The genetic communication and interaction between chronic kidney diseases and frailty
Xiaojun Wang, Xuchao Gu and Yuxin Zhang contributed equally to this work.
[Correction added on 06 December 2023; after first online publication missing sub-sections have been added.]
Abstract
The prevalence of chronic kidney disease (CKD) is increasing due to the global ageing trend. Despite significant advancements in understanding the mechanisms of CKD, little research has focused on the prevention and treatment of associated complications such as frailty. In this review, we provide a summary of the current Genome-Wide Association Studies (GWAS) of kidney diseases based on the unique genetic characteristics of kidneys and a review of the impact of metabolites associated with inflammageing on frailty and CKD. Additionally, the role of circadian rhythm in the intrinsic relationship between kidney and skeletal muscle has also been explored. This article offers insights into the potential communications and interactions between CKD and frailty to facilitate the current clinical treatments and improve the life quality for frail elderly individuals in coexistence of CKD.
1 INTRODUCTION
Frailty serves as a valid phenotype for assessing the extent of ageing in the human body. It encompasses a range of issues including decreased physical function, cognitive dysfunction, psychological and social domain deficits.1 Frailty is commonly associated with a decline in the reserve and function of various physiological systems, and exposure to frailty heightens the adverse health outcomes such as higher mortality rates and reduced life expectancy.2 The primary indicator of frailty is the decrease in physical function. Fried and his colleagues identified five characteristics to describe this indicator: low grip strength, reduced walking speed, self-reported fatigue, low physical activity and unintentional weight loss.3 According to recent epidemiological surveys, China has emerged as one of the countries with the highest number of frail individuals in the world. The prevalence of frailty among elderly individuals aged 60 and above in China ranges from 7.7% to 9.9%.4, 5
Studies have demonstrated a significant correlation between chronic kidney disease (CKD) and frailty.6 As kidney function deteriorates, various pathological processes such as inflammation, oxidative stress, mitochondrial dysfunction and insulin resistance occur.7 These processes contribute to a gradual depletion of protein and energy reserves in the human body, ultimately resulting in a continuous decline in skeletal muscle mass and physical performance.8 Multi-omics technology has provided a reliable approach to gain a comprehensive understanding of the connection between CKD and skeletal muscles. Genomics and epigenome have been instrumental in evaluating the genetic influence of CKD on skeletal muscle,9 while the data obtained through metabolomics are directly related to kidney function and skeletal muscle parameters of patients.10 Currently, numerous genes and metabolites identified through omics technology have been recognised as significant players in the development of CKD. Notably, many of these genes and metabolites overlap with the pathological mechanisms involved in frailty.11
This review will begin by discussing the foundation of kidney heritability. It will then summarise the genetic characteristics and inflammageing-related biochemical markers of CKD, and investigate the biological connections between CKD and the progression of frailty. In addition, this review also provides a summary of the circadian rhythm mechanism in the kidney and skeletal muscles, and explore the potential role of clock genes in facilitating communication between these two systems.
2 THE THEORY OF THE KIDNEY AS THE FOUNDATION OF HERITABILITY WITH GENETIC METHOD
Kidney is the foundation of heritability, as noted in Li Zhongzi's 'Required Readings for Medical Professionals' from the Ming Dynasty, which highlights the strong heritability of kidney's physiological functions, underscoring its essential role in fostering an individual's growth and development. However, this heritability is also suggested to be linked to the likelihood of frailty throughout one's lifetime.12 At present, both Genome-Wide Association Studies (GWAS) and DNA methylation analysis have successfully identified over 800 loci13 and around 100 genetic genes14 that are related to kidney function. These loci and genes are involved in all aspects of kidney physiology and pathology, such as morphological development, transcriptional regulation, cell signal transduction, metabolism and solute transport.15 Mutations and abnormal expression of these loci and genes can lead to a decline in individual kidney function, and accelerate the occurrence of CKD and frailty.
