Biogenesis, function, and landscape of tsRNAs in central nervous system diseases
[Correction added on 04 December 2023; after first online publication missing sub-sections have been added.]
Abstract
Transfer RNA-derived small RNAs (tsRNAs) are fragments that originate from mature or precursor tRNAs and are a subclass of sRNAs. With the development of high-throughput sequencing techniques, the real feature of tsRNAs has gradually been revealed. tsRNAs are functional fragments of distinct lengths produced by the cleavage of mature or precursor tRNAs by different ribonuclease enzymes. tsRNAs exert extensive functions, including gene silencing, translational regulation, and reverse transcriptional regulation, affecting cell viability and differentiation and participating in pathological processes of various diseases, including central nervous system (CNS) diseases. Emerging sequencing evidence indicates that tsRNAs are expressed differently in various CNS diseases, preliminary suggesting that tsRNAs are involved in the occurrence and progression of neurodegenerative diseases, stroke, glioma, epilepsy, and other CNS diseases. In addition, significant differences expression of extracellular tsRNAs in circulating or cerebrospinal fluid between patients and normal individuals have demonstrated the diagnostic and prognostic value of tsRNAs as biomarkers for liquid biopsy. In this review, we provide a detailed summary of the biogenesis, function, and chemical modification of tsRNAs, focusing on the current status and prospects of research on tsRNAs in neurodegenerative diseases, stroke, glioma, epilepsy, and others.
1 BACKGROUND
Transfer RNAs (tRNAs), best known as decoders for messenger RNA (mRNA), are the backbone of protein synthesis.1 The D-loop, anticodon loop, TΨC loop, and stems make up the classic cloverleaf-like stem-loop structure.1 The anticodon loop connects to the corresponding codon on the mRNA according to the principle of complementary base pairing. It transports the amino acid carried by the 3′terminal to the ribosome, completing the extension of the peptide chain.1 In addition to protein synthesis, extensive chemical modifications of tRNAs are also well known.1, 2 Most of the chemical modifications of nucleotides discovered so far are present in tRNAs and are essential for maintaining the stability of tRNAs.1-3 Interestingly, tRNA modification regulates the generation of tRNA-derived small RNAs (tsRNAs), also known as tRNA-derived RNA fragments (tRFs).3
tsRNAs, like other noncoding RNAs (ncRNAs), were initially thought to be meaningless by-products of tRNAs. With advances in high-throughput sequencing techniques, the differential expression of tsRNAs in various diseases has implied their plentiful biological functions.3 In 2009, Lee et al.4 defined and validated tRFs for the first time through in-depth sequencing of prostate cancer cell lines and divided them into three series based on cut sites: tRF-5, tRF-3, and tRF-1. In particular, they found that tRF-1001 was strongly correlated with cell proliferation. Although there is currently no unified nomenclature, the classification and biological function of tsRNAs has recently been fundamentally defined with the deepening of research.5
Expanding studies focus on tsRNAs expression in the central nervous system (CNS). Jehn et al.6 analyzed sRNA sequencing data sets from the human hippocampus, frontal cortex, and cerebellum to characterize the tsRNAs classes and levels in different CNS tissues, demonstrating the presence of a large number of 5′tsRNAs in the primate hippocampus. Extensive sequencing results support the potential regulatory role of tsRNAs in CNS diseases, especially neurodegenerative diseases (ND), stroke, epilepsy, and glioma. Moreover, extracellular tsRNAs increasingly show potential as biomarkers for various CNS diseases. Detection of 5′-tRFAlaTGC in the blood predicts seizures.7 In addition, exosomal tRF-19-INVDRIFU has shown promise in diagnosing and predicting the prognosis of large artery atherosclerotic (LAA) stroke.8
Our review covers the latest research on tsRNAs, specifically their potential role in CNS diseases. We delve into the classification, biogenesis, and importance of chemical modification in regulating their generation. Additionally, we explore the biological functions of tsRNAs, which are the fundament of their disease regulation. Our focus is on the impact of tsRNAs on central nervous system diseases and their potential value as biomarkers in clinical applications.
2 BIOGENESIS AND CLASSIFICATION OF tsRNAs
Mature tRNAs contain 70−90 nucleotides (nt). They are made in the nucleus from a tRNA gene transcribed by RNA polymerase III (pol III). The precursor tRNA (pre-tRNA) is produced and then cleaved by ribonucleases (RNase P and RNase Z) to remove the 5′ leader and 3′ trailer. The CCA sequence is added at the 3′ end by ligase. After chemical modification and intron splicing, the tRNA matures and translocates to the cytoplasm.3, 5 Based on the restriction site and the tRNA termini in the fragment, tsRNAs are classified as follows: (1) 5′-halves (2) 3′-halves (3) 5′-tRFs (4) 3′ -tRFs (5) i-tRFs (6) 1-tRFs. It should be noted that the remaining tsRNAs are derived from mature tRNAs, except for 1-tRFs, which are derived from pre-tRNAs.3 1-tRFs are poly U sequences distributed 16−48 nt in length and obtained by RNase Z or ELAC2 cleavage of the 3′ trailer of the pre-tRNA. There is still no unified nomenclature for tsRNAs (Figure 1A).
