Signature of click chemistry in exosome modification for cancer therapeutic
Abstract
Exosomes, small extracellular vesicles secreted by cells, have gained attention as potential therapeutic agents due to their natural ability to deliver biomolecules and traverse biological barriers. However, their limited targeting specificity and payload capacity necessitate modifications for improved therapeutic efficacy. Click chemistry, known for its high specificity, efficiency, and mild reaction conditions, offers an innovative solution for modifying exosomal surfaces. This technique enables precise attachment of targeting ligands, imaging agents, and therapeutic molecules, enhancing the targeting, delivery, and overall effectiveness of exosome-based therapies. By addressing cancer heterogeneity, click chemistry-modified exosomes can target diverse cancer cell populations within tumors, improving treatment specificity and reducing drug resistance. The development of copper-free click chemistry, such as strain-promoted azide-alkyne cycloaddition (SPAAC), minimizes toxicity, ensuring biocompatibility and safety. As research progresses, this approach holds great promise for personalized and effective cancer treatment, paving the way for next-generation therapeutics and diagnostics.
Cancer remains the second most communal cause of death worldwide, with millions of new cases being identified each year. In 2023, a total of 1.9 million fresh cancer cases (approximately 5370 cases each day) and 609 820 deaths from cancer are predicted to occur (about 1670 deaths per day) in the United States. Despite an overall decline in cancer mortality rates since 1991, certain cancers, such as breast, prostate, and uterine corpus, are experiencing rising incidence rates, which could potentially impact future progress.1, 2 The high incidence of cancer underscores the need for innovative therapeutic strategies. One promising approach involves using extracellular vesicles (EVs), particularly exosomes, in the tumour microenvironment (TME).3 Exosomes facilitate communication between cancer cells and their surroundings, carrying bioactive molecules like proteins, lipids, and nucleic acids that can affect tumour growth, metastasis, and immune responses.4, 19 By modulating the TME, exosomes can either promote or inhibit cancer progression, presenting both challenges and opportunities for cancer therapy.5, 19 Due to their natural origin and biocompatibility, exosomes are increasingly recognized for their therapeutic potential in cancer. They can deliver various therapeutic agents, such as drugs, RNA, and proteins, directly to cancer cells, enhancing efficacy and reducing treatment side effects. Additionally, their ability to cross biological barriers, such as the blood-brain barrier, further enhances their potential for drug delivery.6, 7 However, unmodified exosomes may have limited targeting ability and therapeutic payload capacity, necessitating the development of modified exosomes for more effective cancer treatment. Click chemistry has revolutionized chemical biology with its high efficiency, specificity, and mild reaction conditions. One promising application of click chemistry is modifying exosomal surfaces. Click chemistry offers a precise and versatile method for these modifications, enabling the attachment of various functional molecules to the exosomal surface.8 The copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) is the most commonly used click reaction for exosomal modification.9 This reaction forms a stable triazole ring by reacting an azide with an alkyne in the presence of a copper(I) catalyst. The reaction proceeds rapidly and quantitatively under mild conditions, making it ideal for biological applications. For exosomal surface modification, exosomes are typically first functionalized with either azide or alkyne groups, achieved through methods such as incorporating azide- or alkyne-functionalized lipids into the exosomal membrane or chemically modifying surface proteins with these groups. Once functionalized, exosomes can be conjugated with various molecules, including targeting ligands, imaging agents, and therapeutic payloads, via the CuAAC reaction.9, 10 The use of click chemistry for exosomal modification has several advantages over traditional chemical modification methods. One of the primary benefits is the high specificity and efficiency of the click reaction, which ensures that the modification occurs only at the desired sites without affecting other functional groups on the exosome surface.10, 11 This high specificity reduces the risk of unwanted side reactions and preserves the biological activity of the exosomes. Additionally, the mild reaction conditions of click chemistry, such as room temperature