CKDGen Alliance Data website (https://ckdgen.imbi.uni-freiburg.de) specifies four main traits related to kidney function: estimated glomerular filtration rate (eGFR), urinary albumin/creatinine ratio (UACR), blood urea nitrogen (BUN) and serum urate acid (SUA). Genetic characteristics vary among these traits,16 and eGFR is considered the best overall indicator of kidney function, with heritability ranging from 36% to 75% in different studies.17 Meanwhile, previous research has consistently demonstrated the association between eGFR and frailty.18-20 Table 1 displays the existing representative genomic studies regarding the inheritance of kidney function, with a focus on eGFR. Upon reviewing these studies, as depicted in Figure 1, we discovered that the genetic genes associated with kidney function are primarily located in the glomerulus, renal vesicles and proximal tubules. Only a limited number of genes associated with kidney function are in the distal tubules,21 such as TRPM6, PCBD1, HNF1B, CLCNKB and FYXD2. The proximal renal tubules contain a large number of genetic genes, which play a crucial role in maintaining kidney function. Currently, there is indirect evidence suggesting a link between these genes and frailty. This link is established by the alteration of expression levels of urine biomarkers of kidney tubular injury. For instance, mutations in PKD2 have been found to increase levels of kidney injury molecule 1 (KIM-1) and monocyte chemoattractant protein 1 (MCP-1) in urine, which have been significantly correlated with frailty index in middle-aged and elderly populations.22, 23 Additionally, recent research has revealed that the endoplasmic reticulum and endolysosomes in proximal renal tubules have a significant impact on determining overall kidney function. Qiu et al. developed an expression quantitative trait loci (eQTL) atlas for the glomerular and tubular compartments of the human kidney. They found that proximal tubular pathogenic genes, which are associated with CKD, are significantly enriched in endolysosomal functions. Subsequent investigation identified DAB2 as a representative gene of these pathogenic genes,24 and Dana et al. confirmed that DAB2 could inhibit protein synthesis in muscles by limiting P13K-Akt-mTOR signalling.25 Lee et al. reported that MARCHF1, a regulator of lipid metabolism and glucose tolerance, could potentially contribute to the progression of CKD by causing oxidative stress in the proximal tubular endoplasmic reticulum.26, 27
| Year | Related genes or SNP | PMID |
|---|---|---|
| 2009 | CACNA1S, CPS1, EDEM3, KLHDC7A, RPL3L, 5LC25A45, SLC47A1, PPM1J, CERS2, C9, etc. | 31152163 |
| 2010 | NRIP1, TPRKB, PKD1, NOS3, APOL1, UMOD, GATM/SPATA5L1, SHROOM3, CPS1, PRKAG2, etc. | 31451708 |
| 2010 | EDEM3, RPL3L, SLC25A45, CACNA1S, HOXD11, CERS2, PKHD1, SPEG, GAB1, PDE7A, etc. | 34272381 |
| 2011 | CASZ1, PPARGC1A, ZNF641, MED4-AS1, ZFHX3, ZGPAT, MAFF, ACVR2B, DCDC2, GRB10, etc. | 31015462 |
| 2012 | TPPP, FAT1-LINC02374 | 36720675 |
| 2012 | rs3128852, rs117744700, rs28366355 | 36627639 |
| 2013 | MKL1, DLG1 | 36223920 |
| 2013 | UMOD, PDILT, PRKAG2 | 36271454 |
| 2013 | MARCHF1 | 36422279 |
| 2013 | APOL1 | 35710995 |
| 2013 | ERBB4 | 35227251 |
| 2014 | ACY1, ACY3, NAT8 | 33838163 |
| 2015 | GATM, HBB | 33783510 |
| 2016 | FANCM | 34067580 |
| 2016 | MANBA | 33441424 |
| 2017 | ACR, CUBN | 35246685 |
| 2018 | COL4A3, BMP7, COLEC11, DDR1 | 31537649 |
| 2018 | GNG7 | 31092297 |
| 2018 | NAT8B, CASP9, MUC1 | 30467309 |
| 2018 | ACADM, SLC7A9 | 29545352 |
| 2018 | PLEKHN1, NADK, RAD51AP2, RREB1, PEX6, GRM8, PRX, APOL1, OTUD7B, IFITM3, etc. | 29989002 |
| 2018 | BCAS3, C17orf82, ALDH2, BCAS3, LRP2, PKD2, SLC2A9, OTUB1, SLC22A12, CDC42BPG, etc. | 29558500 |
| 2019 | BCAS3 | 29779033 |
| 2019 | LINC00923 | 27729571 |
| 2019 | SYPL2, SDCCAG8, LRP2, WNT7A, NFKB1, ZNF204, UNCX, KBTBD2, RNF32, AP5B1, etc. | 26831199 |
| 2019 | COL4A1, ATP2B1, ABCA4 | 23727086 |
| 2019 | PRKAG2, SLC6A13, UBE2Q2, PIP5K1B, WDR72 | 24311711 |
| 2019 | FRMD3, GATM, SPATA5L1 | 23254893 |
| 2021 | HMGA2 | 23111731 |
| 2021 | PTGDS, BTP | 23328707 |
| 2021 | ALPK1, FAM78B, UMODL1 | 23539754 |
| 2021 | LASS2, GCKR, ALMS1, TFDP2, DAB2, SLC34A1, VEGFA, PRKAG2, PIP5K1B, ATXN2, etc. | 20383146 |
| 2021 | RPS12, LIMK2, SFI1 | 21150874 |
| 2021 | UMOD, SHOOM3, GATM-SPATA5L1, CST, STC1 | 19430482 |
| 2022 | KRT40, WDR72, UMOD, PDILT | 35228297 |
| 2022 | UMOD, SPATA7, GALNTL5, TPPP | 35716955 |