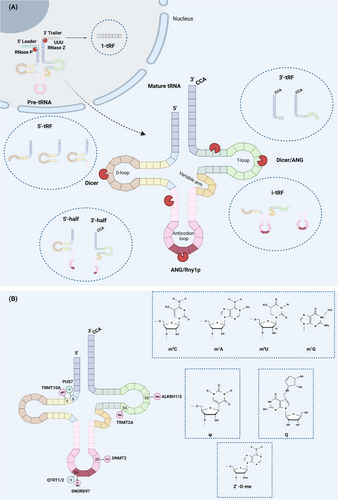
Halves, which are small RNA fragments ranging from 30–40 nt, were first discovered in protists such as Tetrahymena thermophila and Giardia lamblia.9, 10 These fragments are also known as tRNA-derived stress-induced RNA (tiRNA) and are produced when tRNA is cleaved at the anticodon loop by mammalian angiopoietin (ANG)/yeast Rny1p in response to stress conditions like heat shock, hypoxia, and amino acid starvation.11 There are two types of halves: 5′-halves with a 5′-end and 3′-halves with a 3′-end.12 Interestingly, Honda et al.13 identified a class of tiRNAs that are highly expressed in sex hormone-dependent breast and prostate cancers and are produced by sex hormone stimulation: 5′-SHOT-RNA and 3′-SHOT-RNA. Unlike conventional tiRNA, the 3′ end of 5′-SHOT RNA and the 5′ end of 3′-SHOT-RNA contain cyclic phosphates and amino acids, respectively.
The resulting tsRNAs are called tRFs when the cleavage site is close to the D- or T-loop of mature tRNAs.4 The 5′-tRFs are RNA fragments smaller than 30 nt produced by the Dicer cleave D-loop.14 Different 3′ terminal positions (D-loop, D-arm, or anticodon arm) correspond to three subtypes: tRF-5a (14-16 nt), tRF-5b (22-24 nt), or tRF-5c (28-30 nt).15 Similarly, 3′-tRFs are fragments containing a CCA sequence, divided into tRF-3a (−18 nt) and tRF-3b (−22 nt) subsets based on their length.15 Their generation depends mainly on the effects of ANG and Dicer on the T-loop.15 i-tRFs are fragments present only in the anticodon arm and impose an inhibitory effect on the expression of breast cancer genes.16 Additionally, under hypoxic conditions, specific tRNAs (tRNAGlu, tRNAAsp, tRNAGly, and tRNATyr) generate a class of tRFs that contain a complete anticodon stem-loop structure and parts of T-arms and D-arms.16
While the biogenesis and classification of tsRNAs are well understood, the regulatory system for tsRNA biogenesis remains to be fully elucidated. One established regulator of tsRNA biogenesis is the chemical modification of tRNA.
3 ROLES OF CHEMICAL MODIFICATION IN tsRNAs BIOGENESIS
Chemical modifications, such as methylation, queuosine (Q) modification, 2′-O-methylation, and pseudouridine (Ψ), play a crucial role in maintaining the stability of tRNA. Recent studies have also shown that these modifications affect the affinity of ribonucleases for tRNA, thereby regulating the biogenesis of tsRNAs (Figure 1B).
Methylation is a common but significant posttranscriptional modification of tRNAs that affects their decoding activity and stability. Methylated tRNAs are more stable and less likely to form tiRNAs under stress. In contrast, when methylation levels are low, the affinity of ANG for tRNAs is enhanced, leading to an increase in the production of fragments containing the 5′ terminus and, through activation of translation inhibitors, a decrease in protein synthesis. The modifications mainly include 5-methylcytosine (m5C), 5-methyluridine (m5U), N1-methyladenine (m1A), and N1-methylguanine (m1G), depending on the base type and the C or N site of methyl insertion. Among them, the most well-known is m5C, induced by DNA methyltransferase 2 (DNMT2) and NOP2/Sun RNA methyltransferase 2 (NSUN2). DNMT2 acts on cytosine 38 of tRNAAsp, tRNAVal, and tRNAGly, mediating methylation of tRNA and preventing cleavage by endonuclease.17 Similarly, the hypomethylation of tRNA caused by the absence of NSUN2 is ultimately conducive to the accumulation of tiRNAs.18 The tRNA methyltransferase 2 homologous A (TRMT2A) has a similar effect, and Pereira et al.19 first revealed that m5U54 could be used as a protective marker for tRNA cleavage. When TRMT2A is knocked down, m5U54 tRNA hypomodification causes an increase in ANG-dependent 5′tiRNA-GlyGCC and -GluCTC and inhibits global protein translation. However, no definitive evidence suggests a relationship between translation suppression effects and 5′tiRNA-GlyGCC and -GluCTC levels. In addition, tRNA methyltransferase 10 homologous A (TRMT10A) reduces tsRNAGln production in pancreatic β-cell by m1G modification of the tRNAGln position 9.20 Notably, the m1A and m3C modification can be removed by the demethylating enzymes ALKBH1 and ALKBH3,21, 22 resulting in high tRNA sensitivity to ANG.
