Clinical trial status of exosomes-based cancer theranostics
Abstract
Exosomes, are a subpopulation of extracellular vesicles, that originate from endosomes. The major role of exosomes is cellular communication (in this process exosomes display the status of the parental cell's nature, healthy or the cell suffers any pathological complication). In recent decades, research evidence highlighted that exosomes are the masterminds of cancer development and they appear as smart solutions for early diagnosis, prognosis and therapeutic (eg. stem cell, plant and immune cell-derived exosomes) approaches. Exosomes transform the cancer liquid biopsy into a new orientation. Several biofluids (blood, plasma, serum, saliva, urine, CSF (Cerebrospinal Fluid) and cancer tissue are used for exosome-based cancer biomarkers detection. Liquid biopsy becomes more efficient for exosomes, compared to tissue biopsy. Exosomes biocompatibility, low toxicity, and ability to cross biological membranes make it a potential tool for cancer therapeutic development. Exosome-based cancer therapeutics introduce a cutting-edge era of cell-free cancer therapy. This article explores the critical role of exosomes in cancer development, progression, treatment, and clinical trials. Exosome-based clinical trials indicate that we are close to cancer precision medicine.
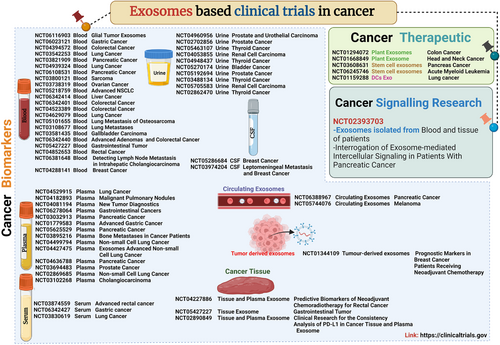
Cancer is a major health complication that results in death globally and is the second most highlighted cause of death due to late diagnosis, poor prognosis accuracy and high possibility of metastasis.1 The challenge of cancer treatment is compounded by therapeutic and drug resistance, making it a progressively difficult disease to manage.2 Annually, a significant portion of the global population is affected by cancer, highlighting the urgent need for improved medical strategies. Recent research has, revealed the impact of exosomes (a subpopulation of extracellular vesicles (EVs) size between 30 and 200 nm) in cancer development and progression that leads to metastasis. It become a next-generation cancer thanostics.3 The biogenesis of exosomes involve endosomes maturation in multi-vesicular bodies (MVBs), which carry several intraluminal vesicles. These exosomes are released into the extracellular space when the MVBs fuse with the plasma membrane.4 Exosomes serve as vital messengers in cancer, facilitating communication between cancer cells and their surrounding environment. They transport bioactive molecules such as proteins, DNA, RNAs, and lipids that influence tumour progression by reprogramming nearby cells and promote metastasis. This interplay between exosomes and cancer cells highlights their role in shaping the tumour microenvironment (TME), promoting angiogenesis, modulating immune responses, and enhancing metastatic potential.3 Tumor-derived exosomes (TEXs) are the key factor for cellular reprogramming in TME for tumor growth. TEXs mediated tmour growth factor-beta protein stimulates mesenchymal stem cells to differentiate into myofibroblasts, promoting cancer proliferation and invasiveness in prostate cancer.5 In pancreatic cancer, Snail protein and microRNA (miRNA)-146 increase cancer cell proliferation and resistance to drugs, enhancing survival capabilities.6 In breast cancer, miRNA-9 upregulates cancer-associated fibroblast-like properties, supporting cancer growth. Moreover, miRNA-105 reduces tight junction protein ZO-1 levels, compromising vascular endothelial barriers and facilitating metastasis in breast cancer. These examples highlight how exosomes bioactive molecules contribute to the complexity of cancer progression by influencing cellular behaviours and interactions within the tumour microenvironment.3 Tumour-derived exosomes play a pivotal role in drug resistance development.7 On the other hand, it has significant importance in cancer diagnostic biomarkers investigation. Various sources of exosomes are utilized for biomarker research including blood, plasma, serum, saliva, urine, etc.8 Exosomes isolated from various cancers reveal distinct biomarkers that reflect the molecular characteristics of their originating tumours. CD63-positive exosomes from breast cancer are enriched with miRNA-21, miRNA-1246 and HER2. CD81-positive exosomes in lung cancer carry EGFRvIII, epidermal growth factor receptor (EGFR) L858R and EGFR T790M variants. EpCAM-positive exosomes from pancreatic cancer are marked by KRAS mutations. Hsp70-positive exosomes in colorectal cancer carry CD44v6, CD133 and EpCAM. Annexin V-positive exosomes in prostate cancer contain PSA and PSMA. Glypican-1-positive exosomes in melanoma express S100B and MIA. These diverse biomarkers indicate that potential of exosomes as valuable sources of diagnostic and prognostic biomarkers of multiple cancers .9 Recent advances in cancer biomarker research have identified several miRNAs in various body fluids that are associated with metastasis and prognosis across different cancer types. In breast cancer, miRNA-222 is highly expressed in plasma and is interlinked with lymphatic metastasis, suggesting its potential as a diagnostic and prognostic biomarker. For lung cancer, plasma levels of miRNA-451 have been found to participate in lymph node metastasis, highlighting its role in the metastatic process. In colon cancer, miRNA-203 is highly expressed in serum and is associated with metastasis, including liver metastasis in in-vivo models, indicating its relevance in disease progression. Prostate cancer studies reveal that miRNA-1290 and miRNA-375 are highly expressed in plasma and are related to castration-resistant prostate cancer and poor overall survival, marking them as critical biomarkers for aggressive disease identification. Additionally, miRNA-1262 in serum has been identified as an efficient prognostic biomarker for liver cancer, providing valuable insights into patient outcomes. These discoveries underscore the importance of miRNAs in cancer diagnosis, prognosis, and the understanding of metastatic mechanisms.10 Moreover, exosomes, known for their stability and compatibility with biological systems, can be customized chemically or biologically to improve the delivery and effectiveness of chemotherapy drugs while minimizing toxicity. Studies have indicated that exosomes can specifically transport doxorubicin to tumour sites via intravenous injection, reducing tumour growth with minimal side effects. Exosomes can effectively deliver let-7a miRNA to breast cancer cells expressing EGFR in animal models. Genetically altered exosomes have also been reducing tumour growth via the transport of therapeutic RNA and proteins. Additionally, research involving zebrafish has shown that exosomes can enhance drug delivery to the brain, highlighting their potential as carriers for anticancer drugs targeting brain metastases.11 Modified exosomes are more effective compared to cell therapy in cancer.12 Resent time several methods have been used for exosomes functionalization such as click chemistry,12 surface modification,14 and development of chimeric exosomes15 make a smart platform for future exosomes-based cancer medicine. Worldwide clinical (Figure 1) trials based on exosomes are classified into three sections biomarkers, therapeutics and signalling research. In clinical trials a huge number focus on early diagnosis and prognostic biomarkers of cancer (biomarker based clinical trials used human biofluids such as blood-this term used in the clinical trials, where the trial does not reveal the source of exosomes, plasma, serum, urine, circulating extracellular vesicles, tumour-derived exosomes, and cancer tissue). In therapeutic clinical trials plants, stem cells, and dendritic cells-derived exosomes get the priority. Hope, in the future exosome-based clinical trials, develop a better solution for cancer.
ACKNOWLEDGEMENTS
Not applicable
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
FUNDING INFORMATION
There is no funding for this study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.