| 2022 | TENM2, COL20A1, DCLK1, EIF4E, PTPRN-RESP18, GPR158, INIP-SNX30, LSM14A, MFF | 35763030 |
| 2022 | UMOD, PDILT, TPPP, MED1, NEUROD2, CSRNP1, DCDC5, NRIP1, SLC22A2 | 35697829 |
| 2022 | PYROXD2, PHYHD1, FADS1-3, ACOT2, MYRF, FAAH, LIPC | 35120996 |
| 2022 | UMOD, PDILT, PRKAG2, WDR72, OR2S2, GATM, LARP4B | 33137338 |
| 2022 | APOL1 | 29885931 |
| 2022 | PMF1, NT5C1B, C2orf73 ORC4, NFE2L2, XYLB, AK125311, SHH, NRG1, etc. | 30604766 |
| 2022 | CD2AP, MMP2, T2D-ESKD, APOL1, TTC21B, COL4A3, NPHP3-ACAD11, CLDN8, ARHGAP24 | 27461219 |
| 2022 | GALNT11, CDH23 | 25493955 |
| 2022 | LRP2, TSC1, PALB2, ROBO2 | 24029420 |
| 2023 | INHBC, LRP2, PLEKHA1, SLC3A2, SLC7A6, PLEKHA1, FBXL20 | 22962313 |
| 2023 | MECOM, UNCX, MPPED2, DCDC5, WDR72, ALDH2, C12orf51, BCAS3 | 22797727 |
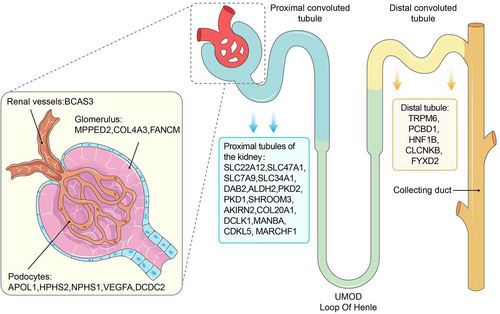
Hereditary CKD refers to CKD that is caused by genetic factors. With the continuous advancement of genetic sequencing, candidate genes for hereditary CKD have been steadily discovered, thus expanding the scope of this disease. Numerous studies have supported the familial and ethnic clustering characteristics of hereditary CKD.28 Epigenetic modifications have a strong impact on CKD susceptibility, primarily through inflammation, oxidative stress, uremia and hyperhomocysteinemia. Webster et al. reported that epigenetic modifications have a greater impact on children, increasing their susceptibility to CKD in the future compared to adults.29 Graham et al. indicated that mutations in DCDC2 gene are associated with hereditary CKD.15 The overexpression of DCDC2 has been found to affect the formation and development of nephrons and ureters via Wnt signals.16 UMOD is the most prominent gene associated with hereditary CKD. Li et al. reported that UMOD is highly heritable and encodes urinary regulatory protein (Tamm Horsfall protein) in the Henle ring region.30 The dysregulation of UMOD expression can result in infiltration of peritubal leukocyte in the kidneys and consequently cause kidney inflammation, which has a strong correlation with the development of frailty.31
Diabetic kidney disease (DKD) and hypertensive kidney disease (HKD) are prevalent forms of CKD, which are linked to the heredity of the kidneys and the decline in kidney function. Many clinical studies revealed that both DKD and HKD are correlated with an elevated risk of frailty.32, 33 Kakio et al. discovered that end-stage renal disease patients with DKD had weaker muscle strength and physical function, as well as higher frailty phenotype scores compared to those without DKD.34 Lee et al. conducted a research on Japanese CKD patients and found that hypertension can significantly increase the risk of frailty in this population.35 DKD is a disease that exhibits clear familial aggregation characteristics. According to a longitudinal cohort study, individuals with DKD in their immediate family are at twice the risk of developing DKD compared to those without DKD in their immediate family.36 Sandholm et al. discovered that the heritability of DKD is approximately 35% through extensive genomics and epigenetics studies.37 Identifying stable and reproducible genetic genes associated with DKD susceptibility is challenging due to the complexity of its pathological mechanism.38 Nonetheless, recent studies suggest that COL4A3, a gene highly expressed in the glomerular basement membrane, may be a stable genetic gene for DKD.39 Mutations in COL4A3 are able to cause thickening of the glomerular basement membrane, renal damage and impaired kidney function.40 Knocking out COL4A3 can also lead to hypertension and muscle mitochondrial dysfunction, all of which are risk factors of frailty.41 Sandholm et al. found that AKIRIN2 is linked to severe DKD. This gene is primarily expressed in proximal tubules, which triggers inflammation and the development of renal fibrosis, and ultimately lead to a decline in kidney function and an increase in frailty risk.42 There is a potential genetic connection between hypertension and susceptibility to kidney disease. Zhang et al. found that variations in UMOD, APOL1, SHROOM3 and DAB2 were linked to both hypertension and decreased renal function.43 SHROOM3 is also proved to play a role in muscle function signal transduction through its regulation of serum magnesium levels.44 However, there is a limited amount of genomics research directly related to HKD. Kim et al. conducted a large GWAS study of over 20 000 Korean men, and identified FANCM as the genetic gene associated with HKD. This research found that FANCM variation predominantly occurred in the glomerulus, tibial artery and aorta, and was strongly correlated with eGFR and creatinine ratio.45