There is a profoundly modified 7-de aza-guanosine ——Q modification at position 34 of tRNAHis/Asn/Tyr/Asp containing the 5′ GUN, which is located in the oscillating anticodon. Q modification is accomplished by QTRT1/QTRT2 heterodimer.23 In humans, Queuosine, produced by gut microbes, is absorbed by cells and replaces guanylate at position 34 under the action of QTRT1/ QTRT2. Q-modification protects tRNAHis and tRNAAsn. The amount of tRNAHis and tRNAAsn detected in HEK293T cells with Q depletion increased by two times and nine times, respectively, compared with that in entirely Q-modified cells.24 It must be mentioned that Q-modification can facilitate the modification of DNMT2 on m5C38-tRNAAsp.25 Vitaili and Kiss demonstrated that human SNORD97 and SCARNA97 cooperated to complete the 2′-O-methylation of cytidine at position 34 of tRNAMet (CAT), preventing ANG from cutting tRNAMet.26
In contrast to the stable tRNA modification methods mentioned above, pseudourinylation (Ψ) promotes the creation of tsRNAs. Pseudouridine is a natural structural analog of uracil, except that the ribose is linked by 5C of the pyrimidine ring instead of 1N. Pseudouridine synthetase 7 (PUS7) mediates Ψ at U8, affects the biogenesis of 5′-tRFs of shared terminal oligonucleotides (TOG) in embryonic stem cells, and is associated with translational regulation.27 In summary, chemical modification plays a regulatory role in tsRNAs production and largely influences tsRNAs biogenesis.
4 BIOLOGICAL FUNCTIONS OF tsRNAs
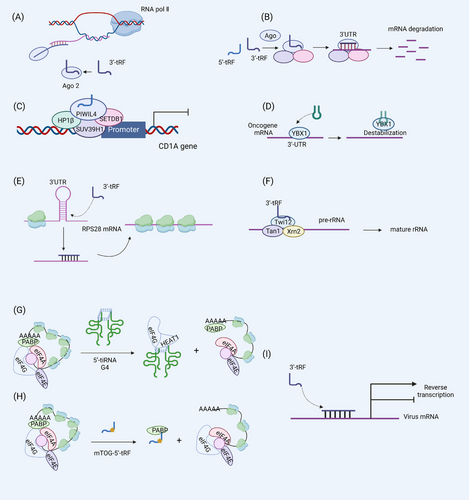
tsRNAs are ancient and evolutionarily conserved small RNAs widely present in several species, including protozoa lacking miRNA, siRNA, and piRNA.3 tsRNA is involved in various cellular biological processes, particularly the regulation of gene expression.28 Its regulatory role can be broadly divided into the following three points: gene silencing, translation, and reverse transcription. (Figure 2)
4.1 Regulation of gene silencing
tsRNAs can induce gene silencing, mainly including pretranscriptional gene silencing (TGS), which is related to the repressive epigenetics of the target site, and posttranscriptional gene silencing (PTGS), which can mediate mRNA cleavage to achieve gene silencing. The 5′-tRF derived from tRNAGlu is highly expressed in human monocytes and has the properties of a piRNA. It interacts with PIWIL4 to recruit H3K9 methyltransferases SUV39H1, SETDB1, and HP1β to the CD1A promoter region, thereby promoting H3K9 methylation-mediated repression of CD1A gene transcription.29 Notably, CD1A expression is closely associated with autoimmune diseases.30 The CD1A gene silencing effect of tsRNAs also highlights the immunomodulatory role of tsRNAs. tRFs participate in PTSG via a miRNA-like effect.31 The main link of PTSG is the assembly of RNA-induced silencing complex (RISC). tRFs bind to Ago2, the RISC's core protein, and transform into RISC capable of silencing mRNA. Furthermore, the RISC is directed to the 3′UTR of the target mRNA, where the tRF binds to the mRNA in an incomplete complementary base pairing. At this time, the PIWI domain of Ago2 protein plays a cleavage activity and degrades the mRNA.31 Nevertheless, tsRNA binds to Ago1, Ago3, and Ago4 more than Ago2.15 In addition to exerting gene silencing effects through RISC, i-tRF has been shown to compete for the mRNA-stabilizing YB-1 protein and repress oncogene expression, thereby repressing oncogene expression.16 Consistent with YB-1, IGFBP1 also plays a role in stabilizing mRNA, while tsRNAGlnCTG interacts with IGFBP1 protein to inhibit the stability of transcripts.32 Besides TGS and PTGS, Dicer-induced tRFs can also promote gene silencing by the mechanism of nascent RNA silencing.33 Dicer-dependent tsRNA binds and guides Ago2 into the nucleus. Subsequently, tsRNA is complementary to intronic pairing of nascent RNA to ensure that Ago2 can be targeted to cleave the target mRNA.33 Although ago is also required, this nascent RNA silencing mechanism occurs in the nucleus and does not affect the transcription of genes, so it is different from PTGS and TGS.