and aqueous environments, help maintain the structural framework and functionality of the exosomes, which is vital for their therapeutic applications. Functionalizing exosomes with targeting ligands through click chemistry significantly enhances their therapeutic efficacy. By attaching ligands that specifically bind to receptors on target cells, modified exosomes can be directed to specific tissues or cell types. This targeted delivery increases the concentration of therapeutic agents at the desired site, thereby improving their therapeutic effects and reducing off-target effects. For example, exosomes functionalized with folic acid, which binds to folate receptors overexpressed on certain cancer cells, have shown improved targeting and uptake by these cells.12
This targeted delivery approach is particularly beneficial in cancer therapy, where selective aiming of tumour cells while sparing healthy tissues is critical.8, 13, 14, 18 In addition to targeting ligands, click chemistry can be used to conjugate imaging agents to exosomes, enabling their use as diagnostic tools.15 By attaching fluorophores, radioisotopes, or magnetic nanoparticles to the exosomal surface, researchers can track the biodistribution and uptake of exosomes in vivo using various imaging modalities. This capability is invaluable for evaluating the pharmacokinetics and therapeutic efficacy of exosome-based therapies. Furthermore, imaging-modified exosomes can serve as non-invasive diagnostic agents for detecting and monitoring diseases.15, 16 Exosomes labelled with fluorescent dyes can be used for real-time imaging of tumour progression and response to therapy. The therapeutic applications of click chemistry-modified exosomes extend beyond targeted drug delivery and diagnostics.17, 18 These modified exosomes can also be used for gene therapy by loading them with nucleic acids, such as siRNA or miRNA. Click chemistry enables the stable and efficient conjugation of nucleic acids to exosomes, protecting them from degradation and ensuring their delivery to target cells.20 Once inside the target cells, the nucleic acids can modulate gene expression, providing a therapeutic effect. This approach has shown promise in treating various genetic disorders and diseases, including cancer, by silencing oncogenes or restoring the function of tumour suppressor genes. Unmodified exosomes may not efficiently target specific cells or tissues, leading to suboptimal therapeutic outcomes therefore, by modifying the surface of exosomes with targeting ligands, therapeutic agents, or imaging molecules, their specificity, efficacy, and safety can be significantly improved.21 Click chemistry offers a versatile and efficient method for achieving these modifications, allowing for the precise and controlled conjugation of a wide range of functional molecules. This enhances the ability of exosomes to deliver therapeutic agents directly to cancer cells, improving treatment outcomes and reducing side effects. Unmodified exosomes are already in the pipeline of clinical trials22 but it's also essential for translating the potential of click chemistry-modified exosomes into clinical practice. Several clinical trials are currently underway to evaluate the safety and efficacy of normal exosome-based therapies in cancer treatment22 (Table 1). These trials are exploring various aspects, including the use of modified exosomes for drug delivery, gene therapy and immune modulation. Early results from these trials are promising, demonstrating the potential of modified exosomes to improve cancer treatment outcomes (Figure 1).
| Aspect | Normal exosomes | Click chemistry-based surface-modified exosomes |
|---|---|---|
| Definition | Natural extracellular vesicles secreted by cells | Exosomes chemically modified using click chemistry |
| Size | 30–150 nm | 30–150 nm (size maintained post-modification) |
| Origin | Numerous cell types, cancer cells and immune cells | Same as normal exosomes |
| Composition | Proteins, lipids, RNAs, DNA and metabolites | Proteins, lipids, RNAs, DNA, metabolites and click-conjugated molecules |
| Isolation methods | Ultracentrifugation, size exclusion chromatography and immunoaffinity capture | The same methods as normal exosomes |
| Purity | Can contain contaminants such as other EVs and protein aggregates | Enhanced with click chemistry purification |
| Natural function | Intercellular communication, transfer of bioactive molecules | Enhanced delivery of therapeutic agents and targeted delivery |
| Biocompatibility | High | High (retained post-modification) |
| Immunogenicity | Low to moderate | Potentially lower with targeted modifications |
| Capability to cross biological barriers | Yes (e.g. BBB) | Yes (enhanced targeting) |