Despite the considerable strides made in genetic research on kidney function, there remains numerous difficulties in translating these findings into clinical practice for preventing or treating CKD and frailty. It is important to acknowledge that genetic diversity can vary based on race and ethnicity, which can impact the efficacy of drugs across different groups. As such, it is crucial to pay close attention to the 'cross-racial' and 'non-cross-racial' characteristics of genetic genes related to kidney function. Khan et al. conducted a comprehensive GWAS across four lineages and 15 independent cohorts. Their findings highlighted the significant role of mutations in APOL1 in predicting cross-racial inherited CKD, particularly in individuals of African descent.46 The origin of APOL1 mutations can be traced back to the historical struggle between Africans and the Trypanosoma parasite. This mutation enables the human body to effectively combat the virulence of Trypanosoma. As a result, the survival rate of humans significantly increases. APOL1 gene mutation is therefore prevalent in humans and is distributed worldwide.47 However, this mutation poses a significant risk for individuals to suffer from various forms of kidney disease and frailty. What is more, according to Hellwege et al.,48 LDB2 and PRK1 are transracial genetic genes that are linked to decreased kidney function in both non-Hispanic Blacks and non-Hispanic Whites, and LDB2 has been found to be correlated with skeletal muscle mass.49
3 INFLAMMAGEING-RELATED METABOLITES ARE RISK FACTORS OF CKD AND FRAILTY
Inflammageing is a recognised indicator of frailty on a global scale. Frailty is an age-related clinical syndrome characterised by reduced physiological reserves, loss of function and reduced resilience across multiple systems and organs, which increases the vulnerability to disability and death.50 Current research suggests that high levels of inflammatory markers in the blood are closely associated with the key aspects of frailty such as loss of skeletal muscle mass and strength, and poor lower extremity performance.51, 52 Inflammation can also accelerate the ageing process of comorbidities and play a role in mediating the connection between comorbidities and disability.53 And also, several chronic inflammatory diseases, such as CKD54 and congestive heart failure,55 frequently result in clinical symptoms that are characterised by frailty, including weight loss, physical decline and fatigue. Observational studies have indicated that reducing inflammation can help prevent and alleviate frailty. Ntanasi et al. have conducted a study on Greek elderly individuals and found that a higher compliance with the Mediterranean diet is related to a lower risk of frailty.56 This association may be attributed to the anti-inflammatory feature of this diet.57 Similarly, Landi and his colleagues have investigated the relationship between the use of non-steroidal anti-inflammatory drugs (NSAIDs) and the skeletal muscle mass and function among Italian elderly individuals, and revealed that users of NSAIDs have a significantly lower risk of frailty and sarcopenia compared to non-users.58
Recent studies indicate that inflammageing is highly susceptible to genetic influences. The accumulation of genetic effects can increase the concentration of circulating inflammatory markers, ultimately impacting the risk of frailty and kidney diseases. Moreover, the deterioration of kidney function is a known risk factor for frailty. Reducing inflammation can lead to significant improvements in enhancing the physiological reserves of kidney and avoid the occurrence of frailty.59 Metabolomics is a valuable tool for monitoring metabolic changes caused by in vivo and in vitro triggers. This allows for better understanding of complex diseases and identification of relevant biochemical characteristics.60 At present, metabolomics has become a common method for studying kidney diseases. Kalim et al. summarised representative metabonomic studies in kidney disease research.61 In this section, we briefly explored the metabolites associated with inflammageing and frailty in CKD and their pathogenesis (Figure 2). These metabolites include tryptophan, taurine, carnitine and sphingolipids.

