Plant-derived exosomes: A new frontier in nano-medicine for cancer and microbial infection therapy
Swastika Maitra and Subham Sarkar contributed equally.
Abstract
Exosomes, small extracellular vesicles secreted by cells, have emerged as pivotal players in cell-to-cell communication. Plant-derived exosomes, in particular, are gaining attention for their potential therapeutic applications in nano-medicine. These vesicles are naturally occurring nanoparticles that carry bioactive molecules such as proteins, lipids, and nucleic acids. Due to their biocompatibility, low toxicity, and ability to traverse biological barriers, plant-derived exosomes present a promising alternative to synthetic nanoparticles for drug delivery, especially in cancer and microbial infection therapy. Exosomes are secreted by almost every cell and are profusely present in all living organisms, making them excellent candidates for a large spectrum of research and applications. This paper describes the highly organized and regulated biosynthesis of exosomes and the prospects of their application in cancer therapy and treatment of microbial infections.
Plant-derived exosomes (PDEs) enter the inside the cells by endocytosis and are eventually released as endosomes from the plasma membrane.1 The budding of exosomes from the plasma membrane takes place in three ways. First, each endosome is transformed into a completely developed multivesicular body (MVB) and the ensuing exosome is released by exocytosis. Second, the exosomes can be directly and squarely released by the vesicles budding from the plasma membrane. Lastly, the budding of exosomes can directly take place from plasma membrane-connected compartments (IPMC) due to dysregulation such as lack of coordination, discipline and synchronisation of one or more physiological events.2 The MVBs are rich in various proteins and lipids. Studies have elucidated compartmentalisation of MVBs, which comprises of many intraluminal vesicles (ILVs).2 The ILVs mature into exosomes that are mainly released outside of the cell by means of the endosomal sorting complex required for transport (ESCRT). The mechanisms by which exosomes are secreted outside of the cell are pathways involving tetraspanins and ESCRT. The proteins of ESCRT such as TSG101, Hrs and STAM1 are indispensable for biosynthesis of exosomes and they bind to ubiquitin to carry out their functions.2 The ILVs become more enriched due to the presence of plenty of ubiquitinated soluble compounds, such as MHC-II in the cells. In released exosomes, besides ubiquitinated MCH-II, non-ubiquitinated MHC-II is also found. Exosome biosynthesis is not always dependent on ESCRT and tetraspanin pathways. Biosynthesis of exosomes mediated by lipids is regulated by GTPase and Rab31 and do not involve ESCRT or tetraspanin.2 However, the most commonly witnessed mechanism of exosome secretion is by the ESCRT pathway, which has a cytoplasmic system made up of multiple subunits that is important for the membrane rearrangement. This enables the budding of the vesicles and sorting of the constituents into MVBs, which depends on ESCRT related proteins such as VPS4, TSG101 and ALIX and four core ESCRT complexes—ESCRT-0, ESCRT-I, ESCRT-II and ESCRT-III. ESCRT plays a crucial role in biosynthesis of exosomes through the development of ILVs.2 Their PLD2 and phosphatidic acid aggregates (PPA) allow the entry of endosomes inside the cell via ESCRT-I complex, which is followed by exosome secretion from those cells. The interaction between PLD2-PPA and MVB12B-MAP (MM) not only aids in secretion of exosomes but also interrupts and impedes the MM-PPA pathway. This results in delayed budding of endosomes which considerably reduces the number of secreted exosomes.2 ALIX aggregates ESCRT-III into late endosomes, in contrast to in vitro transportation of the late endosomes and transmembrane proteins of MVBs as observed in more familiar ESCRT pathway. This enables secretion of transmembrane proteins to the extracellular milieu through the plasma membrane. Interestingly, in this pathway, interaction between ESCRT-III and lysobisphosphatidic acid (LBPA) allows ESCRT-III to avoid the complicated ESCRT pathway.2