4.2 Regulation of protein translation
A growing number of studies have revealed the bidirectional regulation of protein translation processes by tsRNAs. While 5′-tiRNA and 5′-tRF inhibit translation by inhibiting the cap-dependent translation initiation mechanism, the boosting effect of 3′ -tRFs on ribosomal biogenesis determines its positive regulatory effect on translation. Ivanov et al.34 demonstrated that the 5′-tiRNA-YBX-1 complex displaces eIF4G bound to the m7G cap and inhibits translation initiation. The 5′-end of 5′tiRNAAla has a terminal oligomeric guanine (TOG) motif that promotes the formation of G-quadruplex (G4) (containing four tiRNAs). By binding to the HEAT1 domain of eIF4G, G4 disrupts the assembly of the eIF4F complex on the m7G cap of mRNA, resulting in the scanning of the 48S ribosome and ultimately inhibition of translation.35 The mechanism of the inhibitory effect of 5′-tRF (tRNAAla,tRNACys, and tRNAVal) on translation initiation is the formation of the mini TOG (mTOG)-5′-tRF-poly adenosine binding protein 1 (PABP1) complex. PABP1C initiates translation by binding to the tail of the mature mRNA and eIF4F. However, mTOG-5′-tRF-PABP1C sequesters PABP1C from eIF4F, inhibiting translation initiation. mTOG depends on the Ψ modification of PUS7 at U8, and embryonic stem cells lacking PUS7 show increased protein synthesis and defective germ layer specification.27
The regulatory effect of tsRNAs on protein translation is bidirectional. In addition to inhibiting global protein translation, tsRNAs can promote protein translation. The mechanisms involved mainly promote ribosomal biogenesis and positively regulate the production of ribosomal proteins and ribosomal RNA. 3′-tRF derived from tRNALeuCAG(3′tsRNALeuCAG) has been shown to promote ribosome production by binding to and enhancing the translation of ribosomal protein mRNAs RPS28 and RPS15. Specifically, 3′tsRNALeuCAG promotes the translation of the RPS28 mRNA to maintain the number of ribosomes by binding to the 3′UTR and coding region of the RPS28 mRNA to unfold the double-stranded structure of the mRNA. Inhibition of 3′tsRNALeuCAG promotes Hela cell apoptosis and inhibits tumor growth in an orthotopic mouse model of hepatocellular carcinoma, suggesting a different target for cancer therapy.36 In addition, 3′-tRF specifically binds to Twi12, a PIWI family protein essential for Tetrahymena growth, allowing it to enter the nucleus and assemble with Xrn2 and Tan1 exonucleases to form a TXT complex to process pre-rRNA to mature rRNA.37
4.3 Regulation of reverse transcription
Transposable elements (TE) are a class of DNA sequences that can move across host genes. In 1989, Finnegan first classified TE into two classes: retrotransposons and DNA transposons. The former uses RNA as an intermediary and reverse-transcribes RNA into DNA for integration into the host genome.38 Numerous retroviruses use tRNA as a primer to bind to viral RNA's prime-binding site (PBS) to initiate reverse transcription. Recent studies have shown that 18 nt tRF can compete with tRNA for PBS binding to LTR-retrotransposons and restrict the movement of transposable elements in mouse ES cells, whereas 22 nt 3′-tRFs inhibit translation of LTR-retrotransposons capable of encoding.39 Furthermore, the 18-nt 3′-tRF of a dsRNA hybrid consisting of the 3′ -terminus of tRNA3Lys derived from HIV-1 PBS could interact with Ago2 to inhibit HIV-1 reverse transcription.40 tRF also has a regulatory effect on human T-cell leukemia virus type 1 (HTLV-1) infection. tRF-3019 acts as a primer for reverse transcription of HTLV-1, enhancing viral infection. In summary, tsRNA has a regulatory role in viral reverse transcription, and an in-depth study and use of tsRNAs regulation could be a potential way to control viral infection.41 tsRNAs play a crucial role in regulating cell growth and apoptosis through gene regulation and protein synthesis. These biological functions also make tsRNAs important in managing various diseases.