Metabonomic studies have revealed that tryptophan metabolites are often elevated in patients with CKD, regardless of race. For instance, the Cooperative Health Research in the Region of Augsburg S4/F4 (KORA) Study and the Framingham Heart Study (FHS) demonstrated a significant correlation between baseline tryptophan levels and the development of future CKD.62-64 According to Mohib et al.,65 the reduction of tryptophan and the excessive production of downstream metabolites are significant indicators of kidney injury and frailty. Tryptophan is an aromatic amino acid that plays a crucial role in protein synthesis and mitochondrial energy metabolism. It is involved in regulating inflammation and oxidative stress through the aromatic hydrocarbon receptor (AhR) pathway, leading to frailty.66, 67 In addition, Hsu et al. have highlighted the close correlation between tryptophan and the development of renal function.68 In short, disorders in tryptophan metabolism during pregnancy can trigger AhR signalling pathway and result in kidney disease during development.69 On the other hand, if there is a lack of tryptophan in newborns, it can impact the renin angiotensin system, renal tubular sodium processing and renal sympathetic nerves, leading to inferior developmental programming and decreased kidney function.70
Kynurenic acid, serotonin and indole derivatives are the main metabolites of tryptophan regulated by the intestinal microbiota. Kynurenic acid is synthesised from tryptophan through the activity of tryptophan 2,3-dioxygenase (TDO) or indoleamine 2,3-dioxygenase (IDO).71 Kynurenic acid and indole derivatives participate in AhR signal transduction and cause a decrease in kidney function.66, 72 Indoxyl sulfate is a prime example. It has been found to cause inflammation, fibrosis and oxidative stress in renal proximal tubular cells, impair endothelial cell proliferation, promote calcification of vascular smooth muscle cells and ultimately contribute to the development of CKD.73 Multiple studies have established a correlation between kynurenic acid and indole sulfate with frailty. Westbrook et al. and Jang et al. indicated that levels of kynurenic acid significantly increased in elderly individuals diagnosed with frailty, and were inversely related to grip strength and walking speed.67, 74 Chao and Lin have demonstrated that the increase in indole sulfate levels is linked to a decrease in the secretion of myogenic factors and the activation of inflammation in glial cells, which can result in the development of frailty.75
Taurine has been linked to CKD and serves as a vital biomarker for monitoring the progression of the disease.76 A decrease in taurine levels in bloodstream may lead to CKD, as taurine plays a crucial role in providing anti-inflammatory and antioxidant benefits. Odobasic et al. demonstrated that taurine can inhibit the function of antigen-presenting cells and T cells during glomerular inflammation, block inflammatory pathways, and ease kidney injury.77 Gossai et al. have reported that taurine and its metabolites have the ability to decrease the production of intracellular oxidants, enhance the resilience of glomerular capillaries and maintain kidney function.78 Two studies on amino acid metabolomics have indicated that non-frail individuals have significantly higher levels of circulating taurine compared to frail individuals.79, 80
Abnormal blood lipids have been found to be linked to CKD and frailty. In 2015, Yamada et al. found that elevated levels of triglycerides, high-density lipoprotein and low-density lipoprotein represented abnormal lipoprotein levels in the Japanese population. These abnormal levels were significantly correlated with the prevalence of CKD and frailty.81, 82 In recent years, with the rapid development of kidney metabolomics research, lipid metabolism disorders have been identified as a major risk factor for kidney disease.83 Research has demonstrated that excessive ingestion of lipids or impaired consumption of lipids in human body can lead to buildup of lipids in large quantities in renal tubular epithelial cells (RTEC). This causes the continuous release of pro-inflammatory factors and reactive oxygen species (ROS), ultimately promoting RTEC apoptosis.84
The carnitine system is closely related to RTEC. As the most energy-demanding part of the kidney, RTEC is highly dependent on mitochondrial fatty acid oxidation activity. Carnitine can transport fatty acids from cytosol to mitochondria for follow-up β-oxidation, and thereby regulates adenosine triphosphate synthesis.85 Therefore, the carnitine system is an essential component in mitochondrial fatty acid metabolism. Multiple studies have confirmed that high levels of acetyl-carnitine are associated with decreased kidney function.86, 87 Furthermore, several short-chain acylcarnitines, including propionyl-carnitine and butyryl-carnitine, are shown to be negatively correlated with human renal function.64, 88 In vitro experiments conducted by Ottria et al. have shown that elevated levels of circulating carnitine can stimulate fatty acid oxidation and trigger the release of inflammatory factors.89 According to Rattray et al., carnitine is a significant metabolite that differentiates between frailty and non-frailty phenotypes, and the carnitine shuttle pathway is also identified as a crucial factor leading to increased risk of developing frailty.90