The therapeutic advantages of PDEs are manifold. Their inherent non-toxicity and biocompatibility reduce the risk of adverse immune reactions, allowing for repeated administration without significant side effects. The presence of natural bioactive compounds with known anticancer properties enhances their therapeutic efficacy, providing a dual approach of direct cancer cell targeting and modulation of the tumour microenvironment.3 The nano-sized structure of exosomes allows for efficient encapsulation of therapeutic agents and targeted delivery to cancer cells, improving bioavailability and reducing off-target effects.4 Additionally, the production of PDEs is more cost-effective and sustainable compared to synthetic nano-medicines, making them viable for large-scale clinical applications. Utilising plant sources also mitigates ethical issues related to the use of animal or human cells, simplifying regulatory approval processes. (See Figure 1)
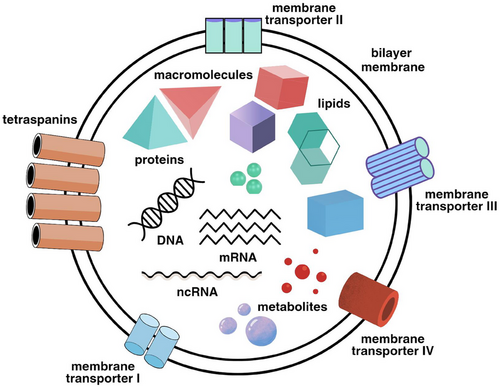
The utilisation of PDEs in the cancer therapy is a burgeoning area of research. These exosomes can be loaded with chemotherapeutic agents, enhancing their delivery to tumour cells while minimising systemic toxicity.5 For instance, exosomes derived from ginger and grape have been shown to carry anti-cancer compounds effectively. Their natural origin ensures that they are less likely to elicit immune responses compared to synthetic carriers.4 Moreover, PDEs can be engineered to target specific cancer cells, providing a means for the precise and personalised treatment.6 This targeting capability is due to the presence of surface molecules on exosomes that can be modified to recognise and bind to cancer-specific markers. These exosomes exhibit multiple mechanisms of action in the cancer therapy, including the inhibition of cell proliferation by down regulating cell-cycle regulatory proteins, induction of apoptosis through intrinsic and extrinsic pathways, suppression of migration and invasion by inhibiting matrix metalloproteinases (MMPs), enhancement of chemo-sensitivity by modulating drug efflux pumps and increasing drug uptake, and providing anti-inflammatory and antioxidant effects to create a tumour microenvironment less conducive to cancer progression.5, 7 The potential of PDEs in the cancer therapy is promising, yet several challenges need to be addressed for successful clinical translation. Standardisation of isolation and characterisation techniques is crucial to ensure consistency and reproducibility. Further research is needed to elucidate the detailed mechanisms of action of PDEs and their interaction with cancer cells. Navigating the regulatory landscape to achieve approval for clinical use will require rigorous testing to ensure safety and efficacy.
In addition to the cancer therapy, PDEs hold promise in combating microbial infections. These exosomes can be engineered to deliver antimicrobial agents directly to the site of infection, enhancing their efficacy while reducing potential side effects.8, 9 For example, exosomes from edible plants like ginger have demonstrated the ability to encapsulate and deliver antimicrobial peptides. This targeted delivery system not only ensures higher concentrations of the therapeutic agents at the infection site but also minimises the risk of developing antibiotic resistance by reducing the need for systemic administration of high-dose antibiotics.
The development and application of PDEs in nano-medicine require further research to overcome existing challenges. These challenges include optimising the isolation and purification processes, understanding the bio distribution and pharmacokinetics of exosomes, and ensuring the scalability of production for clinical applications. Advances in biotechnology and nanotechnology are expected to address these issues, paving the way for the integration of PDEs into mainstream medical treatments. As the research community continues to explore and harness the potential of these natural nanoparticles, plant-derived exosomes may revolutionise the way we approach the treatment of cancer and microbial infections, offering safer and more effective therapeutic options.
AUTHOR CONTRIBUTIONS
Swastika Maitra, Subham Sarkar, and Bikram Dhara equally contributed in preparing this paper.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
FUNDING INFORMATION
The authors did not receive any funding for this study.
ETHICS STATEMENT
Ethical approval is not appliacable for this article as no human or animal data represented in the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this paper as no new data were created in this study.