5 ROLES OF TSRNAS IN THE OCCURRENCE AND DEVELOPMENT OF CENTRAL NERVOUS SYSTEM DISEASES
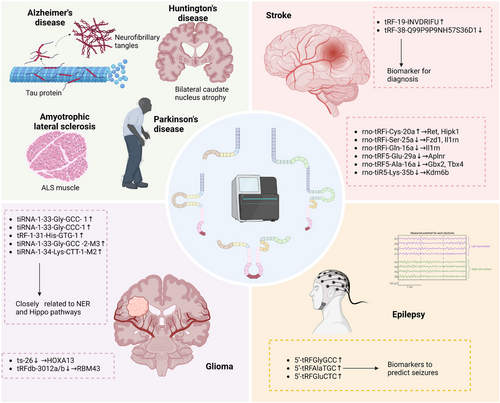
Extensive research has revealed that tsRNAs contribute to the development of cancer,42-44 paternal inheritance,45-47 and autoimmune diseases.48-50 Additionally, studies have shown that tsRNAs also have impacts on CNS diseases.8, 51, 52 Here we shed light on the role of tsRNAs in ND, stroke, glioma, and other neurological disorders (Table 1; Figure 3).
| Diseases | Samples | Expression | Description/Mechanism/Target gene | Ref. |
|---|---|---|---|---|
| AD | SAMP8 mouse brain | AS-tDR-011389↓ | P2ry1, Camk2n1 | 55 |
| AS-tDR-013428↓ | Rpsa | |||
| AD | Sprague-Dawley rats hippocampus | tiRNA-Gln-CTG-002↑ | ERCC5 | 57 |
| tiRNA-Val-AAC-002↑ | ERCC5 | |||
| tRF-Gly-CCC-011↑ | / | |||
| tRF-Thr-CGT-019↑ | GRIA4 | |||
| AD | APP/PS1 transgenic mice hippocampus | tRF-Thr-CGT-003↑ | CACNG2 | 58 |
| tRF-Leu-CAA-004↓ | CYP2C40, CYP2C68, CYP2S1, RYR1 | |||
| AD | Prefrontal lobe cortex samples of AD patients | tiRNA-Tyr↓ | Enhance the vulnerability to oxidative stress on neurons | 51 |
| tiRNA-Arg↓ | Involved in synapse formation in AD | |||
| PD | SAMP8 mouse brain | AS-tDR-005058↑ | Erc1 | 55 |
| AS-tDR-011775↑ | Mobp, Park2 | |||
| ALS | Eukaryotic cells | tiRNACys↑ | Inhibit translation initiation by displacing eIF4F from cap structures | 73 |
| tiRNAAla↑ | Inhibit translation initiation by displacing eIF4F from cap structures and induce the assembly of stress granules | |||
| HD | Human and mouse brain | 5′-tRFAlaCGC-3 | Impaired cell viability of primary neurons in the striatum | 79 |
| LAA stroke | Human Circulating exosome | tRF-19-INVDRIFU↑ | Biomarker for diagnosis, short-term poor prognostic grade stroke severity and plaque rupture risk assessment |
8 |
| tRF-38-Q99P9P9NH57S36D1↓ | Biomarker for diagnosis | |||
| ICH | Right globus pallidus of the rat brain | rno-tRFi-Cys-20a↑ | Ret, Hipk1 | 85 |
| rno-tRFi-Ser-25a↓ | Fzd1, Il1rn | |||
| rno-tRFi-Gln-16a↓ | Il1rn | |||
| rno-tRF5-Glu-29a↓ | Aplnr | |||
| rno-tRF5-Ala-16a↓ | Gbx2, Tbx4 | |||
| rno-tiR5-Lys-35b↓ | Kdm6b | |||
| Ischemia-reperfusion | Rat neuronal PC12 cells | 5′tiRNA-Gly-GCC↑ | Correlated with the degree of cell injury and can be used as a marker of cell injury and death induced by ischemia-reperfusion | 85 |
| 3′tiRNA-Gly-GCC↑ | ||||
| 5′tiRNA-Cys-GCA↑ | ||||
| 5′tiRNA-Ala-AGC↑ | ||||
| GBM | Tumor tissue | tRF-1-32-chrM.Lys-TTT↓ | / | 91 |
| tiRNA-1-33-Gly-GCC- 1↑ | Closely related to NER and Hippo pathways | |||
| tiRNA-1-33-Gly-CCC-1↑ | ||||
| tRF-1-31-His-GTG-1↑ | ||||
| tiRNA-1-33-Gly-GCC−2-M3↑ | ||||
| tiRNA-1−34-Lys-CTT-1-M2↑ | ||||
| Diffuse glioma | Human brain glioma tissue | ts-26↓ | HOXA13 | 92 |
| tRFdb-3012a↓ | RBM43 | |||
| tRFdb-3012b↓ | RBM43 | |||
| Epilepsy (preseizure) | Human plasma | 5′-tRFGlyGCC↑ | Biomarkers to predict seizures | 7 |
| 5′-tRFAlaTGC↑ | ||||
| 5′-tRFGluCTC↑ |
5.1 Neurodegeneration diseases
5.1.1 Alzheimer's disease
Alzheimer's disease (AD), the leading cause of dementia,53 is a ND characterized by the deposition of β-amyloid (Aβ) in the brain and neuronal fibrillary tangles composed of hyperphosphorylated tau. Recent high-throughput sequencing studies have identified differential expression of ncRNAs, including tsRNAs,54 in the brain and blood between AD and normal control. A study comparing the differential expression profiles of tsRNAs in senescence-accelerated mouse susceptible 8 (SAMP8) and senescence-accelerated mouse resistant 1 (SAMR1) identified eight differentially expressed tsRNAs and 110 target genes.55 Rpsa, the target gene of AS-tDR-013428, can promote the production and internalization of Aβ.55, 56 Moreover, genome-wide analysis of hippocampal samples from AD rats (induced by intracerebroventricular injection of oligomeric Aβ1-42) has shown that 3′-tiRNAs, 5′-tiRNAs, and 1-tRFs are significantly up-regulated in the AD group compared with the sham group.57 Additionally, Zhang et al. identified 27 differentially expressed tsRNAs in the hippocampus of APP/PS1 transgenic mice and WT mice by transcriptome analysis, followed by qPCR validation of 2 tsRNAs. After bioinformatics analysis, qPCR and Western blot verification of the target genes, tRF-Thr-CGT-003 and tRF-Leu-CAA-004 were found to be involved in the regulation of CACNG2 and RYR1 genes related to calcium regulation.58 Numerous studies have focused on the pathogenic role of calcium dysregulation caused by RyR channel leakage in AD.59-63 An intriguing recent study revealed the contribution of