Recent evidence suggests that changes in lipid metabolism is also related to the development of proximal tubular cells, distal tubular cells and podocytes. Wang et al. found that the sphingolipids metabolic pathways of cells in these three parts are significantly increased compared to cells in other areas of the kidney.91 Sphingolipid is a type of adipokines that links perirenal fat to the kidney. Its role is essential in preserving regular kidney function and facilitating the healing process of damaged kidneys.92 Ceramide is a sphingolipid that is generated from free fatty acid precursors. It plays a crucial role in regulating inflammation, apoptosis and maintaining mitochondrial homeostasis. According to current evidence, ceramide is capable of inducing ROS production, inflammatory activation, caspase activation and apoptosis by enhancing the expression of proapoptotic Bcl-2 protein and reducing the expression of antiapoptotic Bcl-2 protein in the mitochondrial membrane of renal tubular cells, which ultimately leads to a decline in kidney function.93 Yeoh et al. have utilised mass spectrometry in conjunction with lipidomic analysis to determine that there is a significant correlation between ceramide and both the degree of frailty and quality of life among elderly males.82
4 THE POTENTIAL LINK BETWEEN KIDNEY AND SKELETAL MUSCLE METABOLISM THROUGH THE REGULATION OF CIRCADIAN RHYTHM
As individuals age, skeletal muscles naturally undergo a process of atrophy. This atrophy is characterised by increased levels of muscle fibrosis and the accumulation of intramuscular adipose tissue.94 The primary cause of this phenomenon is an imbalance in skeletal muscle protein metabolism and impairment of muscle cell regeneration.95 Recent studies have revealed that individuals aged 75 and above experience a median decrease in skeletal muscle mass ranging from 0.64% to 0.98% and that a decline in kidney function significantly accelerates the loss of skeletal muscle.96 The decline in kidney function can lead to solute retention in Uremia, which results in inflammation, oxidative stress and insulin resistance. These factors disrupt skeletal muscle mitochondrial metabolism and compromise the health of skeletal muscle.95 Furthermore, the decline in kidney function is also associated with lipid metabolism disorder in muscle cells (as discussed earlier) as well as insulin resistance in skeletal muscle.97 As CKD advances, the accumulation of uremia solute hampers protein hydrolysis through the IRS1-PI3K-AKT signalling pathway, leading to a persistent deterioration of skeletal muscle mass and function.98
The circadian rhythm system, also referred to as the biological clock, serves as a fundamental regulatory mechanism for human physiological functions. This system originates from the central clock in the suprachiasmatic nucleus (SCN) of the hypothalamus. This central clock can control various brain regions and clocks in peripheral organs through body fluid and neuron signals to synchronise daily behaviour and physiological rhythm, allowing human body to better adapt to external environment.99 Numerous studies have demonstrated the presence of circadian rhythm oscillations in the physiological functions of kidney and skeletal muscle. Specifically, the renal plasma flow, glomerular filtration rate and tubular secretion/reabsorption process exhibit peak values during wakefulness and decline during sleep.100 Skeletal muscle strength and endurance are typically at their highest in the afternoon and evening, and reach their lowest levels in the morning.101
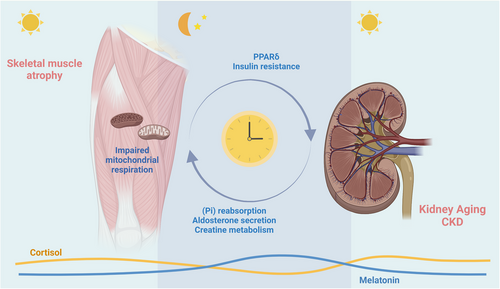
Previously, it was believed that peripheral clock is only influenced by the SCN and self-regulation mechanisms under physiological conditions.102, 103 However, Greco et al. discovered that the peripheral clock of the liver requires communication with the peripheral clock of skeletal muscles to maintain its normal physiological function.104 This study suggests the possibility of crosstalk between human peripheral clocks. While there is no concrete evidence indicating a biological connection between peripheral clocks of kidney and skeletal muscle, there are instances where the circadian rhythm of both organs exhibits synchronous changes.105 These changes are associated with oxidative stress, lipid metabolism and glucose homeostasis damage.106 The expression of certain clock genes may serve as a means of communication between the physiological function of kidney and skeletal muscle. A brief overview of clock genes found in both organs and their mechanisms of action is provided in this section. As shown in the Figure 3, we aim to explore the potential communication role of clock genes between these two organs.
