chemotherapeutic agents, such as sorafenib, to effectively target and kill hepatocellular carcinoma cells to inhibit tumour angiogenesis and disrupt growth signalling pathways in hepatocellular carcinoma.4, 3 Camel milk exosomes can efficiently encapsulate and deliver natural compounds such as curcumin to pancreatic cancer cells, enhancing their bioavailability and therapeutic efficacy by targeting the NF-κB and Akt signalling pathways, involved in pancreatic cancer progression.4 Caprine (goat) milk exosomes can be engineered to carry tumour-suppressive microRNAs, such as miRNA-34a, which can induce apoptosis and inhibit proliferation in prostate cancer cells by targeting androgen receptor signalling and cell cycle regulation. Caprine milk exosomes can also be utilized to deliver therapeutic enzymes, such as L-asparaginase, to target asparagine metabolism in osteosarcoma cells, leading to their selective starvation and death.3 Equine (horse) milk exosomes can deliver anti-cancer peptides, such as Lytic Peptide-1, to induce apoptosis and inhibit proliferation in breast cancer cells by targeting cell survival and proliferation signalling pathways.5 Milk exosomes demonstrate promising potential as natural nanoscale delivery vehicles for cancer therapy, althouogh a recent study on bovine milk revealed their dual effect on tumour progression which necessitates cautious investigation (Figure 1).
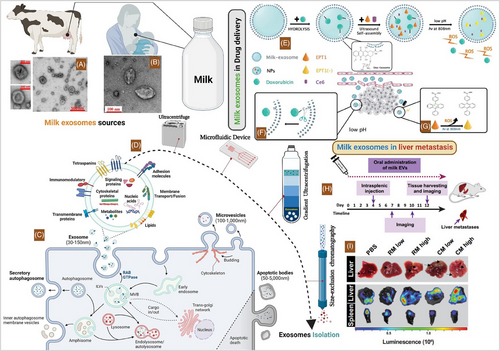
While they may inhibit primary tumour growth, evidence suggests a simultaneous potential for promoting metastasis by inducing epithelial-to-mesenchymal transition (EMT).11 This duality underscores the critical need for comprehensive research to elucidate the underlying mechanisms of action, and optimize isolation and engineering for enhanced stability and targeted drug delivery.4 In conclusion, a comprehensive understanding of their mechanisms of action, particularly their influence on both primary tumour growth and metastasis, is crucial for safe and effective clinical translation. This involves investigating and clarifying the intricate interplay between milk EVs and cancer cells, recognizing that differential cargo composition, cellular context, receptor expression, microenvironmental factors, and cross-talk with signalling pathways, all contribute to the specific effects observed. Milk EVs have dynamic and multifaceted interactions in cancer biology, emphasizing the need for further research to elucidate the precise molecular mechanisms underlying their effects and to explore their therapeutic potential while mitigating potential risks and ultimately determining the safety and efficacy of milk-derived EVs in clinical cancertreatment. Decoding the full potential of milk exosomes, however, requires embracing their complexity- a challenge that, if met, could rewrite the future of precision cancer therapy.
AUTHOR CONTRIBUTIONS
Asmit Das: Original manuscript writing. Swarup Sonar: Scientific illustrationdevelopment. Ketki Kalele: Original manuscript writing. Vetriselvan Subramaniyan: Review and Editing.
ACKNOWLEDGEMENTS
Not applicable.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
There is no funding for this study.
ETHICS STATEMENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.