cholinergic targeting tRFs in the nucleus accumbens to the phenomenon of accelerated dementia and cholinergic neuron loss in female AD patients, suggesting the potential of sex-adjusted anticholinesterase therapy in the treatment of AD.64 As mentioned above, chemical modifications regulate the production of tsRNAs and are involved in the stress response. Zhang et al. measured small RNA modification profiles in autopsy samples of prefrontal cortex tissue from AD patients and controls and found that modifications in 30–40-nt fragments (with the majority of tsRNAs), compared with controls, the levels of 2′-O-methylcytidine, 2′-O-methyluridine, and 7-methylguanosine were higher in the AD group. At the same time, m1G, N2, N2-dimethylguanosine, and Ψ were opposite. These results suggest that tsRNAs and their modifications may be involved in AD.51 Another study suggested that tRNA methylation is involved in the regulation of AD, based on the lower levels of mitochondrial and cytoplasmic tRNA m1A in AD mouse models and the more severe phenotype induced by the knockdown of the methyltransferases TRMT10C, HSD17B10 and TRMT61A in the Drosophila tau model.65
5.1.2 Parkinson's disease
Parkinson's disease (PD) is a ND caused by the degeneration and death of dopaminergic (DA) neurons in the substantia nigra of the midbrain, resulting in a significant reduction of DA content in the striatum. It is clinically characterized by bradykinesia, resting tremor, and myotonia.66 ANG mutations have been found in PD and are directly associated with DA neuron degeneration.67 Since ANG is a critical enzyme in the production of tiRNAs, it is reasonable to suspect a regulatory relationship between ANG, tsRNAs, and PD. At present, there are still few studies on tsRNAs in PD, and most of them focus on biomarkers.55, 68, 69 For instance, sequencing analysis of CSF and serum samples from PD patients and NC showed that tRFs are a potential non-invasive biomarker of PD.68 The differential expression of tsRNAs between PD and NC indirectly reflects the possible regulation of tsRNAs in PD. More mechanistic studies are needed in the future to confirm this. In addition, studies on the brain tissue of SAMP8 mice found that AS-tDR-011775 targeted Park2, one of the most common pathogenic genes in PD.55
5.1.3 Amyotrophic lateral sclerosis
ANG mutations have been found in some amyotrophic lateral sclerosis (ALS) patients,70, 71 a severe ND characterized by the progressive degeneration and loss of motor neurons (NM), and eventual respiratory muscle paralysis.72 Recombinant ANG treatment has shown to rescue stress-induced death in ANG-depleted neurons.73 In 2014, Ivanov et al.73 proposed that DNA analogs of 5′-tiRNAAla containing G4 could spontaneously enter NM and protect NM from stress-induced death in a YB-1-dependent manner, providing a new direction for ND treatment, especially ALS. In addition, 5′-tiRNAAla and 5′-tRNACys have been found to interact with the G4 structure formed by the 23 GGGGCC RNA repeats (2–20 repeats in average human) of the C9ORF72 gene of ALS to interfere with SG assembly.73 A study also validated the indicative role of 5′-tRFValCAC in ALS, suggesting its prognostic value in ALS patients and mouse models.74
5.1.4 Huntington's disease
Huntington's disease (HD) is an autosomal dominant ND that causes slowly progressive choreiform involuntary movements, psychiatric abnormalities, and dementia. The disease is caused by a single gene defect leading to an abnormal increase in the Huntingtin (HTT) gene's CAG copy number. This increase causes the HTT protein to aggregate, leading to the destruction of protein balance, interfering with transcription, and affecting the release of neurotransmitters.75 However, recent studies have focused on the toxic effects of HTT at the RNA level.76-78 The injection of putamen sRNAs from HD patients into the striatum of WT mice induced motor deficits that worsened with the number of injections. These sRNAs up-regulated genes in neuroinflammatory and immune-related pathways. The neurotoxic effect of HD-sRNA-PT was demonstrated by a decrease in Nissl+ and DARPP-32+ cells and an increase in cleaved caspase-3+ cells in the striatum of HD-sRNA-PT mice compared to mice receiving vehicle-injected and CTL-sRNA-PT mice. In particular, sequencing results showed that tRNAs were more abundant in HD-sRNA-PT than other sRNAs, and overexpression of 5′-tRFAlaCGC-3 was responsible for reduced neuronal viability indicated by in vitro.79 This study provides a basis for targeting tRFs for HD treatment strategies.