| Circulation time | Short to moderate | Prolonged with PEGylation or other surface modifications |
| Targeting specificity | Limited | Enhanced with targeting ligands (e.g. folic acid and antibodies) |
| Therapeutic payload capacity | Limited (natural loading mechanisms) | Increased due to chemical modifications (higher loading efficiency) |
| Stability of Cargo | Moderate (cargo may degrade) | High (due to protective chemical conjugation) |
| Surface modification method | None (natural composition) | Copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) and strain-promoted azide-alkyne cycloaddition (SPAAC) |
| Attachment of therapeutic molecules | Limited (endogenous loading) | Efficient and stable via click chemistry (e.g. drugs and RNA) |
| Attachment of imaging agents | Limited | Enhanced for diagnostic applications (e.g. fluorophores and radioisotopes) |
| Use in drug delivery | Yes, but less efficient | Highly efficient targeted drug delivery |
| Use in gene therapy | Possible, but with limitations | Enhanced gene delivery with stable nucleic acid conjugation (e.g. siRNA and mRNA) |
| Immune modulation | Natural immune signalling | Enhanced with immune modulators (e.g. cytokines) |
| Role in tumour microenviornment (TME) | Natural modulation | Tunable for specific effects on TME (e.g. promoting anti-tumor immunity) |
| In-vivo tracking and Imaging | Limited capability | Advanced tracking with conjugated imaging agents (e.g. MRI and PET) |
| Potential for off-target effects | Higher due to non-specific distribution | Reduced with targeted modifications (lower systemic toxicity) |
| Scalability for therapeutic use | Moderate (challenges in large-scale production) | High potential with standardized click chemistry protocols |
| Current clinical trials | Limited (mostly exploratory) | Several ongoing trials for cancer therapy and diagnostics |
| Overall therapeutic efficacy | Moderate | Significantly enhanced (targeted and efficient delivery) |
| Safety profile | High (natural biocompatibility) | High, but dependent on click chemistry conditions (e.g. copper toxicity) |
| Challenges | Limited targeting, lower therapeutic payload and potential contaminants | Copper toxicity, scalability, maintaining exosome integrity and regulatory approval |
| Future directions | Exploring natural exosome functions, enhancing isolation and purity | Development of biocompatible click reactions, novel ligands and disabling regulatory challenges |
- Abbreviations: BBB, blood brain barrier; CuAAC, copper(I)-catalyzed azide-alkyne cycloaddition; EVs, extracellular vesicles; SPAAC, Strain-promoted azide-alkyne cycloaddition; TME, tumor microenvironment.
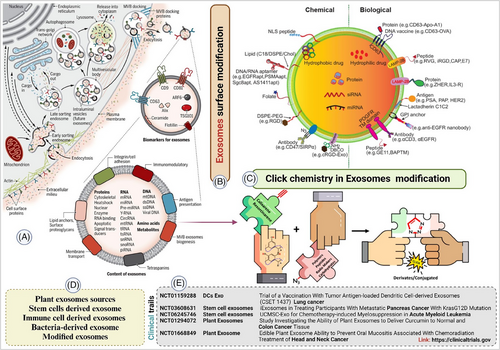
Click chemistry is a powerful technique for modifying exosomes to enhance their therapeutic potential. Due to its high specificity, efficiency, and mild reaction conditions, it is ideal for attaching targeting ligands, imaging agents, and therapeutic molecules to exosomes. These modifications greatly improve the targeting, delivery, and effectiveness of exosome-based therapies, opening up new possibilities for cancer treatment. Cancer heterogeneity, which involves diverse cell populations, complicates treatment and contributes to drug resistance.23, 24 Exosomes, naturally proficient at delivering therapeutic agents, show promise in tackling this complexity. Click chemistry allows for precise modifications, enabling exosomes to target different cancer cell subtypes, thus enhancing treatment specificity and efficacy. Modified exosomes can carry a range of therapeutic agents, offering a comprehensive approach to treating heterogeneous tumours and reducing resistance. The advent of copper-free click chemistry, such as strain-promoted azide-alkyne cycloaddition, minimizes toxicity and ensures biocompatibility and safety.25 This approach is highly promising for personalized and effective cancer treatment. As research and clinical trials advance, exosomes modified through click chemistry are expected to play an increasingly important role in next-generation cancer therapeutics and diagnostics. By overcoming challenges related to targeting specificity, therapeutic payload capacity, and toxicity, these innovative exosomes hold great promise for personalized and effective cancer treatments. This strategy has the potential to significantly improve treatment outcomes, providing hope for better management of this complex and devastating disease.
AUTHOR CONTRIBUTIONS
Nobendu Mukerjee: Conceptualization; original draft writing; illustrations. Swarup Sonar: Review and editing.
ACKNOWLEDGEMENTS
Not applicable
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
There is no funding for this study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.