BMAL1 and CLOCK are the primary activators of circadian rhythm in kidney and skeletal muscles. The BMAL1-CLOCK heterodimer activates the transcription of various clock-controlled genes (CCG), which regulate the physiological functions of these organs. Lack of BMAL1 can lead to various functional deficiencies. Xie et al. observed the disappearance of circadian rhythm in renal cortex medulla osmotic pressure gradient in BMAL1-null mice.107 The study conducted by Nikolaeva et al. revealed that the expression of organic anion transporters (SLC22a8 and OAT3) decreased by approximately 80% in the nephrons of BMAL1-null mice,108 which severely impacted the normal secretion of creatinine and the effectiveness of loop diuretics such as furosemide. Additionally, it has been observed that the excretion rate of urinary creatinine is linked to skeletal muscle mass in various populations.109 BMAL1 plays a crucial role in regulating mitochondrial function, lipid metabolism and transcriptional programming of amino acid metabolism in skeletal muscle. Dyar et al. found that the absence of BMAL1 can result in weakened mitochondrial respiration, reduced glycolysis and increased insulin resistance in mouse skeletal muscles.110 In mouse skeletal muscles, the absence of BMAL1 also results in elevated levels of CD36 and FABP3, both of which are associated with the progression of renal fibrosis.111, 112 Chatterjee et al. have reported that BMAL1-deficient mice exhibit an increase in mTORC1 activity.113 mTORC1 is responsible for protein synthesis in skeletal muscles. However, excessive activation of mTORC1 can lead to damage of renal podocytes and tubular cells, ultimately causing the development of CKD.114
Period proteins (Per1, Per2 and Per3) and cryptochrome (Cry1 and Cry2) play a crucial role in regulating the circadian rhythm mechanism. These proteins form the PER-CRY heterodimer, which inhibits the transcriptional activity of BMAL1-CLOCK, thus providing negative feedback.115 Additionally, PER and CRY jointly regulate aldosterone synthesis in adrenal cells and plasma aldosterone levels.116 Aldosterone is a type of salt corticosteroid that plays a crucial role in maintaining sodium balance and regulating blood pressure in the human body. However, excessive levels of aldosterone can lead to harmful reactions, such as kidney damage, hypertension, sensory disorders and skeletal muscle atrophy.117 Recent studies have found that knocking out PER1 in rats and mice or knocking out CRY in mice can increase plasma aldosterone levels.118 There is limited research on the function of PER in skeletal muscles, but studies have found that CRY regulates insulin resistance in skeletal muscles119 and may lead to renal dysfunction.120 CRY can also inhibit the activity of lipid-sensing peroxisome-proliferator-associated receptor δ (PPARδ) in skeletal muscles, which is believed to be related to kidney ageing.121
GLUT4 is a CCG regulated by BMAL1, and is expressed in both skeletal muscle cells and distal tubules of kidney. GLUT4 is able to increase insulin sensitivity and glucose absorption to promote muscle health.122 However, the circadian rhythm oscillation can lead to insulin resistance and a decrease in the expression of GLUT4.123 A study showed that transgenic mice with muscle GLUT4KO displayed a diabetes phenotype and a 55% reduction in insulin-stimulated systemic glucose uptake. This is believed to be connected to the development of DKD.124
NAMPT is vital to kidney and skeletal muscle metabolism. It acts as a rate-limiting enzyme in the NAD salvage synthesis pathway and indirectly regulates oxidative metabolism and glucose-stimulated insulin secretion by controlling intracellular NAD levels. Furthermore, NAMPT-mediated NAD synthesis is partially regulated by the circadian rhythm mechanism, providing a critical link from the clock oscillator to metabolic pathways.125 Studies have demonstrated that absence of NAMPT can lead to a significant decrease in the reabsorption of inorganic phosphate in kidney,126 and inorganic phosphate plays a critical role in the mitochondrial respiration of skeletal muscles.127 Regarding skeletal muscles, NAMPT can provide an ecological niche for muscle stem cells, maintaining the stability of skeletal muscle mitochondria, and regulating the level of lipid mediators in skeletal muscles to enhance muscle function. This finding has significant implications for the development of interventions that can improve skeletal muscle health and function.128
Although there is no conclusive evidence that cortisol and melatonin are regulated by specific clock genes, the crucial role of cortisol and melatonin in regulating circadian rhythm deserves particular mention.129 Cortisol levels typically peak during the day, promoting wakefulness and activity, while melatonin increases at night to aid in sleep. Research has shown a correlation between cortisol and melatonin metabolism, and the functioning of the kidneys and skeletal muscles. When cortisol levels are elevated or metabolic rhythms are abnormal, it can lead to decreased insulin sensitivity and signalling in skeletal muscle cells,130 which in turn increases the risk of sarcopenia.131 Additional studies have found that high levels of cortisol can contribute to higher mortality rates among individuals with CKD.132 Melatonin has the ability to prevent mitochondrial dysfunction and insulin resistance in skeletal muscles.133 while also restoring muscle regeneration and enhancing muscle function.134 Research also has found that melatonin can help maintain the normal physiological function of the kidneys and prevent the progression of CKD135 through the mediation of the renin-angiotensin system.136 Overall, maintaining a healthy circadian rhythm is crucial for the proper function of skeletal muscles and kidney. However, additional research is required to comprehend the roles of cortisol and melatonin between skeletal muscles and kidney to promote better health outcomes for individuals with CKD and frailty.