5.2 Stroke
Stroke remains the third most common disease affecting human health and the leading cause of disability.80 Therefore, early diagnosis and treatment of stroke are essential to reduce disability and mortality. However, high-resolution imaging methods are not accessible in all hospitals, and simple, non-invasive, and easy-to-use detection methods such as body fluid biopsies are gaining increasing attention. Many recent studies have described ncRNAs as potential stroke biomarkers.81, 82 Several studies have also focused on the diagnostic value of emerging tsRNAs for stroke. In a study involving 75 ischemic stroke patients, 66 hemorrhagic stroke patients, and 22 healthy volunteers, plasma tsRNAs levels were measured using anti-m1A antibodies on 1d, 7d, and 30d after stroke. The results showed that plasma tRFs levels at admission were significantly higher in stroke patients than in controls. Moreover, they correlated with infarct size, hematoma volume, and poor outcome.83 Another study screened and validated the differences in tRFs between acute ischemic stroke, intracerebral hemorrhage (ICH), stroke mimics, and healthy controls by RNA-seq and proposed that plasma tRFs could be used as biomarkers to distinguish acute stroke subtypes.84 By sequencing and extensive sample validation (120 LAA: 105 NC: 110 SAO: 105AS), we identified the potential of circulating exosomal tsRNAs as biomarkers for LAA stroke, and importantly, we found that the diagnostic efficacy of circulating exosomal tsRNAs was better than that of plasma tsRNAs.8 In addition to plasma sequencing, transcriptome sequencing of brain tissue samples from chronic CH rats also revealed the involvement of tsRNAs in ICH. Although the specific biological function of tsRNAs is still unclear, enrichment analysis results suggest that tsRNAs may be involved in the oxidative stress response associated with ICH.85 In addition, tsRNAs may also be involved in regulating ischemia-reperfusion injury.86
5.3 Glioma
Gliomas are tumors that originate in glial cells or their precursors. According to the fifth edition of the World Health Organization classification of central nervous system tumors, adult diffuse gliomas include three histological types: astrocytoma, oligodendroglioma, and glioblastoma (GBM), with four grades.87 The latest authoritative statistics from The Central Brain Tumor Registry of the United States (CBTRUS) showed that GBM is the most common primary malignant tumor of the central nervous system in the U.S. population,88 with a median survival time of less than 15 months.89 Since the discovery of tsRNAs in tumor cells in the 1970s, a growing body of research has revealed the regulatory role of tsRNAs in tumors. Gyuris et al. analyzed extracellular vesicular and non-vesicular ribonucleoprotein complexes (RNPs) in mouse glioblastoma cells. They found that RNPs are enriched in tRFs and may be involved in intercellular communication.90 A recent study using sequencing analysis of tumor tissues revealed differentially expressed tsRNAs between GBM and low-grade glioma (LG). They propose tRF-1-32-chrM.Lys-TTT, tiRNA-1-33-Gly-GCC-1, and tiRNA-1-33-Gly-CCC-1 associated with nucleotide excision repair, Hippo signaling, and cancer-related pathways, tRF-1-31-His-GTG-1, tiRNA-1-33-Gly-GCC-2-M3, and tiRNA-1-34-Lys-CTT-1-M2 may be responsible for the higher malignant grade of GBM.91 tRNALeuCAA derived tRFdb-3012-a/b is linked to IDH mutation status in the glioma,92 which is one of the important markers for LG and GBM and is associated with well survival outcome.93 Additionally, ts-26 may regulate glioma by binding to the 3′UTR of HOXA13, which is significantly associated with poor survival outcome of glioma patients.92
5.4 Epilepsy
Epilepsy is a chronic, recurrent brain disorder caused by the abnormal firing of neurons. Seizures and the abnormal brain electrical activity they cause can rarely be captured during diagnosis due to the uncertainty of seizures. Currently, the diagnosis of epilepsy mainly relies on medical history and electroencephalographic monitoring. Recently, biomarker-based liquid biopsy methods have attracted much attention. Several studies on sRNAs have revealed the potential of sRNAs as novel diagnostic biomarkers.94 However, there have been limited studies on the development of seizure-predictive biomarkers. A fast and straightforward method for accurate seizure prediction is beneficial in reducing the impact of epilepsy on daily life and the incidence of trauma caused by uncontrolled acute seizure states. Hogg et al. conducted a study comparing tRFs levels in the blood of patients with epilepsy (preseizure and postseizure) and healthy volunteers. They found that 5′-tRFGlyGCC, 5′-tRFAlaTGC, and 5′-tRFGluCTC had the highest expression levels in the preseizure phase and showed statistically significant differences in the validation phase. Further ROC analysis revealed that 5′-tRFAlaTGC had the highest seizure diagnostic efficiency (AUC = 0.916).7 This study opens up new possibilities for seizure prediction. To enable tRF-based seizure detection at home or in the clinic, the same group developed an electrochemical direct detection method based on platinum nanoparticles to quantify the three tRFs. Compared with the traditional detection method for tRFs, this method has the advantages of sensitivity, specificity, and simplicity.95 An essential step in the clinical translation of seizure biomarkers has been taken.
6 CONCLUSION
Advances in sequencing technology have gradually expanded our knowledge of the transcriptome. Since 2009, tsRNAs are no longer considered degradation products. More importantly, their various biological regulatory functions, such as gene silencing and translational regulation, have been gradually understood. tsRNAs are different types of fragments produced by the cleavage action of ribonuclease on tRNA. The biogenesis of tsRNAs is regulated by chemical modifications such as tRNA methylation, Q modification, 2′-O-methylation, and Ψ modification. tsRNA participates in gene silencing utilizing nascent RNA silencing, TGS, and PTGS. However, its regulation of the translation process is bidirectional. In addition, tsRNAs also play an influential role in reverse transcriptional regulation. tsRNAs are widely found in a variety of CNS diseases. The differential expression of tsRNAs between disease and healthy individuals suggests their role in CNS disease regulation, and the intrinsic regulatory mechanism has been gradually revealed. In addition, accumulated studies depict the promise of extracellular tsRNAs as novel biomarkers for various diseases, and we summarize the current progress of tsRNAs detection based on body fluid biopsy in diagnosing CNS diseases such as stroke, epilepsy, and glioma.
Although tsRNAs are older than other ncRNAs and exist widely in all kinds of life, they are still in the earlier research stage. The current deficiencies in the field of tsRNAs must be pointed out. First of all, lacking a unified naming convention is a limitation in the field of tsRNAs. Currently, the detection of tsRNAs relies on high-throughput sequencing, and different sequencing platforms have different naming methods for tsRNAs. The results presented here may confuse readers, and the same molecule may be named differently. A unified nomenclature system is a crucial step to accelerate the progress of tsRNAs research. Second, as mentioned above, studies on tsRNAs in CNS mainly stop at the step of searching for differentially expressed tsRNAs between patients and normal controls. However, the specific regulatory mechanisms of candidate tsRNAs in diseases still need to be clarified, and more in-depth mechanistic studies must be made available. In addition, based on the stability of tsRNAs and the universality of their expression in diseases, the humoral biopsy strategy of extracellular tsRNAs is the focus of much research. Many studies have highlighted the diagnostic potential of tsRNAs as humoral biomarkers for CNS diseases such as stroke, glioma, and epilepsy. However, these studies have geographical and sample size limitations, which inevitably lead to bias. As diagnostic biomarkers, the specificity and sensitivity of tsRNAs still need to be improved. More importantly, detecting tsRNAs based on high-throughput sequencing is currently relatively expensive. In addition to sensitivity and specificity, the criteria for becoming a biomarker in fluid body biopsy should also focus on simplicity and economy. As mentioned above, for seizure detection, the development of a tsRNAs detection method that can be implemented in community hospitals or even at home is a goal to achieve the promotion of tsRNAs-based fluid biopsy. Of course, this goal presupposition that the tested biomarkers are reliable. Therefore, improving the sensitivity and specificity of tsRNAs as biomarkers and developing cost-effective detection methods are necessary to realize the application of tsRNAs as biomarkers of CNS diseases and other diseases.
In summary, with the deepening of more precise, the clearer picture of tsRNAs is exciting. The dysregulation of tsRNAs in CNS diseases also indicates that tsRNAs are related to CNS diseases’ progression and prompts researchers to further investigate how tsRNAs regulate CNS diseases. More efforts are needed to achieve clinical translation of tsRNAs in the future.
AUTHOR CONTRIBUTIONS
KYY drew the manuscript and images, YS and JZ contributed to the conception of the article, and XDP and XYZ proofread the manuscript.
ACKNOWLEDGEMENTS
We are grateful to Biorender for the drawing support.
FUNDING
Not applicable.
CONFLICTS OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS APPROVAL
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