5 SUMMARY AND FUTURE DIRECTION
There is a strong epidemiological and pathophysiological link between CKD and frailty. This connection involves kidney genetics, inflammageing and circadian rhythm disruption.137, 138 Various omics studies related to CKD have been reviewed, highlighting how kidney genetics and inflammageing contribute to the pathogenesis of CKD, and how these factors lead to frailty. Additionally, the role of circadian rhythm disruption in promoting the development of CKD and frailty has been discussed, along with the relationship between circadian rhythm disruption and these two conditions. This review aims to provide new perspectives for enhancing the management of CKD and frailty.
It is worth emphasising that although genetic factors related to kidney function partly determine the risk of frailty in CKD patients, not all CKD patients with genetic defects in kidney function will experience frailty. This is because kidney function can be restored through treatment or intervention. The genetic defects in kidney function often result in persistent inflammation within the renal tubules, but acquired anti-inflammatory measures can help improve this condition. Current research suggests that following a Mediterranean dietary pattern, which is known for its anti-inflammatory properties, can effectively reduce inflammation and oxidative stress levels, delay the progression of CKD in specific populations and improve frailty symptoms.139 Furthermore, it has been proposed that high-frequency or extended dialysis regimens may be an effective approach for patients with end-stage renal disease (ESRD). These regimens can help reduce uremic toxin accumulation, decrease inflammation levels and alleviate frailty.75 However, further experimental studies are necessary to confirm this hypothesis.
The kidneys play a crucial role in regulating skeletal muscle metabolism. Previous clinical research shows that compared to healthy elderly individuals of the same age and gender, CKD patients generally exhibit lower exercise capacity and poorer physical function, increasing their risk of frailty.140 Numerous randomised controlled trials (RCT) have demonstrated that exercise training can improve skeletal muscle mass and physical performance in CKD patients,141 but there is limited research on whether kidney function can recover after such interventions. Lima et al. have indicated that combined resistance and aerobic training can improve the decline of kidney function in kidney transplant recipients,142 but the specific mechanisms remain unclear. Therefore, investigating the conditions under which skeletal muscle status can be improved to promote kidney function recovery and exploring the underlying mechanisms hold significant academic importance.
Circadian rhythm disruption may also be a potential solution to address this issue. In this review, we suggest that circadian rhythm disruption may mediate the physiological mechanisms of muscle–kidney crosstalk, and disrupted circadian rhythms can promote the development of CKD and frailty. Skeletal muscles make up approximately 40% of bodyweight and represent the largest peripheral clock collection in the human body.143 Meanwhile, the kidneys are among the organs with the most circadian rhythm transcripts.102 Increasing evidence has illustrated the potential role of circadian rhythms in the interconnectedness between kidney and skeletal muscles, and further research is needed to fully understand this specific relationship, which can contribute to the development of new clinical treatment approaches.
In summary, despite the extensive research conducted on CKD and frailty, we are still far from translating these research findings into clinical practice. Given the background of global population ageing, CKD and frailty will continue to be significant problems affecting many elderly individuals. This review will inspire and provide clues for further exploration of the inherent connections between CKD and frailty, as well as the development of novel treatment modalities.
AUTHOR CONTRIBUTIONS
Zhijun Bao and Xiaojun Wang takes responsibility for the intellectual content in the study. Study concept and design: Zhijun Bao and Xiaojun Wang. Drafting of the manuscript: Xiaojun Wang, Xuchao Gu and Yuxin Zhang.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
FUNDING INFORMATION
National Key R&D Program of China, Grant Number: 2018YFC2002000; National Natural Science Foundation of China, Grant Numbers: 82071581, 82104790; Shanghai Medical Leadership Training Program, Grant Number: 2019LJ09
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Not applicable.




